©The Author(s) 2021.
World J Diabetes. May 15, 2021; 12(5): 524-540
Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.524
Published online May 15, 2021. doi: 10.4239/wjd.v12.i5.524
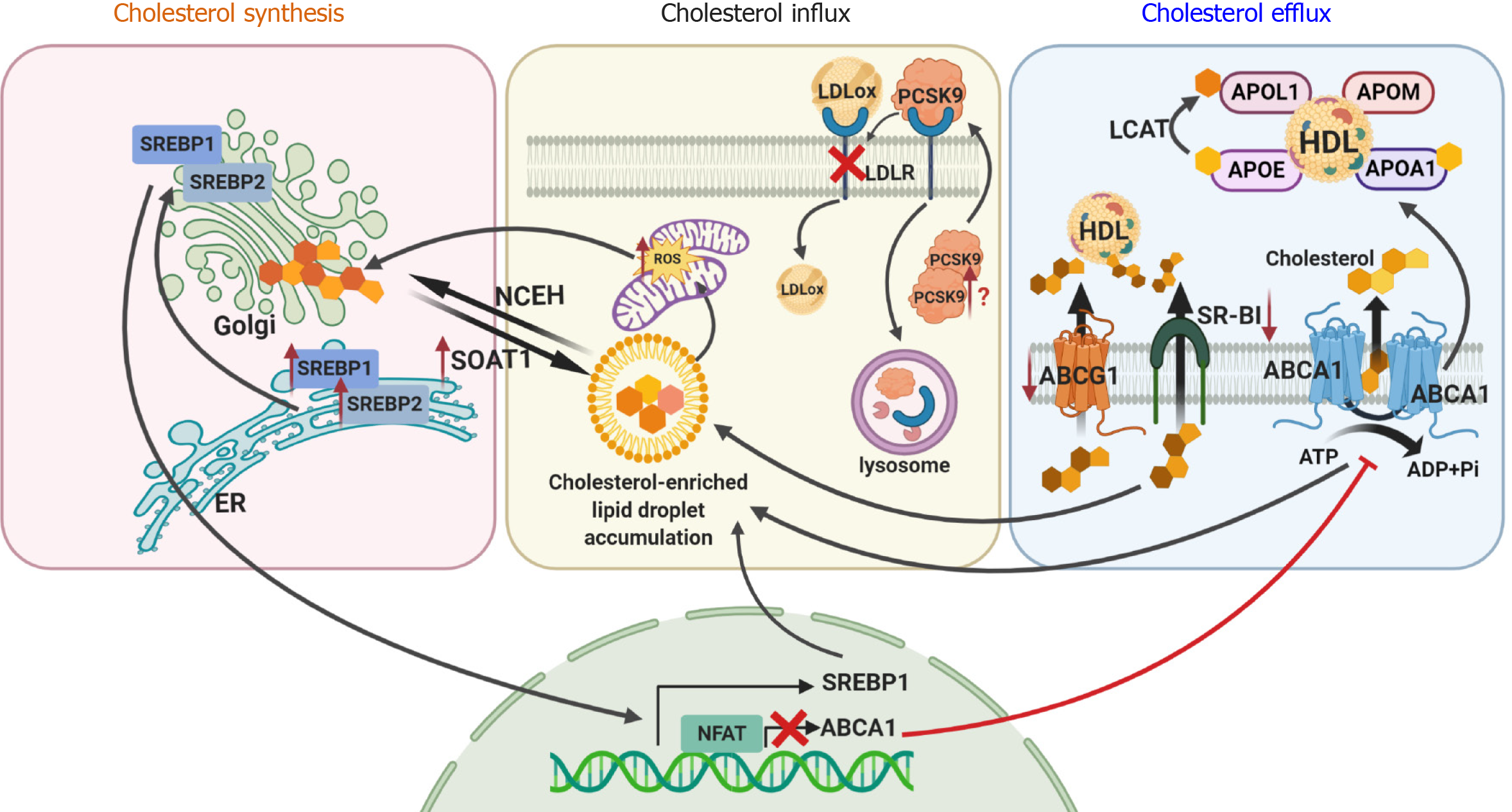
Figure 1 Cellular dyslipidemia in diabetic kidney disease affects cholesterol synthesis, influx and efflux.
Sterol regulatory element-binding protein 1 or 2 (SREBP1 and SREBP2) is transported from the endoplasmic reticulum to the Golgi apparatus, where it is cleaved followed by translocation to the nucleus to initiate cholesterol synthesis. Newly synthesized free cholesterol is then converted into esterified cholesterol by sterol O-acyltransferase 1 (SOAT1) or is transported to the plasma membrane for efflux by ATP-binding cassette subfamily A member 1 (ABCA1), subfamily G member 1 (ABCG1) or scavenger receptor class B type I (SR-BI). Cholesterol uptake from circulating low density lipoproteins (LDLs) is mediated by LDL receptor (LDLR). In diabetic kidney disease (DKD), overexpression of SREBP1 and SREBP2 and decreased expression of ABCA1, ABCG1 and SR-BI results in accumulation of cholesterol inside a cell and increased reactive oxygen species production. Accumulation of free cholesterol activates SOAT1, leading to over-production of esterified cholesterol, which is toxic to cells. Overexpression of proprotein convertase subtilisin kexin 9 may also contribute to DKD via enhanced degradation of the LDLR, resulting in increased levels of circulating LDL cholesterol. This image was created using BioRender software (www.BioRender.com). SREBP1: Sterol regulatory element-binding protein 1; SREBP2: Sterol regulatory element-binding protein 2; ER: Endoplasmic reticulum; SOAT1: Sterol O-acyltransferase 1; NCEH: Neutral cholesterol ester hydrolase; LDLox: Oxidized low density lipoprotein; PCSK9: Proprotein convertase subtilisin kexin 9; LDLR: Low density lipoprotein receptor; ROS: Reactive oxygen species; LCAT: Lecithin:cholesterol acyltransferase; APOL1: Apolipoprotein L1; APOE: Apolipoprotein E; APOM: Apolipoprotein M; APOA1: Apolipoprotein A1; HDL: High density lipoprotein; ABCA1: ATP-binding cassette subfamily A member 1; ABCG1: Subfamily G member 1; SR-BI: Scavenger receptor class B type I; ATP: Adenosine triphosphate; ADP: Adenosine diphosphate; Pi: Inorganic phosphorus.
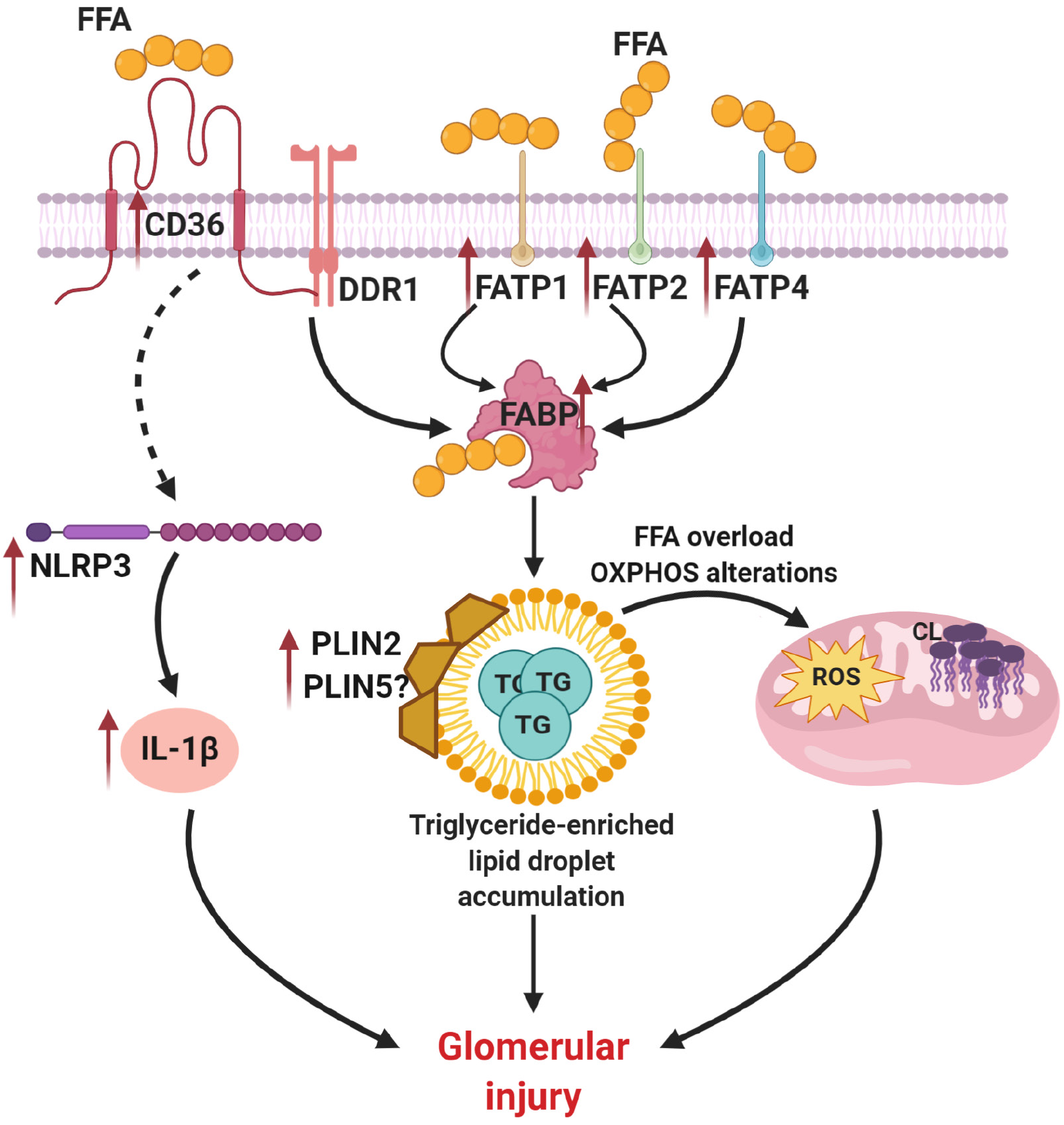
Figure 2 Abnormalities in triglyceride homeostasis contribute to lipid droplet accumulation in diabetic kidney disease.
Increased expression of scavenger receptor class B (CD36), fatty acid transporter protein 1 (FATP1), FATP2, FATP4 and fatty acid-binding protein leads to accumulation of fatty acids inside a cell, abnormalities in triglyceride (TG) metabolism and formation of TG-enriched lipid droplets. Altered activity of perilipin protein family members (PLIN2 and possibly PLIN5) also contributes to lipid droplet formation. In turn, accumulation of TG-enriched lipid droplet causes alteration in oxidative phosphorylation, cardiolipin accumulation and reactive oxygen species overproduction. Together with increased expression of NLR family pyrin domain containing 3 and interleukin 1β CD36 overexpression causes podocyte injury in diabetic kidney disease. This image was created using BioRender software (www.BioRender.com). FFA: Free fatty acid; DDR1: Discoidin domain receptor 1; FATP1: Fatty acid transporter protein 1; FATP2: Fatty acid transporter protein 2; FATP4: Fatty acid transporter protein 4; FABP: Fatty acid transporter protein; NLRP3: NLR family pyrin domain containing 3; PLIN2: Perilipin protein family member 2; PLIN5: Perilipin protein family member 5; TG: Triglyceride; OXPHOS: Oxidative phosphorylation; ROS: Reactive oxygen species; IL-1β: Interleukin 1 beta.
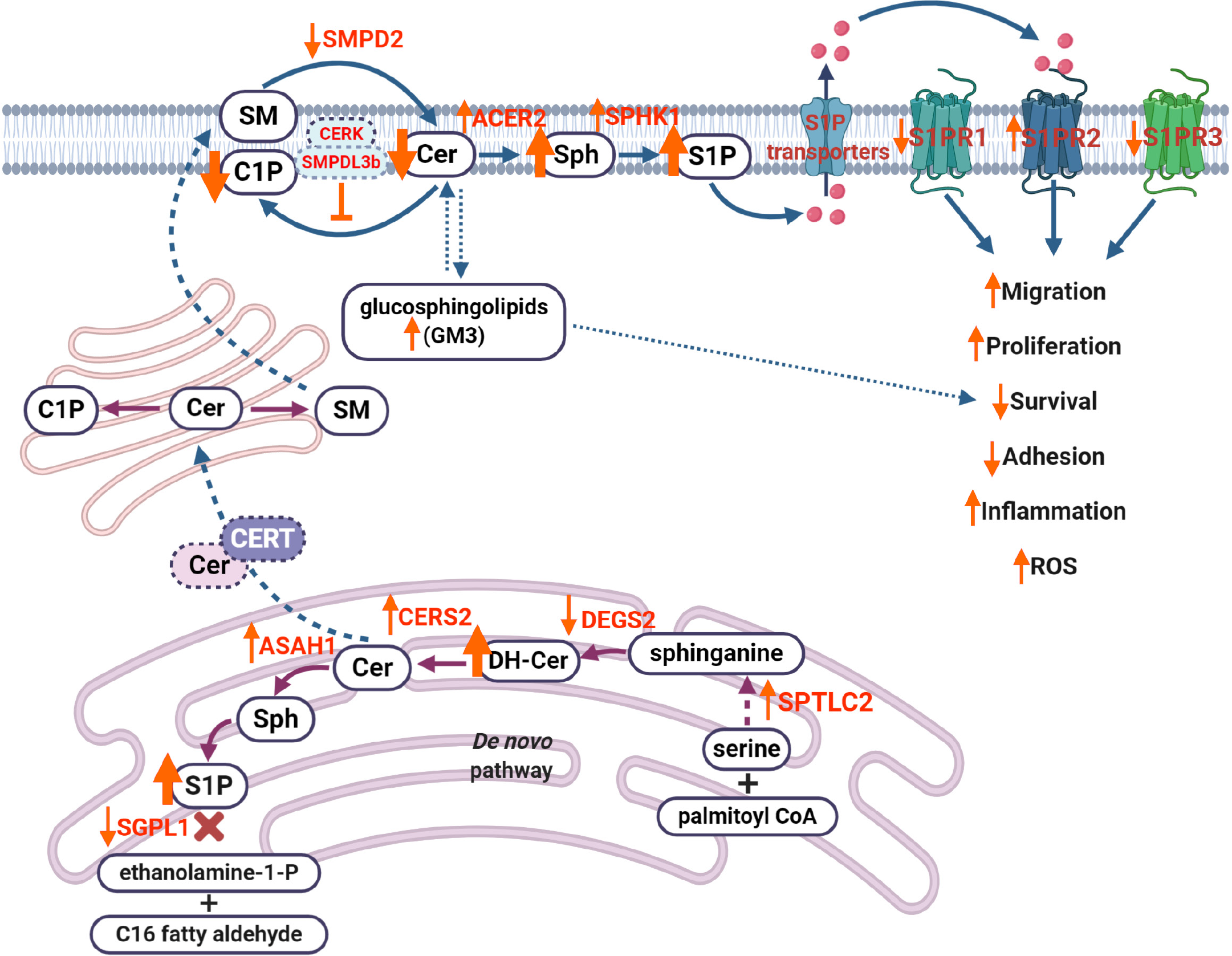
Figure 3 Dysregulation of sphingolipid metabolism contributes to the progression of diabetic kidney disease.
Decreased activity of desaturase (DEGS2) results in the accumulation of dihydroceramides. Increased activity of ceramide synthase 2 (CERS2) leads to increased production of ceramide (Cer), which leads to increased production of sphingosine (Sph) and sphingosine-1-phosphate (S1P) via decreased activity of sphingosine-1-phosphate lyase 1. Cer can also be translocated to the Golgi apparatus via ceramide transport protein, where it results in production of sphingomyelin. At the plasma membrane, decreased activity of sphingomyelin phosphodiesterase 2 affects Cer production, while elevated activity of alkaline ceramidase 2 increases levels of Sph, which, in turn, leads to accumulation of S1P via increased activity of sphingosine kinase 1. Overproduction of S1P results in increased S1P efflux via S1P transporters (such as ATP-binding cassette transporters ABCA1, ABCG1, ABCC1 and S1P transporter SPNS2), where S1P can act as a paracrine factor to activate S1P receptor signaling (primarily, S1P receptors 1-3, S1PR1, S1PR2, S1PR3), leading to dysregulation of many cellular processes, including migration, proliferation, survival or inflammation. Accumulation of gangliosides (GM3) can also affect cell survival in diabetic kidney disease. This image was created using BioRender software (www.BioRender.com). SMPD2: Sphingomyelin phosphodiesterase 2; SM: Sphingomyelin; C1P: Ceramide-1-phosphate; CERK: Ceramide kinase; SMPDL3b: Sphingomyelin phosphodiesterase acid-like 3b; Cer: Ceramide; Sph: Sphingosine; S1P: Sphingosine-1-phosphate; S1PR1: Sphingosine-1-phosphate receptor 1; S1PR2: Sphingosine-1-phosphate receptor 2; S1PR3: Sphingosine-1-phosphate receptor 3; GM3: Ganglioside M3; CERT: Ceramide transport protein; SGPL1: Sphingosine-1-phosphate lyase 1; DH-Cer: Dihydroceramide; ASAH1: N-acylsphingosine amidohydrolase 1; CERS2: Ceramide synthase 2; DEGS2: Delta(4)-desaturase, sphingolipid 2; SPTLC2: Serine palmitoyltransferase 2; CoA: Acyl-coenzyme A; ROS: Reactive oxygen species.
- Citation: Mitrofanova A, Burke G, Merscher S, Fornoni A. New insights into renal lipid dysmetabolism in diabetic kidney disease. World J Diabetes 2021; 12(5): 524-540
- URL: https://www.wjgnet.com/1948-9358/full/v12/i5/524.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i5.524