©The Author(s) 2023.
World J Diabetes. Mar 15, 2023; 14(3): 234-254
Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.234
Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.234
Figure 1 Experimental design.
Tongxinluo (TXL) improved hindlimb appearance and restored blood flow in mice. A: Hindlimb vasculature; B: Ligation site; C: Timeline of the experimental operations; D: The appearance of ischemic hindlimbs in mice 4 wk after surgery; E: The limb appearance scores of mice, assessed weekly after surgery; F: Laser Doppler perfusion imaging was performed before and after surgery and on postoperative Days 7, 14, 21, and 28; G: Quantification of tissue perfusion in the ischemic limb (red box) is expressed as a percentage of perfusion in the nonischemic limb. The data are presented as the means ± SDs. aP < 0.05 compared with the model group, bP < 0.05 compared with the TXL-L group, n = 4-8. TXL: Tongxinluo.
Figure 2 Tongxinluo promoted arteriogenesis in the ischemic hindlimb.
A: Representative whole-mount images of Microfil vascular casting of mouse limbs; B and C: Collateral vessels are indicated by white arrows and were quantified by micro- computed tomography analysis to determine the vessel volume and surface area; D: Immunofluorescence staining for CD31 (red) and α-SMA (green) to evaluate the capillary density and arterioles in the ischemic adductor (200×, bar=100 μm); E-F: Quantification of the mean fluorescence density of CD31 and α-SMA. All the values are shown as the means ± SDs. aP < 0.05 compared with the normal group, bP <0.05 compared with the model group, cP < 0.05 compared with the Tongxinluo group, n = 3-6. TXL: Tongxinluo.
Figure 3 Tongxinluo inhibited endothelial cell pyroptosis.
A: Immunofluorescence staining for assessment of reactive oxygen species (ROS) levels in ischemic hindlimbs (400×, bar = 50 μm); B: Quantification of the mean fluorescence intensity of ROS; C-G: Western blot analysis of the expression of the pyroptosis-associated proteins NLRP3, GSDMD, Caspase-1 and Cleaved caspase-1 in the ischemic adductor; H and I: The pyroptosis-associated inflammatory cytokines IL-18 and IL-1β were analyzed by ELISA. The values are shown as the means ± SDs. aP < 0.05 compared with the normal group, bP < 0.05 compared with the model group, cP < 0.05 compared with the Tongxinluo-L group, n = 3-4. ROS: Reactive oxygen species; TXL: Tongxinluo.
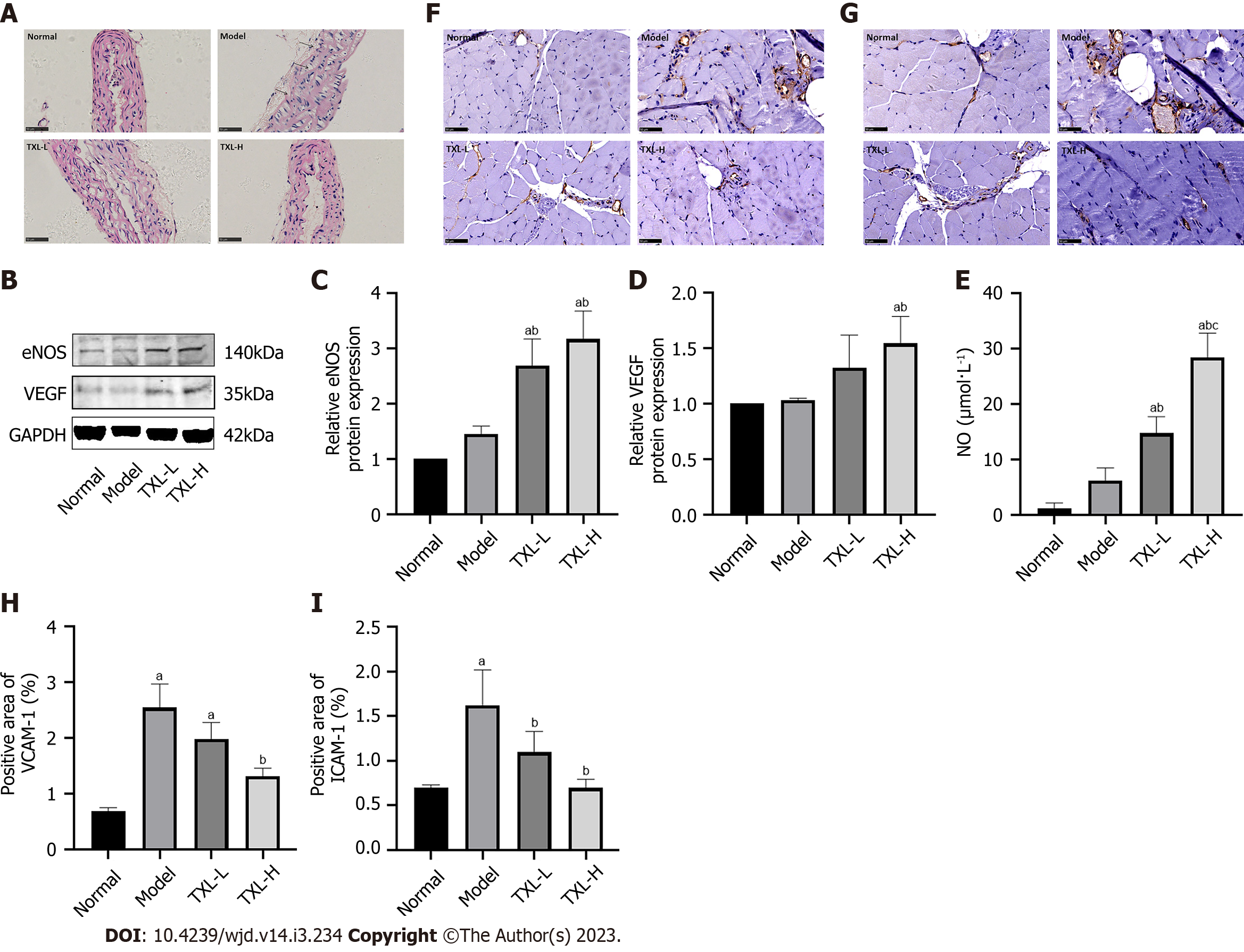
Figure 4 Tongxinluo restored endothelial cell structure and regulated endothelial nitric oxide synthase, vascular endothelial growth factor, nitric oxide, intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 expression.
A: Representative images showing H&E staining of the aortas of mice (400×, bar = 50 μm); B-D: Western blot analysis of the expression of endothelial cell function-related proteins eNOS and VEGF in mice; E: Nitric oxide (NO) kit to detect NO levels in mouse serum; F and G: Immunochemical staining of intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in the ischemic adductor (400×, bar = 50 μm); H and I: Quantification of the percentage areas of ICAM-1 and VCAM-1. The values are shown as the means ± SDs. aP < 0.05 compared with the normal group, bP <0.05 compared with the model group, cP < 0.05 compared with the TXL-L group, n = 3-4. TXL: Tongxinluo; eNOS: Endothelial nitric oxide synthase; VEGF: Vascular endothelial growth factor; ICAM-1: intercellular cell adhesion molecule-1; VCAM-1: Vascular cell adhesion molecule-1; NO: Nitric oxide.
Figure 5 Tongxinluo enhanced the barrier function of endothelial cells to inhibit the inflammatory response and promote macrophage polarization.
A-E: The barrier-related proteins VE-cadherin and β-catenin and the cytokines CD45 and TNF-α were measured and quantified by Western blot analysis; F-G: Measurement of the cytokine levels of IL-6 and C-reactive protein by ELISA; J and K: Immunofluorescence staining of total macrophages (F4/80, green) with M1-type macrophage markers [inducible nitric oxide synthase (iNOS), red] and M2-type macrophage markers (Arg-1, red) (100×, bar=250 μm); H and I: Quantification of the Pearson colocalization coefficients of F4/80 with iNOS and Arg-1. The values are shown as the means ± SDs. aP < 0.05 compared with the normal group, bP <0.05 compared with the model group, cP < 0.05 compared with the TXL-L group, n = 3. TXL: Tongxinluo; CRP: C-reactive protein; iNOS: Inducible nitric oxide synthase.
Figure 6 Tongxinluo strengthened the crosstalk between endothelial cells and smooth muscle cells to induce smooth muscle cell proliferation and migration.
A-D: Western blot analysis of the expression of Jagged-1, Notch-1 and ephrin-B2, which are related to the signaling pathway that mediates endothelial-smooth muscle cells interactions; E: Quantitative analysis of PCNA mRNA expression was determined via RT-PCR; F-H: Western blot analysis of the expression of the cell proliferation-associated proteins matrix metalloproteinase 9 (MMP9) and MMP2 in the ischemic adductor. The values are shown as the means ± SDs. aP < 0.05 compared with the normal group, bP <0.05 compared with the model group, cP < 0.05 compared with the Tongxinluo group, n = 3. TXL: Tongxinluo; MMP9: Matrix metalloproteinase 9.
Figure 7 Tongxinluo promoted human vascular endothelial cell (HUVEC) proliferation, restored HUVEC function, and inhibited pyroptosis.
A: Human vascular endothelial cell (HUVEC) morphology; B: Cell counting Kit-8 assay for HUVEC proliferation rate; C: NO kit to detect NO levels in in HUVEC supernatants; D-G: The expression of the endothelial-associated proteins endothelial nitric oxide synthase, vascular endothelial growth factor, VE-cadherin and β-catenin in the HUVEC supernatants was analyzed by RT-PCR; H: HUVECs for tubule formation experiments; I: Flow cytometry detection of cell pyroptosis; J: Statistical analysis of the percentage of caspase-1 and PI double-stained positive cells in the total cells. The values are shown as the means ± SDs. aP < 0.05 compared with the normal group, bP <0.05 compared with the model group, cP < 0.05 compared with the TXL-L group, n = 3. TXL: Tongxinluo; VEGF: Vascular endothelial growth factor; eNOS: Endothelial nitric oxide synthase.
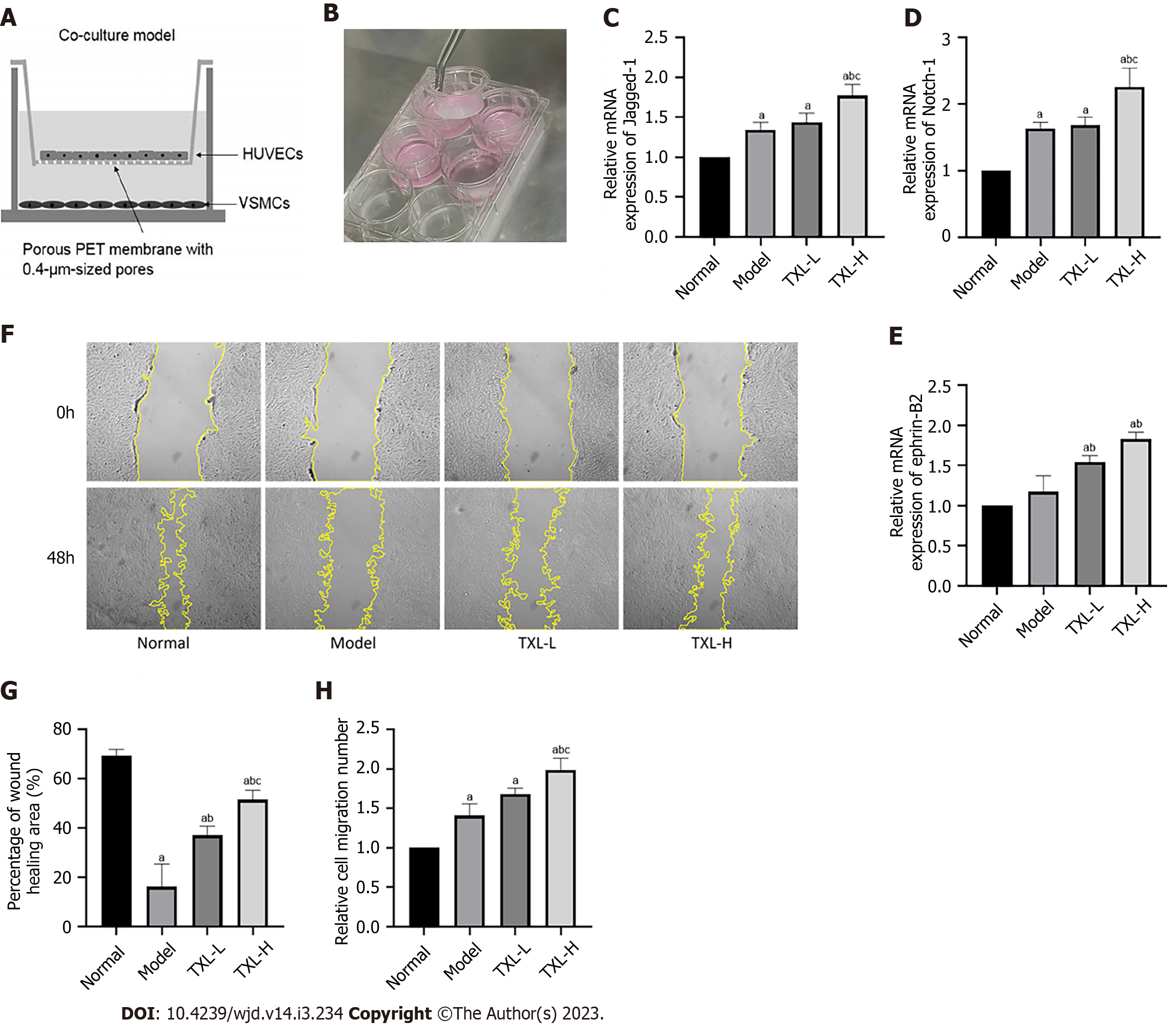
Figure 8 Tongxinluo enhanced the interaction between human vascular endothelial cells and vascular smooth muscle cells (VSMCs) and promoted the migration of VSMCs.
A and B: Schematic diagram and photos of the coculture system; C-E: Quantitative analysis of Jagged-1, Ntoch-1 and ephrin-B2 mRNA expression was determined via RT-PCR; F: Scratch assay to detect VSMC migration ability. G: Percentage of the wound healing area was measured using ImageJ software; H: Transwell assays were performed to detect VSMC migration ability and the relative cell migration number was calculated by ImageJ software. The values are shown as the means ± SDs. aP < 0.05 compared with the normal group, bP <0.05 compared with the model group, cP < 0.05 compared with the TXL-L group, n = 3. TXL: Tongxinluo; HUVECs: Human vascular endothelial cells; VSMCs: Vascular smooth muscle cells.
- Citation: Gu JJ, Hou YL, Yan YH, Li J, Wei YR, Ma K, Wang XQ, Zhang JH, Wang DD, Li CR, Li DQ, Sun LL, Gao HL. Tongxinluo promotes endothelium-dependent arteriogenesis to attenuate diabetic peripheral arterial disease. World J Diabetes 2023; 14(3): 234-254
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/234.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.234