©The Author(s) 2023.
World J Diabetes. Oct 15, 2023; 14(10): 1573-1584
Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1573
Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1573
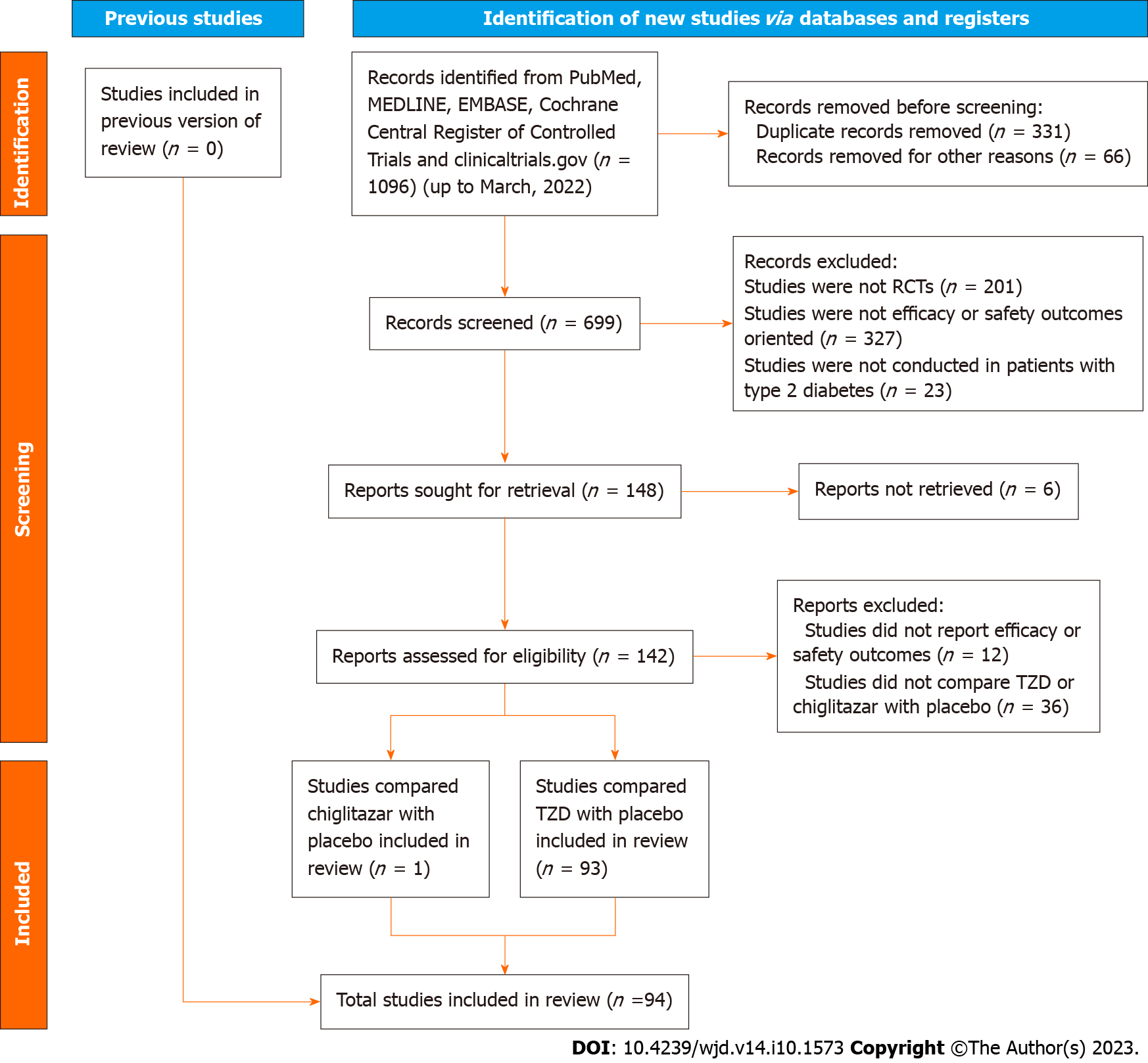
Figure 1 Flow chart of this indirect meta-analysis.
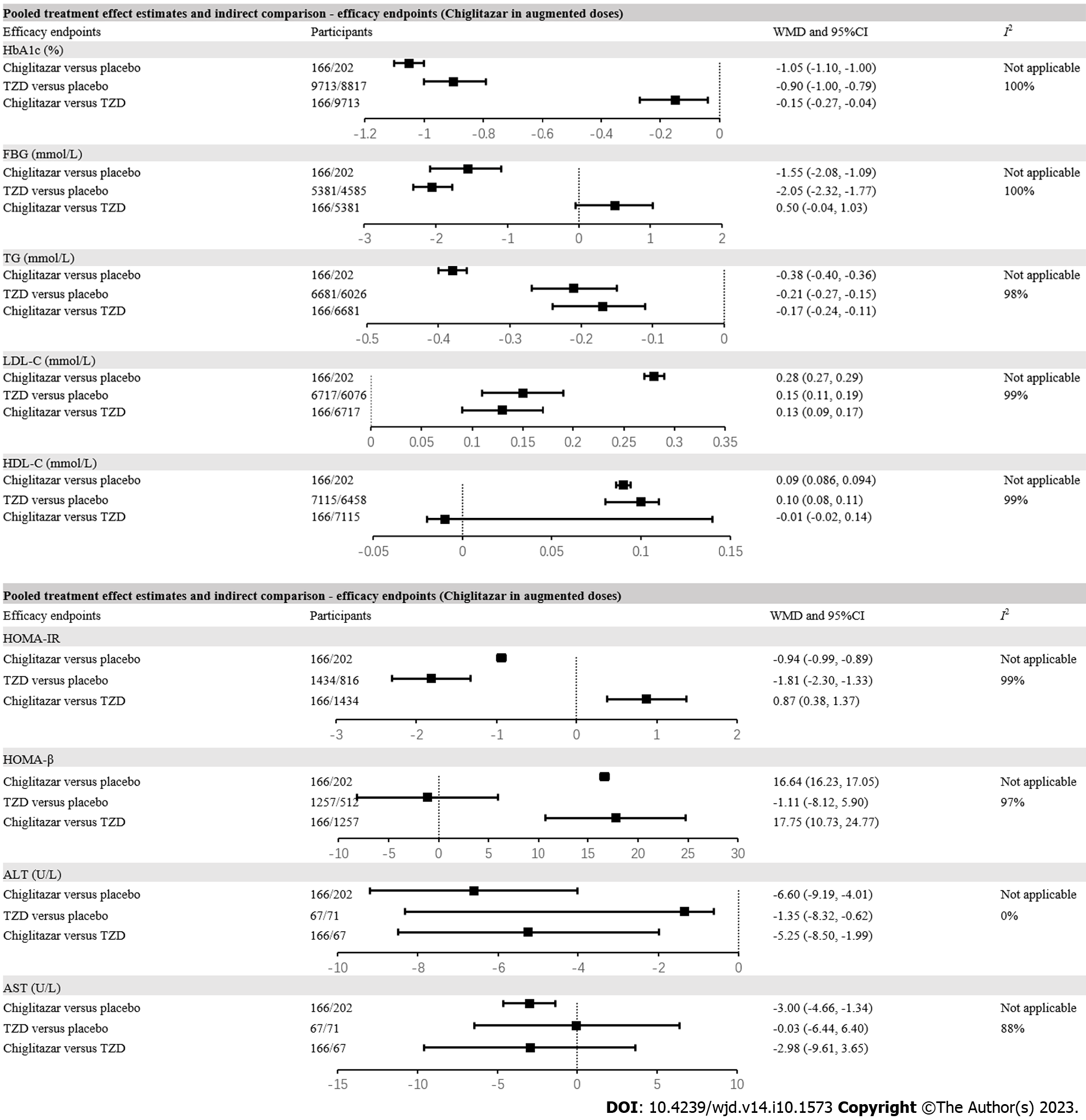
Figure 2 The forest plot exhibiting pooled effect estimates and indirect comparison between chiglitazar and thiazolidinediones on efficacy endpoints including hemoglobin A1c, fasting blood glucose, triglycerides, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, homeostasis model assessment of insulin resistance, homeostasis model assessment of β cell function, alanine aminotransferase and aspartate aminotransferase.
HbA1c: Hemoglobin A1c; FBG: Fasting blood glucose; TG: Triglycerides; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; HOMA-IR: Homeostasis model assessment of insulin resistance; HOMA-β: Homeostasis model assessment of β cell function; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; RR: Risk ratios; 95%CI: 95% confidential intervals; TZD: Thiazolidinedione.
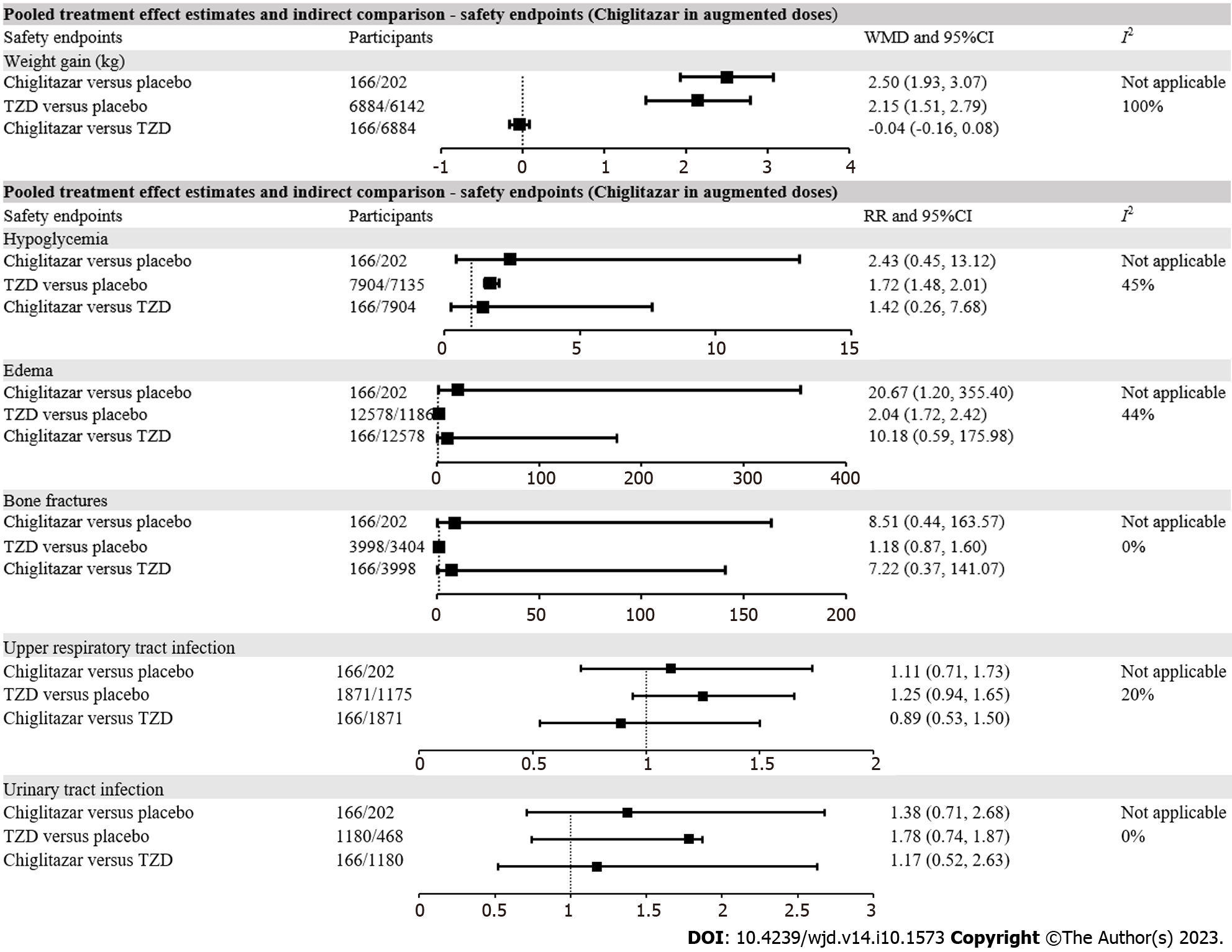
Figure 3 The forest plot exhibiting pooled effect estimates and indirect comparison between chiglitazar and thiazolidinediones on safety endpoints including weight gain, hypoglycemia, edema, bone fractures, upper respiratory tract infection and urinary tract infection.
HbA1c: Hemoglobin A1c; FBG: Fasting blood glucose; TG: Triglycerides; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; HOMA-IR: Homeostasis model assessment of insulin resistance; HOMA-β: Homeostasis model assessment of β cell function; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; RR: Risk ratios; 95%CI: 95% confidential intervals; TZD: Thiazolidinedione.
- Citation: Lin C, Li ZL, Cai XL, Hu SY, Lv F, Yang WJ, Ji LN. Indirect comparison of efficacy and safety of chiglitazar and thiazolidinedione in patients with type 2 diabetes: A meta-analysis. World J Diabetes 2023; 14(10): 1573-1584
- URL: https://www.wjgnet.com/1948-9358/full/v14/i10/1573.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i10.1573