©The Author(s) 2023.
World J Diabetes. Oct 15, 2023; 14(10): 1478-1492
Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1478
Published online Oct 15, 2023. doi: 10.4239/wjd.v14.i10.1478
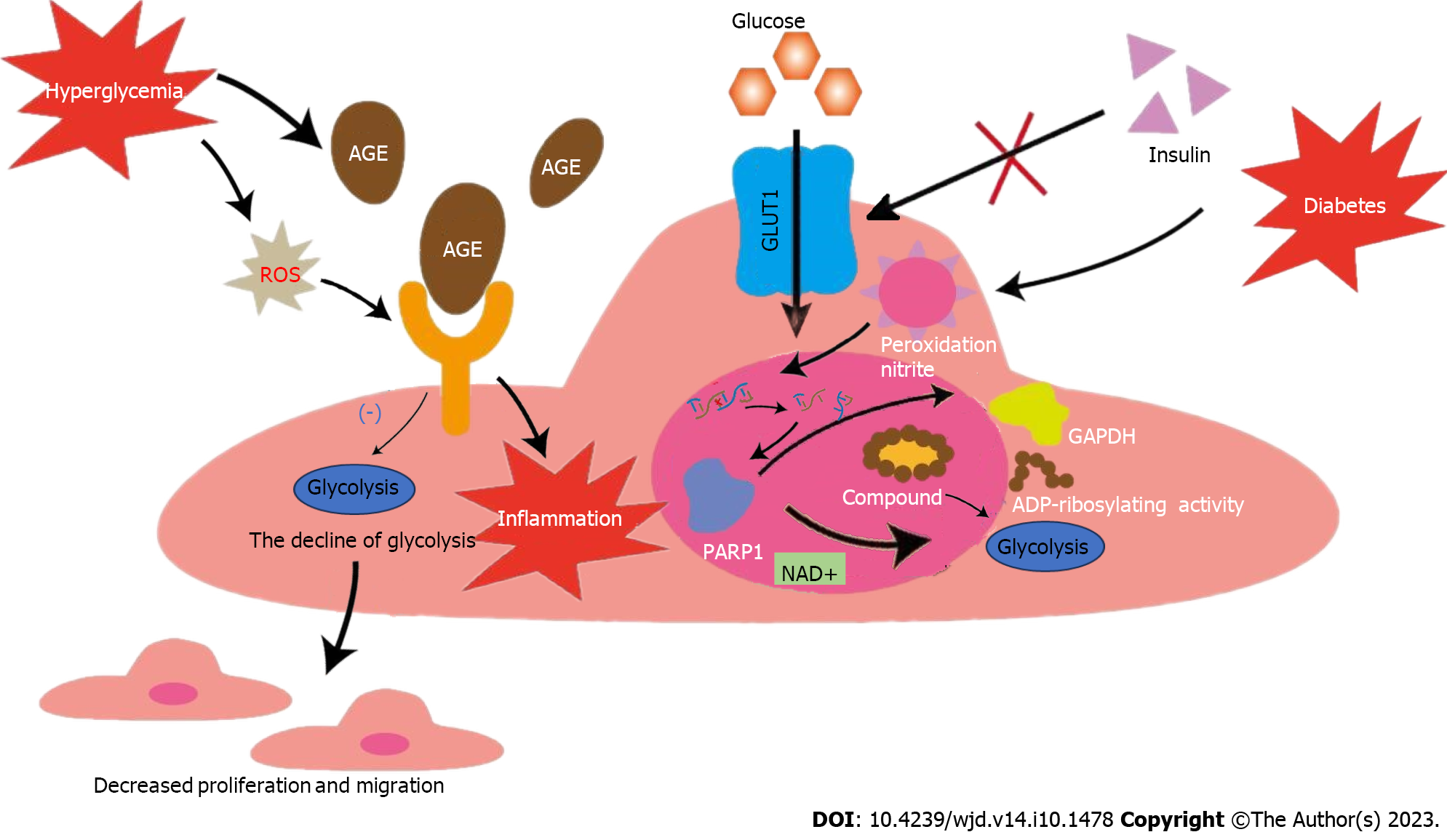
Figure 1 Effects of glycolysis on endothelial cells in hyperglycemic environment.
The activity of glucose transporter 1 is regulated by extracellular glucose concentration, independent of insulin, making endothelial cells in patients with diabetes more vulnerable. Nitrosation stress caused by diabetes can damage DNA and activate poly polymerase 1 (PARP1). PARP1 can not only promote the consumption of NAD+, but also reduce the activity of GAPDH after adenosine diphosphate ribosylation. Both pathways inhibit glycolysis and lead to dysfunction of ECs. Diabetes can promote the accumulation of advanced glycosylation end-products (AGEs), and diabetes-induced reactive oxygen species can increase the expression of receptor for AGE and pro-inflammatory endogenous ligands. The above process leads to the down-regulation of ECs glycolysis and the intensification of inflammation, causing decreased migration and proliferation of ECs. GLUT1: Glucose transporter 1; EC: Endothelial cell; AGE: Advanced glycosylation end-products; ROS: Reactive oxygen species; PARP-1: Poly polymerase 1; ADP: Adenosine diphosphate; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
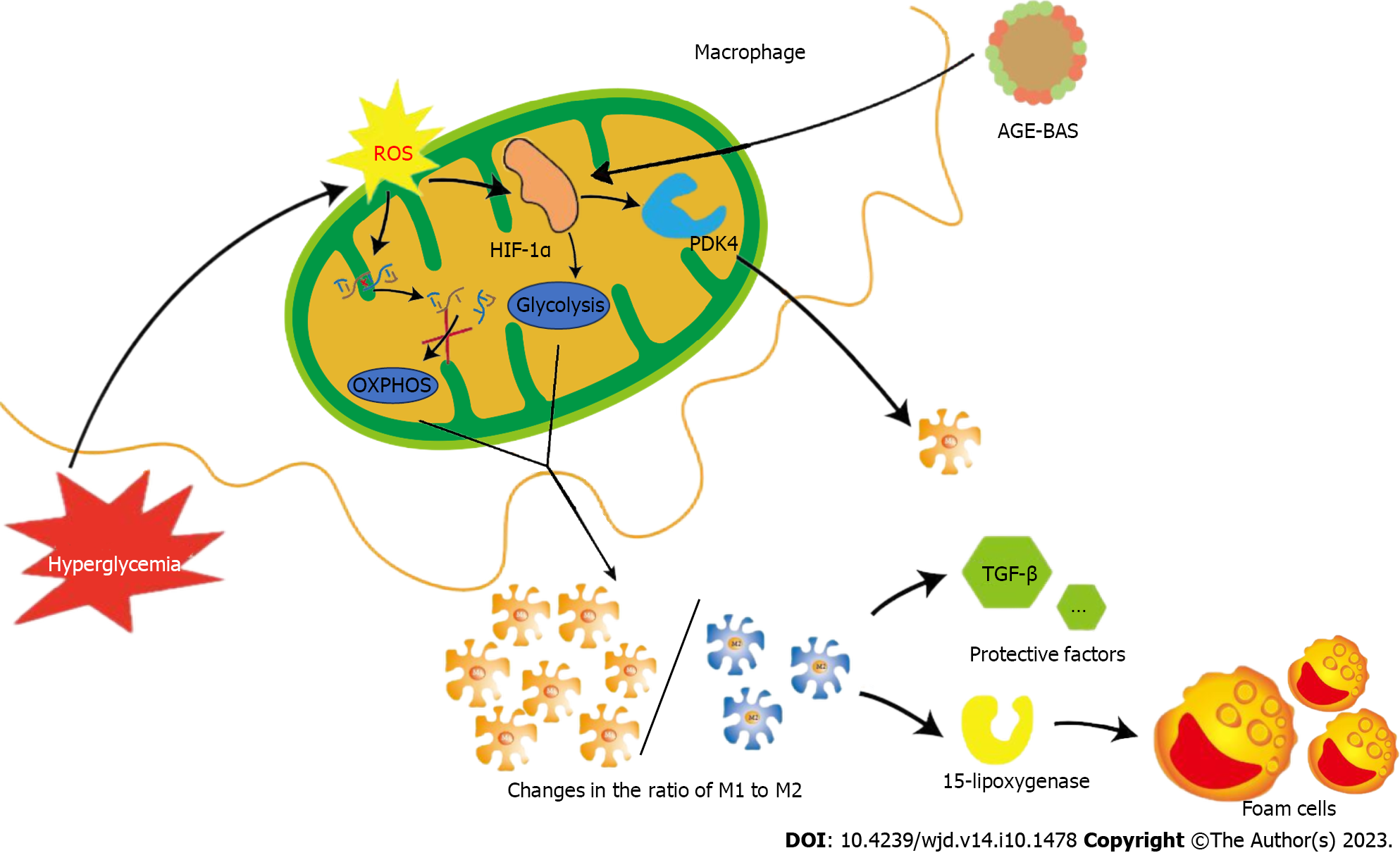
Figure 2 Effects of glycolysis on macrophages in hyperglycemic environment.
Reactive oxygen species (ROS) induced by hyperglycemia can damage mitochondrial DNA and inhibit oxidative phosphorylation. Simultaneously, ROS can induce the expression of hypoxia-inducible factor 1α and promote glycolysis. Under the above two conditions, the ratio of M1 and M2 changes. M2 has the ability to secrete 15-lipoxygenase to promote foam cell formation and atherosclerotic protective factors such as transforming growth factor-beta, which plays an important role in maintaining plaque stability. The increase of M1 and the decrease of M2 ultimately aggravate the instability of diabetic atherosclerotic plaques. OXPHOS: Oxidative phosphorylation; AGE: Advanced glycosylation end-products; BAS: Bovine serum albumin; TGF-β: Transforming growth factor-beta; HIF-1α: Hypoxia-inducible factor 1α.
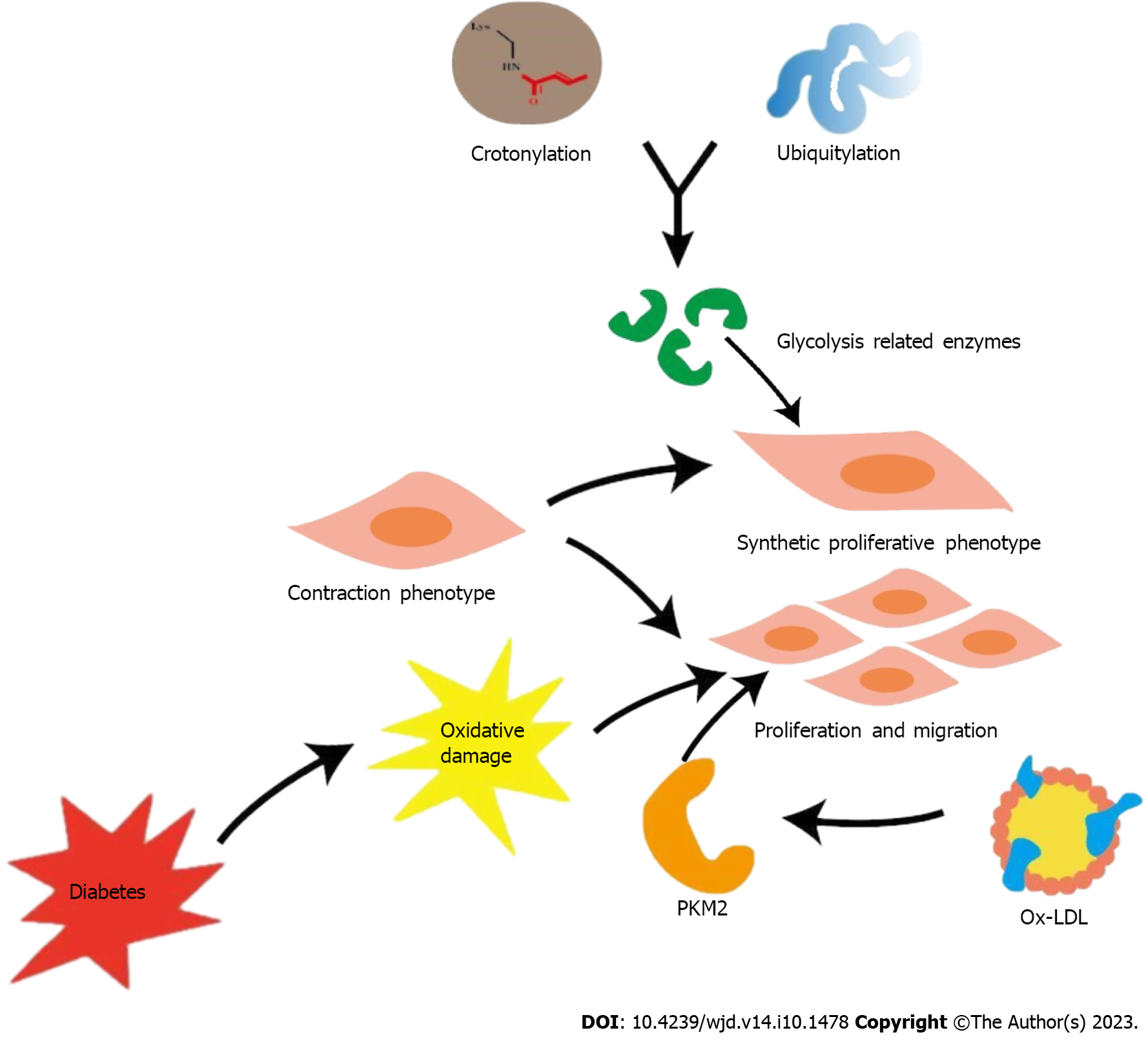
Figure 3 Effects of glycolysis on vascular smooth muscle cells in hyperglycemic environment.
The crosstalk between crotonylation and ubiquitination in glycolysis related enzymes may be a potential mechanism underlying the phenotypic remodeling of vascular smooth muscle cells (VSMCs). Ox-low-density lipoprotein promotes proliferation and migration of VSMC by up-regulating PKM2-related glycolysis. Oxidative damage in the context of diabetes plays an important role in these processes, causing the development of diabetic atherosclerosis. PKM: Pyruvate kinase subtype; LDL: Low-density lipoprotein.
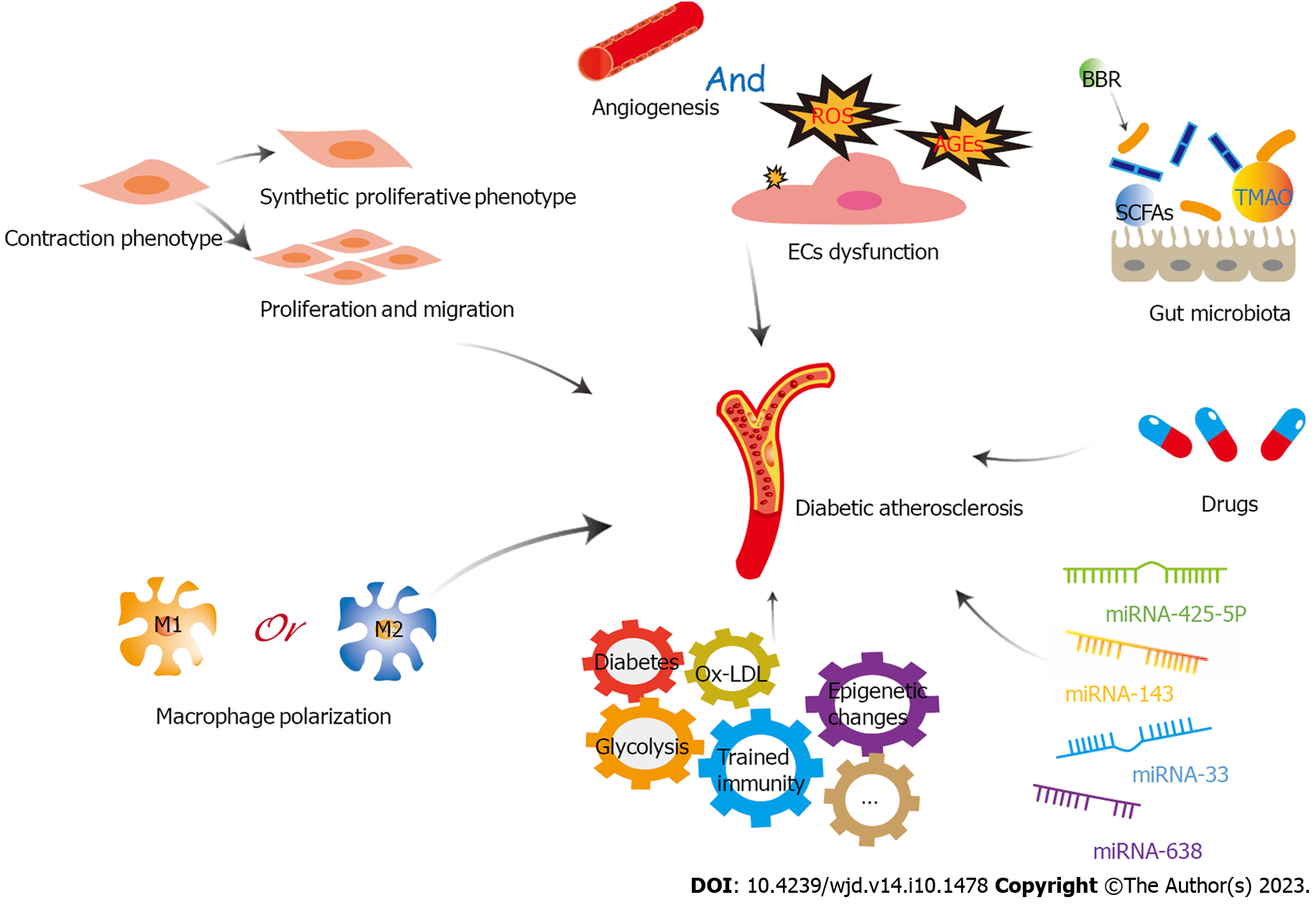
Figure 4 A variety of factors play important roles in diabetic atherosclerosis through glycolysis, including endothelial cells dysfunction, proliferation, migration and phenotypic transformation of vascular smooth muscle cells, macrophage polarization, trained immunity, microRNAs, gut microbiota, and drugs.
EC: Endothelial cell; LDL: Low-density lipoprotein; ROS: Reactive oxygen species; TMAO: Trimethylamine-N-oxide; BBR: Berberine; miRNA: microRNA; SCFAs: short-chain fatty acids.
- Citation: Liu QJ, Yuan W, Yang P, Shao C. Role of glycolysis in diabetic atherosclerosis. World J Diabetes 2023; 14(10): 1478-1492
- URL: https://www.wjgnet.com/1948-9358/full/v14/i10/1478.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i10.1478