©The Author(s) 2025.
World J Diabetes. Oct 15, 2025; 16(10): 109815
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.109815
Published online Oct 15, 2025. doi: 10.4239/wjd.v16.i10.109815
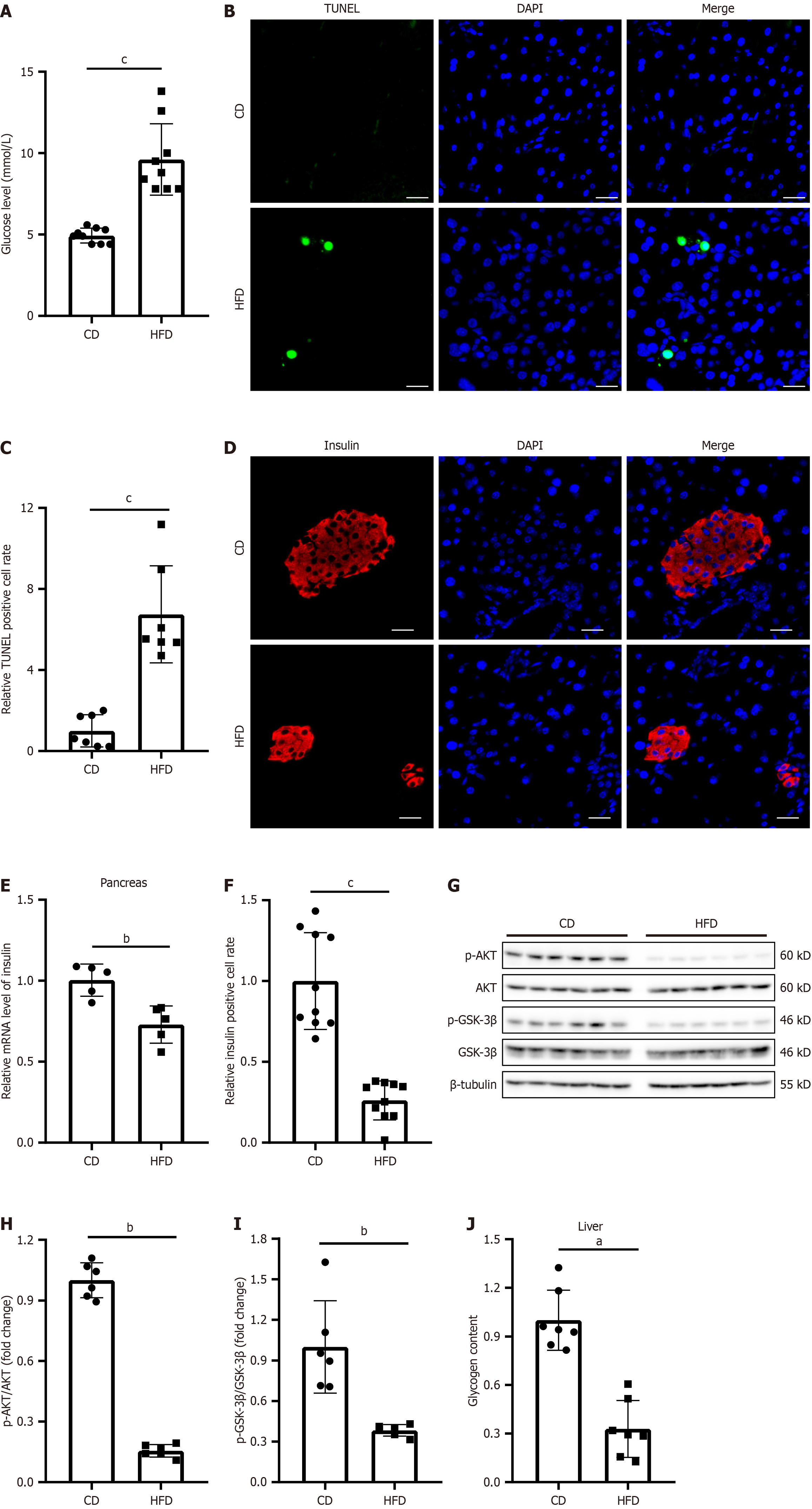
Figure 1 High-fat diet feeding induces pancreas and liver dysfunction in mice.
The mice were fed a chow diet or high-fat diet for 18 weeks. A: Fasting blood glucose levels; B: TUNEL staining for apoptotic cells in the pancreas; C: Quantification of TUNEL-positive cells via ImageJ software; D: Immunofluorescence staining for insulin in the pancreas; E: Insulin mRNA levels in the pancreas; F: Quantification of insulin-positive areas using ImageJ software; G: Western blot analysis of AKT/GSK signaling in the liver; H and I: Densitometric quantification of relative protein levels normalized to β-actin performed using ImageJ; J: Liver glycogen content. The experiments were repeated three times. aP < 0.05, bP < 0.01, cP < 0.001. Scale bar: 20 μm. Western blot raw data in Supplementary material.
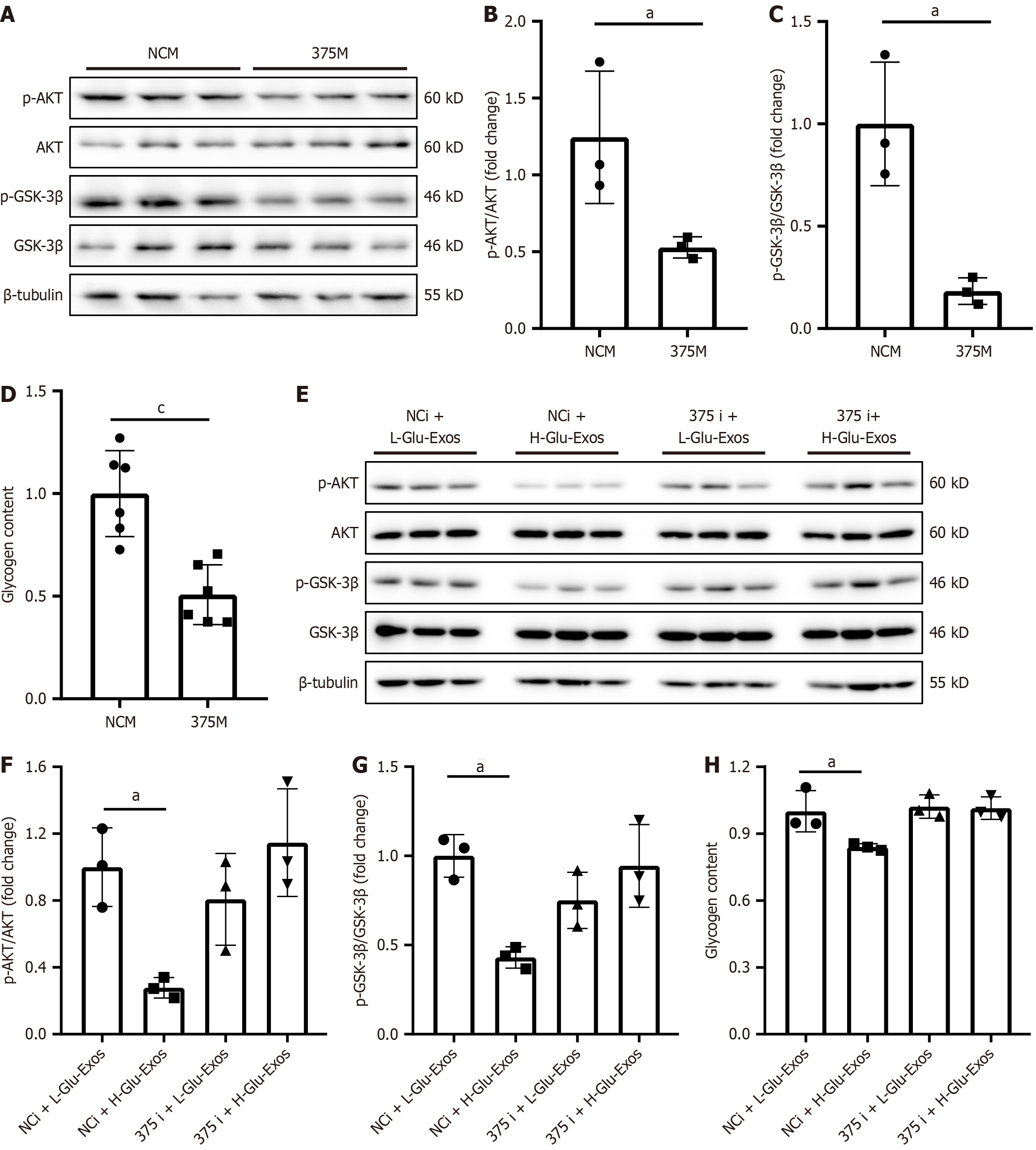
Figure 2 Supernatants of glucotoxic pancreatic cells impair glycogenesis of hepatocytes.
MIN-6 cells were treated with low (5.5 mmol/L) or high (33.5 mmol/L) glucose. A: Cell viability was detected by MTT assay; B and C: TUNEL staining and quantification of apoptotic MIN-6 cells; D: Insulin mRNA expression levels in MIN-6 cells. Hep1-6 cells were treated with supernatant from MIN-6 cells (MIN6-Sup); E-G: Western blot analysis of AKT and GSK-3β phosphorylation levels; H: Detection of the glycogen content. Hep1-6 cells were treated with conditioned media from primary islet cells (IPC-Sup) of high-fat diet-fed or chow diet-fed mice; I-K: Western blot analysis of AKT and GSK-3β phosphorylation levels; L: Detection of the glycogen content. MIN-6 and Hep1-6 cells were cocultured using Transwell plates; M-O: Western blot analysis of AKT and GSK-3β phosphorylation levels; P: Detection of the glycogen content. The experiments were repeated three times. aP < 0.05, bP < 0.01, cP < 0.001. Western blot raw data in Supplementary material.
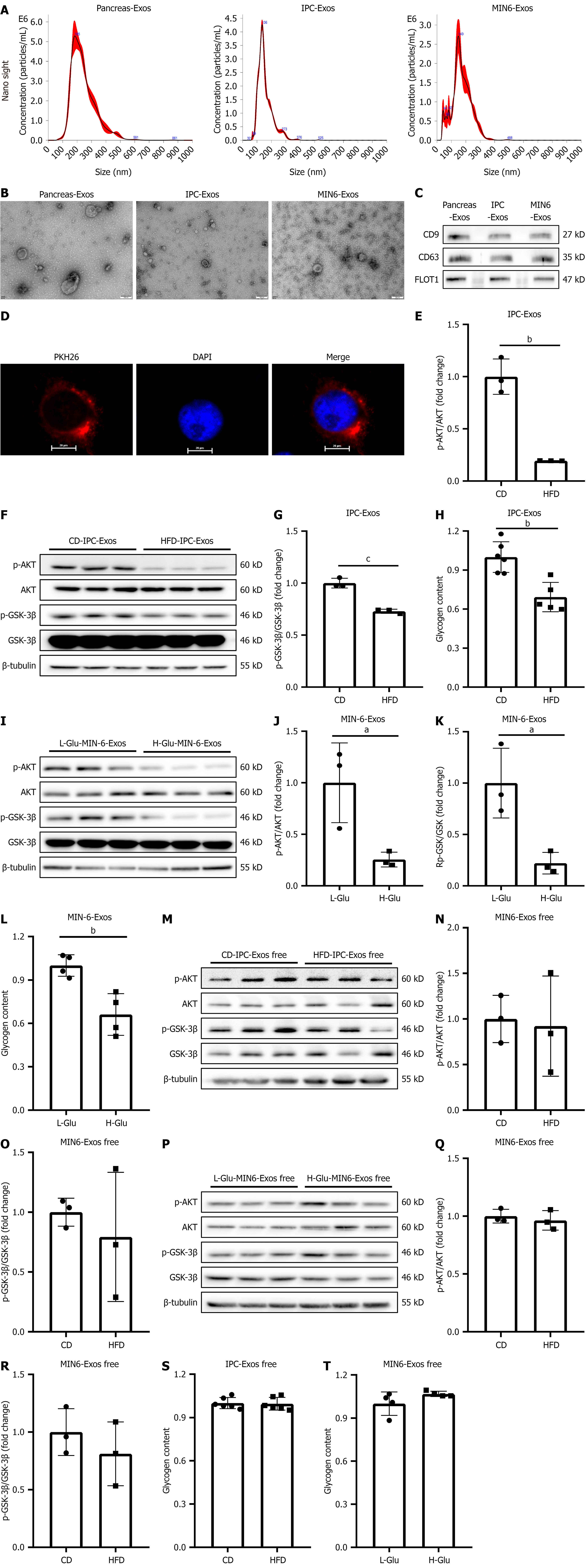
Figure 3 Exosomes secreted by glucotoxic pancreatic cells reduce hepatocyte glycogenesis.
A: Exosome identification by NanoSight particle size analysis; B: Exosome morphology visualized by transmission electron microscopy; C: Western blot analysis of exosomal markers (CD63, CD9, and FLOT1); D: Tracking of pancreatic cell-derived exosomes labeled with PKH26 dye in Hep1-6 cells; E-L: Western blot analysis of AKT/GSK signaling (E-G, I-K) and glycogen content (H and L) in Hep1-6 cells treated with exosomes from High-fat diet (HFD) mouse islet primary cells (IPC-Exos) and MIN-6 cells (MIN-6 Exos); M-T: Western blot analysis of AKT/GSK signaling (M-O, P-R) and glycogen content (S and T) in Hep1-6 cells treated with exosomes-free conditioned media from HFD mouse islet primary cells (IPC-Exos free) and MIN-6 cells (MIN-6 Exos free). The experiments were repeated three times. Western blot raw data in Supplementary material. aP < 0.05, bP < 0.01, cP < 0.001. CD63: Cluster of differentiation 63; CD9: Cluster of differentiation 9; FLOT-1: Flotillin.
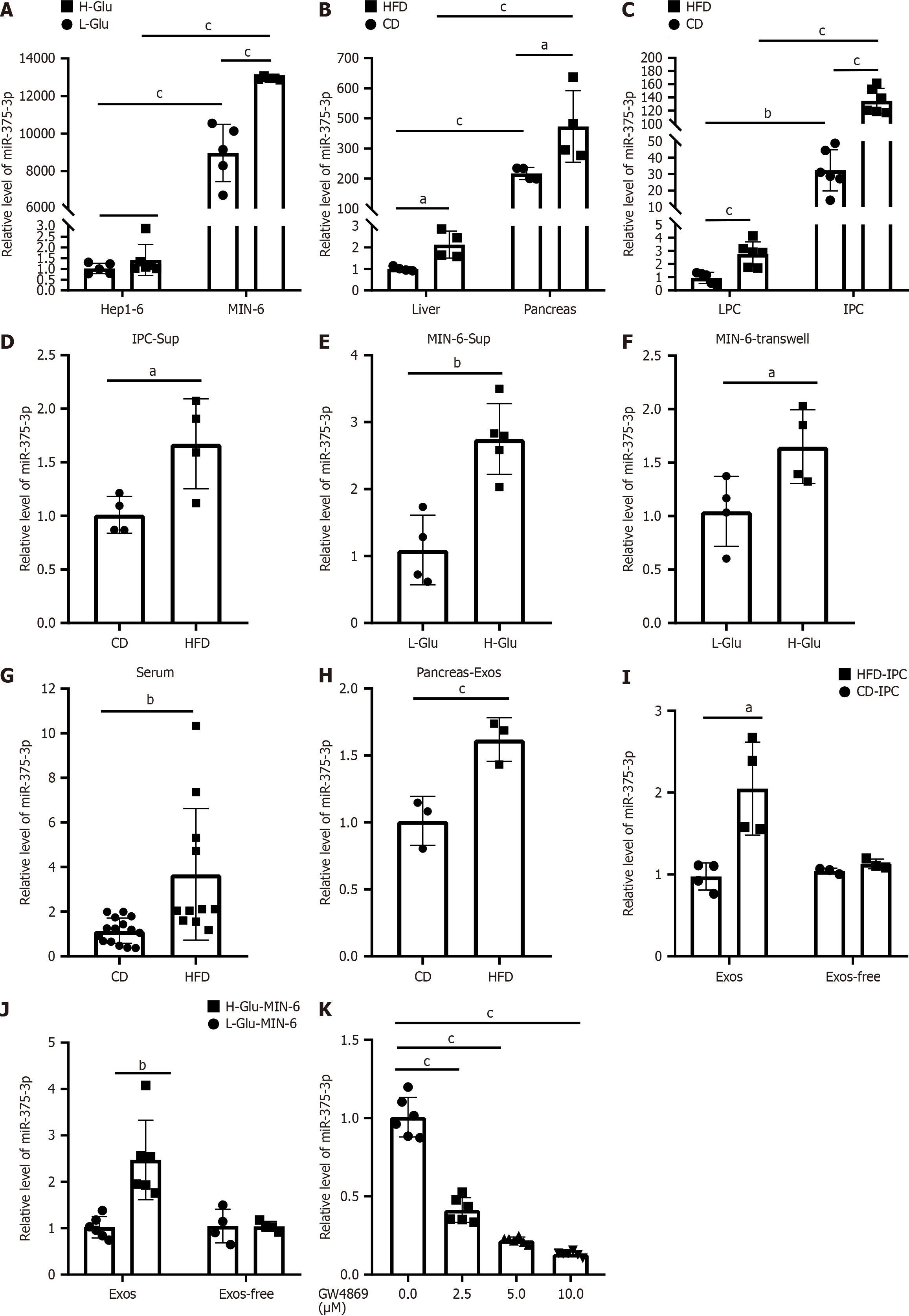
Figure 4 miR-375-3p is transferred from glucotoxic pancreatic cells to hepatocytes via exosomes.
Relative miR-375-3p levels were quantified by quantitative reverse transcription polymerase chain reaction. A: Hep1-6 and MIN-6 cells after low- or high-glucose stimulation; B: Pancreatic and liver tissues of high-fat diet (HFD)-fed or chow diet (CD)-fed mice; C: Primary islet cells (IPCs) and LPCs from HFD-fed or CD-fed mice; D: Hep1-6 cells treated with conditioned media from IPCs (IPC-Sup); E: Hep1-6 cells treated with conditioned media from MIN-6 cells (MIN6-Sup); F: Hep1-6 cells cocultured with MIN-6 cells using Transwell plates (MIN6-Transwell); G: Plasma of HFD-fed or CD-fed mice; H-J: Exosomes or exosome-depleted supernatantsisolated from pancreatic tissues (pancreas-Exos), IPCs (IPC-Exos), and MIN-6 cells (MIN-6 Exos); K: Hep1-6 cells treatment with conditioned media from GW4869-treated MIN-6 cells. The experiments were repeated three times. aP < 0.05, bP < 0.01, cP < 0.001.
Figure 5 miR-375-3p inhibition abrogates the influence of glucotoxic pancreatic cell-derived exosomes on hepatocytes.
Hep1-6 cells were transfected with miR-375-3p mimics or control mimics for 48 hours using RNAi Max. A-C: Western blot analysis of AKT/GSK signaling and quantification of phosphorylated proteins; D: Glycogen content analysis in Hep1-6 cells. Hep1-6 cells were transfected with a miR-375-3p inhibitor or control inhibitor for 48 hours and treated with exosomes from low- or high-glucose-stimulated MIN-6 cells for 24 hours; E and F: Western blot analysis of AKT/GSK signaling and Rbpj protein levels; G: Densitometric quantification of the relative protein levels normalized to β-actin performed using ImageJ; H: Glycogen content analysis in Hep1-6 cells. The experiments were repeated three times. aP < 0.05, cP < 0.001. Western blot raw data in Supplementary material.
Figure 6 miR-375-3p regulates hepatocyte glycogenesis via Rbpj.
A: Schematic diagrams illustrating the miR-375-3p binding site in the Rbpj 3'-untranslated region (UTR) predicted by TargetScan. Hep1-6 cells were transfected with a miR-375-3p inhibitor or control inhibitor for 48 hours; B-E: Western blotting of AKT/GSK signaling and protein level of Rbpj; F: Detection of glycogen content. Hep1-6 cells were cotransfected with miR-375-3p mimics and Rbpj expression plasmid or empty vector for 48 hours; G-J: Western blotting of AKT/GSK signaling and the protein level of Rbpj; K: Detection of the glycogen content; L-N: Quantification of Rbpj protein (L and M) and mRNA (N) levels in Hep1-6 cells transfected with miR-375-3p mimics or control mimics; O-Q: Protein and RNA levels of Rbpj in the livers of high-fat diet-fed mice and chow diet-fed mice; R-U: Protein and RNA levels of Rbpj in Hep1-6 cells treated with exosomes secreted from MIN-6 cells after low- or high-glucose stimulation (R and S) or with miR-375-3p inhibitor (T and U); V: Schematic diagrams of the mutated miR-375-3p binding site in the Rbpj 3'-UTR; W and X: Hep1-6 cells were cotransfected with miR-375-3p mimics and pmirGLO-Rbpj-WT/MUT plasmids, followed by luciferase reporter assay. The experiments were repeated three times. aP < 0.05, bP < 0.01, cP < 0.001. Western blot raw data in Supplementary material.
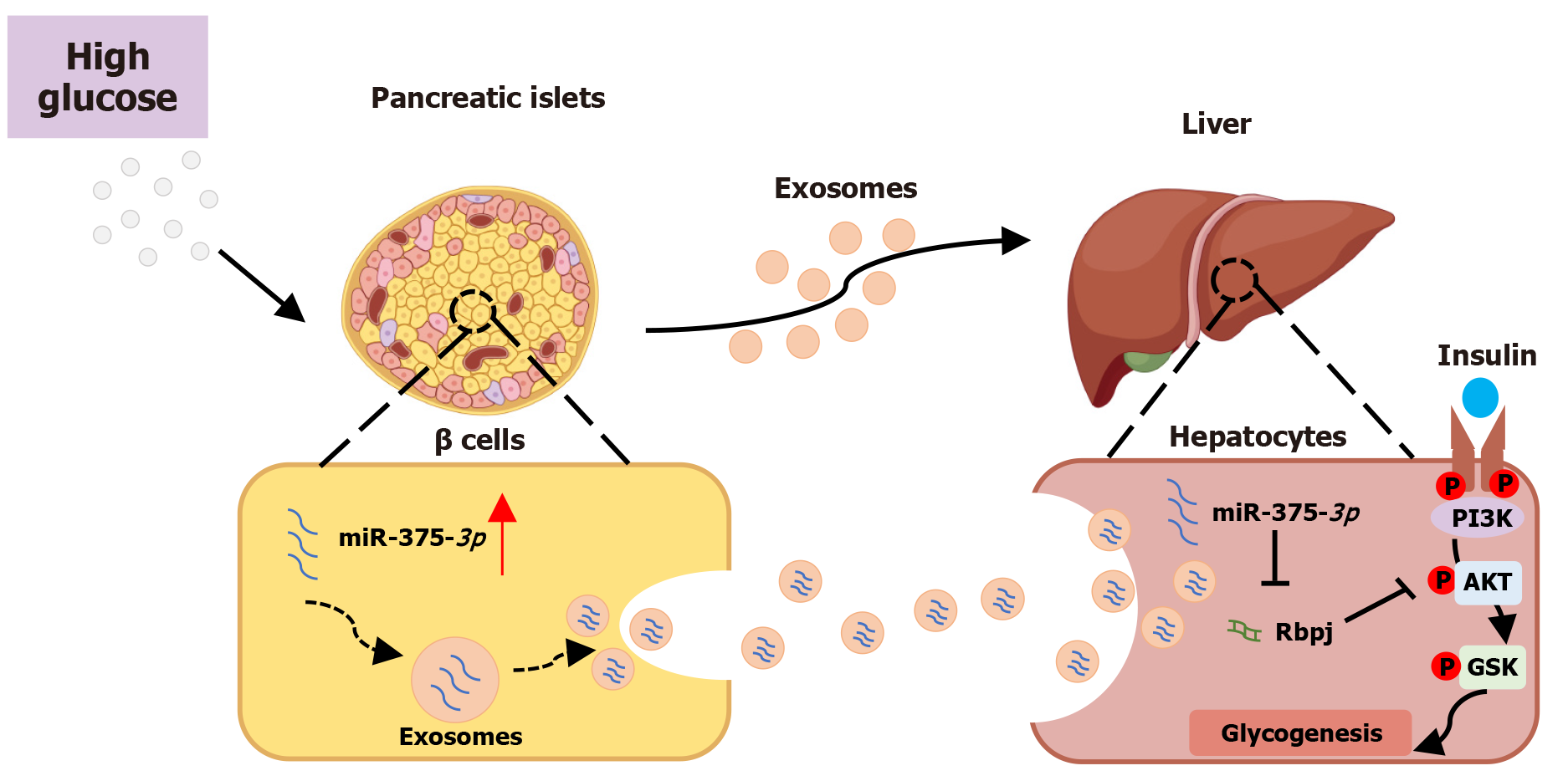
Figure 7 Schematic diagram of the mechanism underlying pancreatic-cell-derived miR-375-3p inducing impairment of hepatocyte glycogenesis.
Glucotoxicity specifically upregulates miR-375-3p in pancreatic cells but not in hepatocytes. Pancreatic cell-derived miR-375-3p is delivered to hepatocytes via exosomes, leading to reduced glycogenesis. miR-375-3p inhibits the AKT/GSK signaling pathway by targeting Rbpj, thereby impairing hepatocyte glycogenesis. Figure created by Figdraw.
- Citation: Xu FZ, Dou L, Wu X, Xia CX, Yu DN, Man Y, Shen T, Huang XQ. Exosomal transfer of miR-375-3p from pancreatic β cells to hepatocytes impairs hepatic glycogenesis via Rbpj repression. World J Diabetes 2025; 16(10): 109815
- URL: https://www.wjgnet.com/1948-9358/full/v16/i10/109815.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i10.109815