©The Author(s) 2025.
World J Diabetes. Feb 15, 2025; 16(2): 97910
Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.97910
Published online Feb 15, 2025. doi: 10.4239/wjd.v16.i2.97910
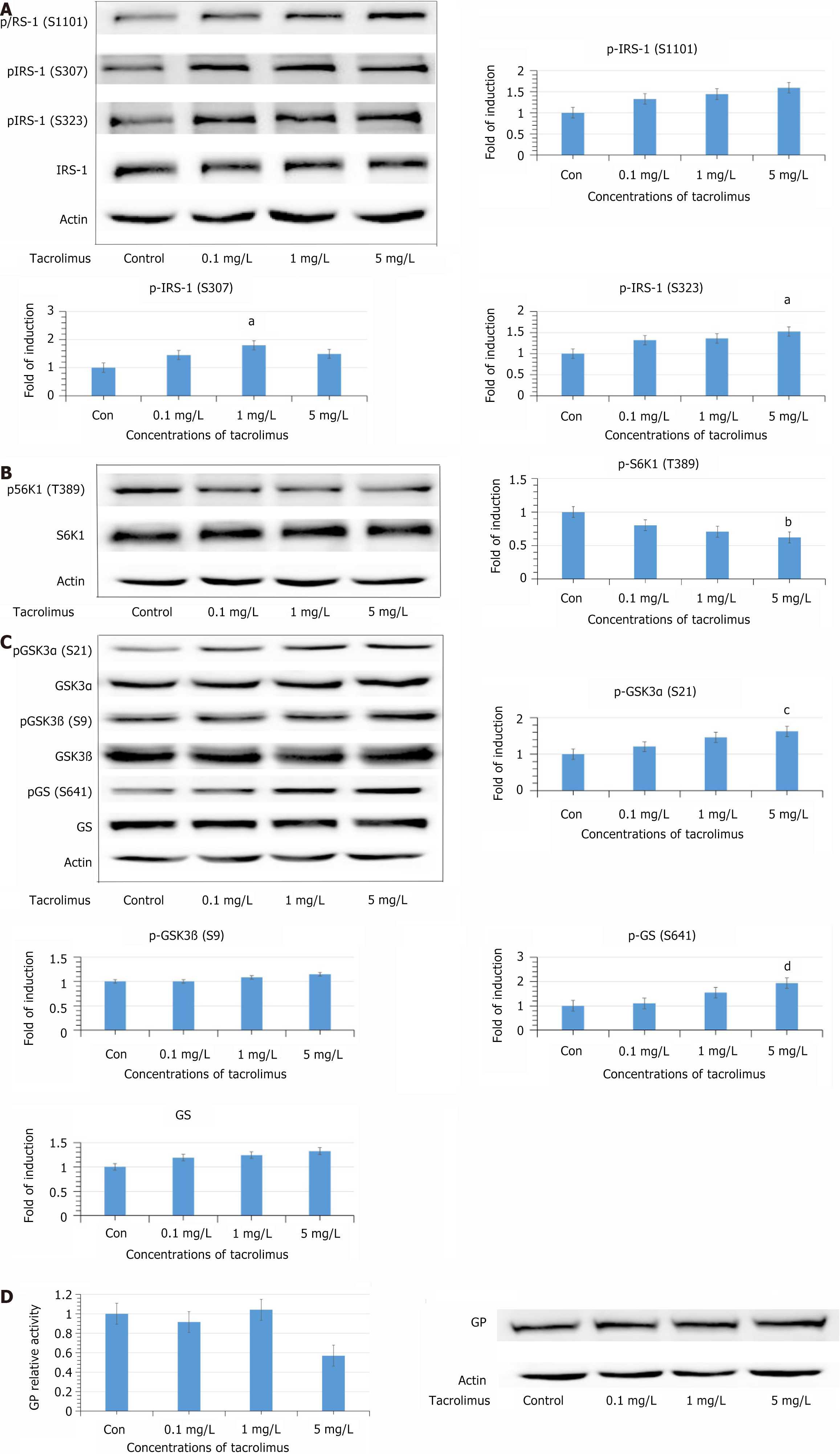
Figure 1 After exposure to tacrolimus for 24 hours.
A: Phosphorylation of insulin receptor substrate (IRS) 1 at Ser 307 and Ser 323 was significantly increased at concentrations of 1 and 5 mg/L (aP < 0.05). Phosphorylation of IRS1 at Ser 1101 was also increased, although not significantly; B: Phosphorylation of S6K1 at Thr 389 was decreased in a concentration-dependent manner (bP < 0.05); C: Phosphorylation of glycogen synthase kinase (GSK) 3α at Ser 21 was increased (cP < 0.05). Phosphorylation of GSK3βat Ser 9 was also increased, but not significantly. Unexpectedly, the phosphorylation of glycogen synthase at Ser 641 was increased (dP < 0.05); D: Measurement of glycogen phosphorylase (GP) activity to ascertain the effects of tacrolimus on glycogen synthesis. After exposure to tacrolimus for 24 hours, there was no significant change in total expression of GP. IRS: Insulin receptor substrate; GSK: Glycogen synthase kinase; GP: Glycogen phosphorylase.
- Citation: Li HY, Wang Y, Ran M, Gao F, Zhu BY, Xiao HY, Xu C. Tacrolimus induces insulin receptor substrate 1 hyperphosphorylation and inhibits mTORc1/S6K1 cascade in HL7702 cells. World J Diabetes 2025; 16(2): 97910
- URL: https://www.wjgnet.com/1948-9358/full/v16/i2/97910.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i2.97910