©The Author(s) 2022.
World J Diabetes. Jun 15, 2022; 13(6): 454-465
Published online Jun 15, 2022. doi: 10.4239/wjd.v13.i6.454
Published online Jun 15, 2022. doi: 10.4239/wjd.v13.i6.454
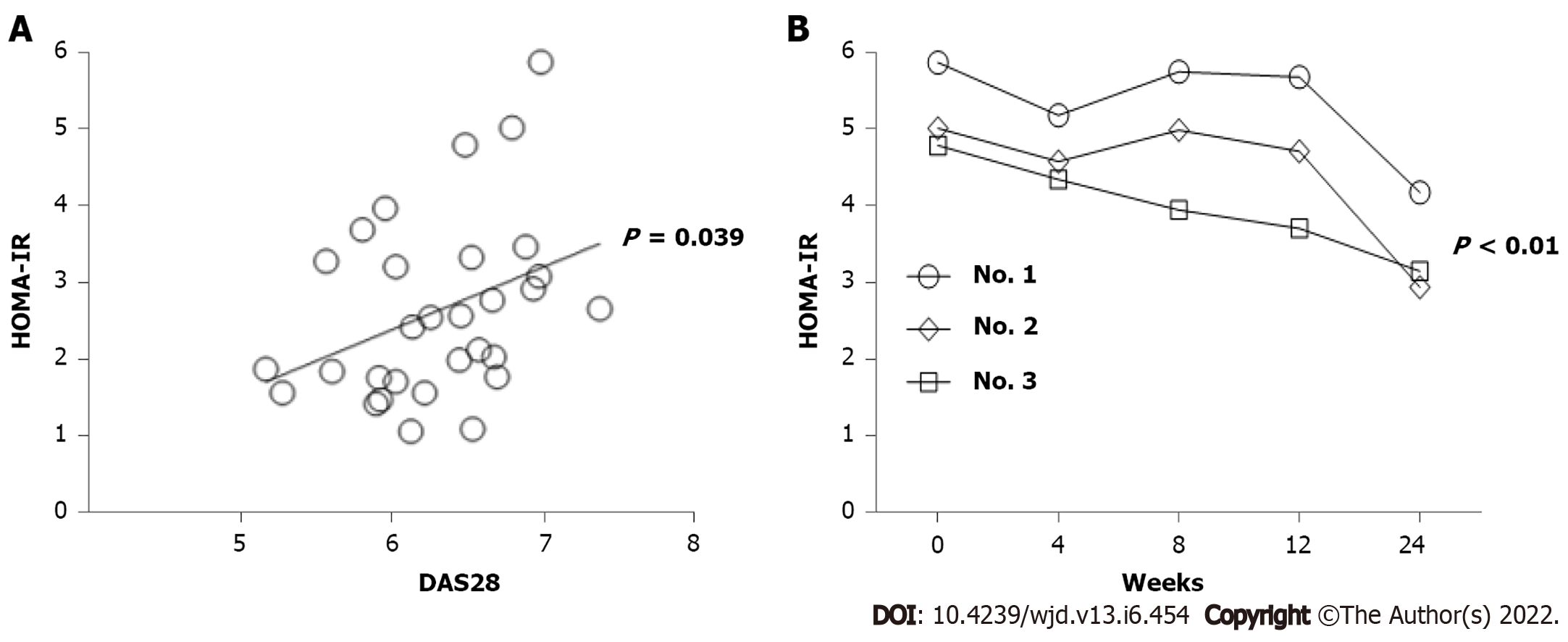
Figure 1 Characteristics of homeostatic model assessment-insulin resistance levels in active rheumatoid arthritis patients naïve to biologic synthetic disease-modifying anti-rheumatic drugs.
A: Positive correlation between 28-joint disease activity score 28 values and homeostatic model assessment (HOMA)-insulin resistance (IR) levels (P = 0.039) before tofacitinib (TOF) therapy; B: Serial calculations of HOMA-IR levels in 3 patients with high baseline IR at weeks 0, 4, 8, 12 and 24 after TOF therapy. There were significantly lower levels at week 24 as compared with those at week 0 (P < 0.01). HOMA-TR: Homeostatic model assessment-insulin resistance.
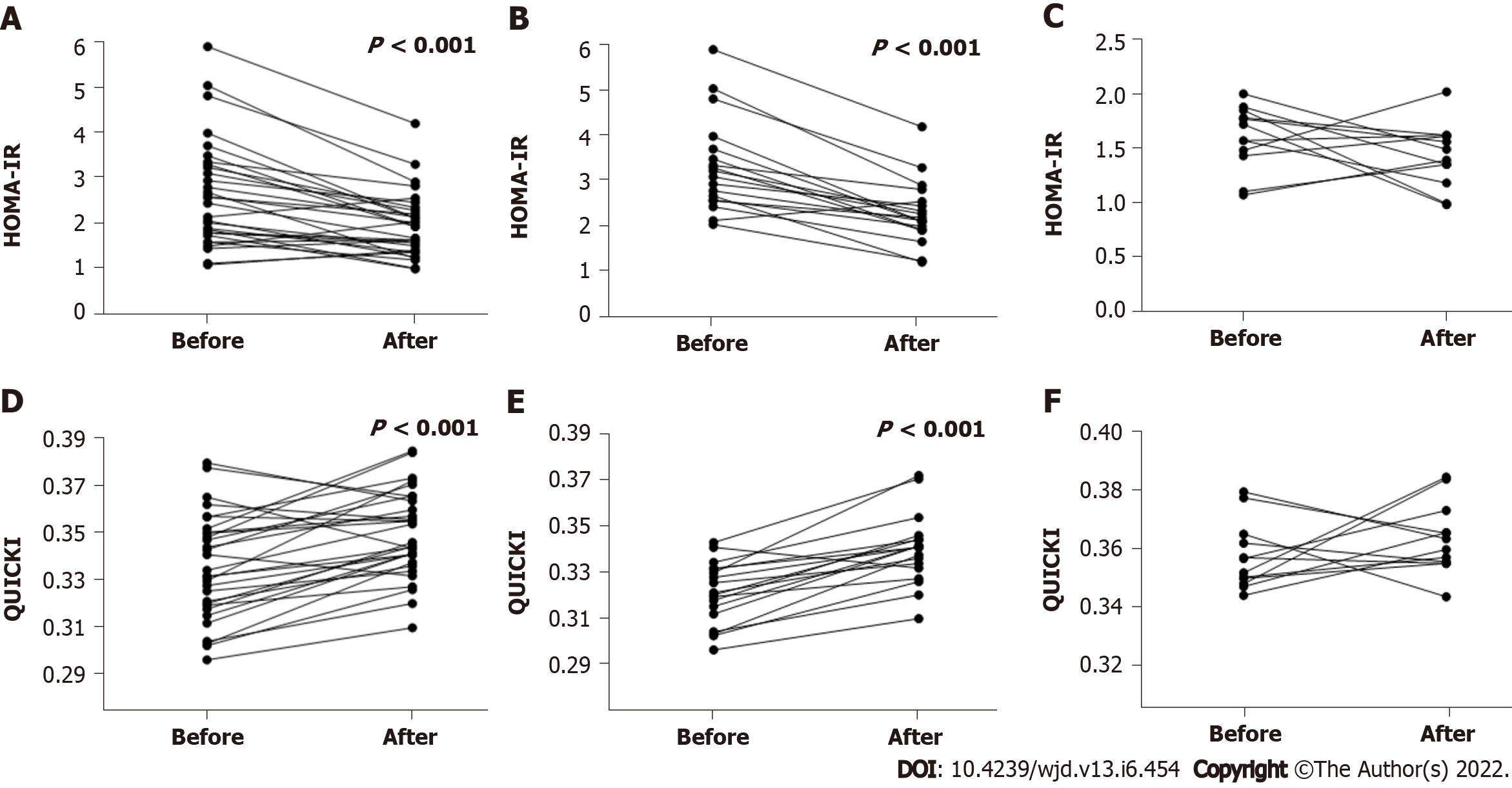
Figure 2 Homeostatic model assessment-insulin resistance and Quantitative Insulin Sensitivity Check Index levels in 30 active rheumatoid arthritis patients naïve to biologic agents before and 24 wk after tofacitinib therapy.
A: Homeostatic model assessment (HOMA)-insulin resistance (IR) levels in all 30 patients at weeks 0 and 24 after tofacitinib (TOF) therapy (P < 0.001); B: HOMA-IR levels in the high-IR group with 18 patients at weeks 0 and 24 after TOF therapy (P < 0.001); C: HOMA-IR levels in the low-IR group with 12 patients at weeks 0 and 24 after TOF therapy; D: Quantitative Insulin Sensitivity Check Index (QUICKI) levels in all 30 patients at weeks 0 and 24 after TOF therapy (P < 0.001); E: QUICKI levels in the high-IR group with 18 patients at weeks 0 and 24 after TOF therapy (P < 0.001); F: QUICKI levels in the low-IR group with 12 patients at weeks 0 and 24 after TOF therapy. QUICKI: Quantitative Insulin Sensitivity Check Index; HOMA-TR: Homeostatic model assessment-insulin resistance.
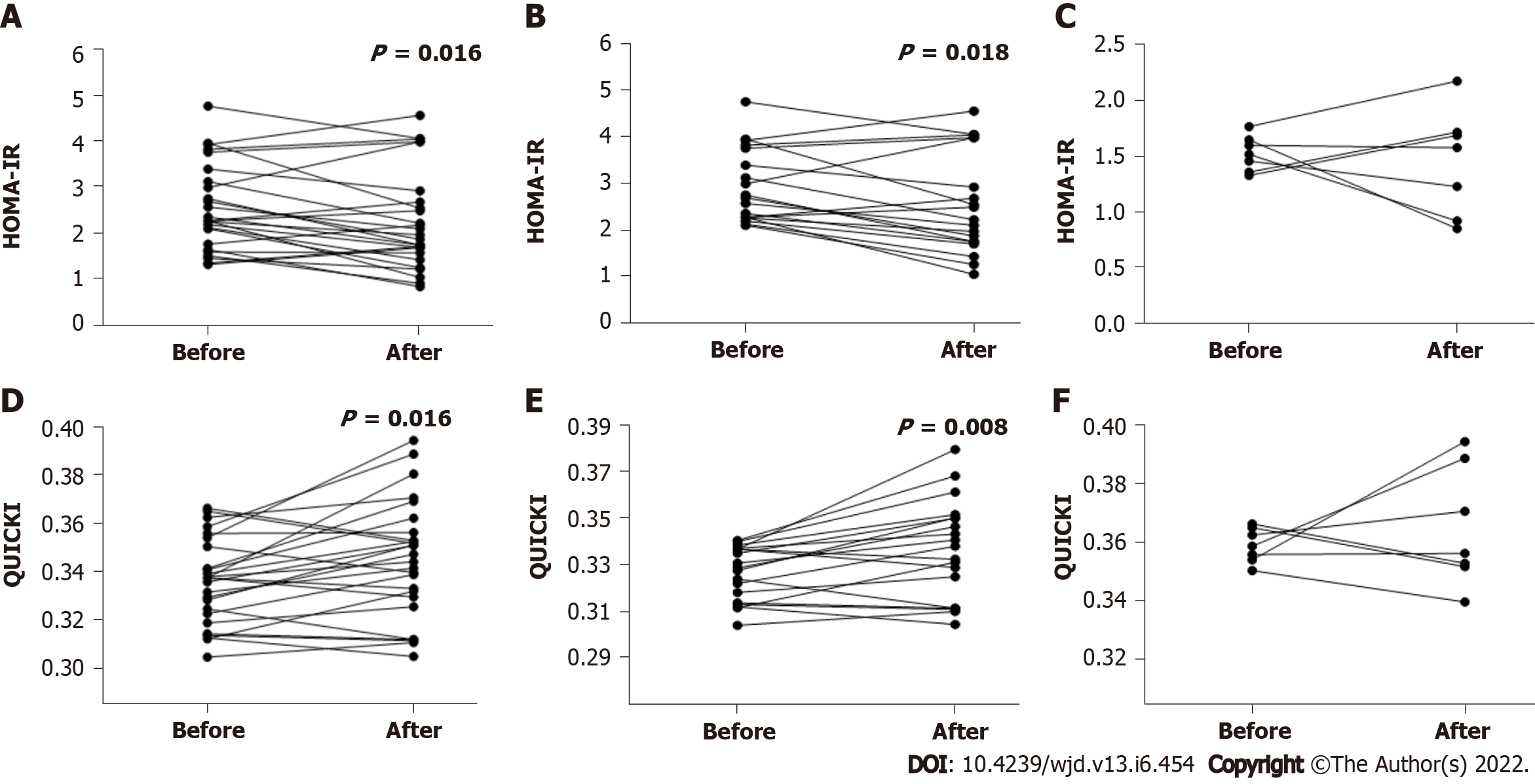
Figure 3 Homeostatic model assessment-insulin resistance and Quantitative Insulin Sensitivity Check Index levels in 26 active rheumatoid arthritis patients exposed to biologic agents before and 24 wk after tofacitinib therapy.
A: Homeostatic model assessment (HOMA)-insulin resistance (IR) levels in all 26 patients at weeks 0 and 24 after tofacitinib (TOF) therapy (P = 0.016); B: HOMA-IR levels in the high-IR group with 19 patients at weeks 0 and 24 after TOF therapy (P = 0.018); C: HOMA-IR levels in the low-IR group with 7 patients at weeks 0 and 24 after TOF therapy; D: Quantitative Insulin Sensitivity Check Index (QUICKI) levels in all 26 patients at weeks 0 and 24 after TOF therapy (P = 0.016); E: QUICKI levels in the high-IR group with 19 patients at weeks 0 and 24 after TOF therapy (P = 0.008); F: QUICKI levels in the low-IR group with 7 patients at weeks 0 and 24 after TOF therapy. QUICKI: Quantitative Insulin Sensitivity Check Index; HOMA-TR: Homeostatic model assessment-insulin resistance.
- Citation: Wang CR, Tsai HW. Immediate-release tofacitinib reduces insulin resistance in non-diabetic active rheumatoid arthritis patients: A single-center retrospective study. World J Diabetes 2022; 13(6): 454-465
- URL: https://www.wjgnet.com/1948-9358/full/v13/i6/454.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i6.454