Published online Jul 15, 2017. doi: 10.4251/wjgo.v9.i7.300
Peer-review started: November 8, 2016
First decision: December 27, 2016
Revised: January 19, 2017
Accepted: March 23, 2017
Article in press: March 24, 2017
Published online: July 15, 2017
Processing time: 246 Days and 15.2 Hours
To evaluate the prognostic significance of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and prognostic nutritional index (PNI) and other clinicopathological factors in patients undergoing curative resection of colon cancer.
183 patients with histologically proven colorectal cancer who had undergone potentially curative resection between 2010 and 2016 at Ankara Numune Training and Research Hospital were retrospectively analyzed and clinicopathological characteristics included age, sex, tumor type, grade, size and localization, the number of metastatic and total number of lymph nodes removed, vascular and perineural invasion of the tumor, TNM stages, tumor marker levels (CEA, CA19-9, AFP, CA-125, CA15-3), complete blood counts, albumin levels, overall survival (months), NLR, PLR, LMR and PNI ratios were retrospectively reviewed and analyzed from the electronic database. The primary outcome measure was overall survival.
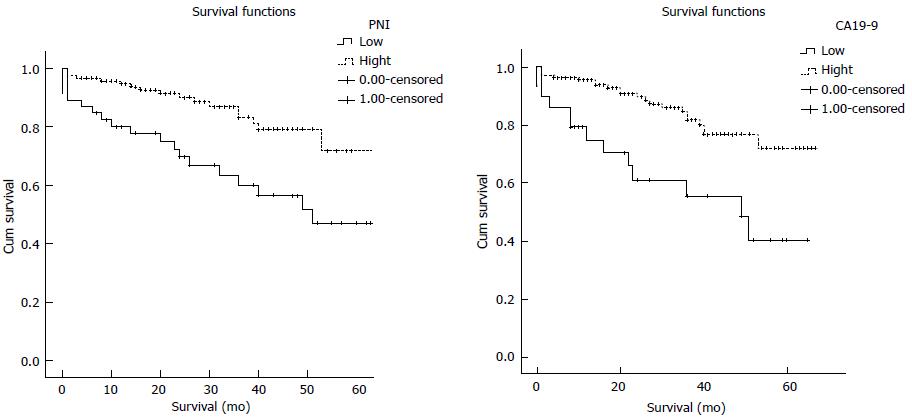
Regarding overall survival, on univariate analysis the following variables were significantly associated with poor outcome following resection: T-stage (P = 0.037), lymph node invasion (P = 0.037), cancer stage (P = 0.034), CEA (P = 0.042), CA19-9 (P = 0.004), and PNI (P = 0.001). To evaluate the independent prognostic value, multivariate Cox proportional hazard analysis to control for other prognostic factors was used. Using cancer-specific death as an end point for NLR, PLR, LMR, PNI and CA19-9 the optimal cut off values were calculated by ROC analysis. Regarding overall survival, on multivariate analysis high CA19-9 (HR = 1.001, 95%CI: 1.00-1.002, P = 0.012) and low PNI (HR = 0.938, 95%CI: 0.891-0.987, P = 0.014) were the only variables independently associated with shortened overall survival. Patients with a PNI < 35 had a median OS of 52.25 mo. In contrast, patients with an PNI > 35 had a median OS of 66 mo. Patients with a CA 19-9 < 17 had a median OS of 66 mo and in patients with a CA19-9 > 17 had a median OS of 53.76 mo.
This study shows that decrease in the PNI and increase in CA 19-9 is associated with poor survival in patients with resectable colon cancer.
Core tip: Predictors of colorectal cancer (CRC) that may determine overall survival are extremely important. Inflammation is now widely recognised to be a key element of disease advancement and survival in CRC. Aim of this study was to evaluate the prognostic significance of clinicopathologic factors and the indicators of systematic inflammatory response by using neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and prognostic nutritional index in patients undergoing curative resection of colon cancer. In the present study, we report, for the first time, a longitudinal comparison of the four systemic inflammation-based prognostic scores and CA19-9 in patients with resectable CRC.
- Citation: Akgül Ö, Çetinkaya E, Yalaza M, Özden S, Tez M. Prognostic efficacy of inflammation-based markers in patients with curative colorectal cancer resection. World J Gastrointest Oncol 2017; 9(7): 300-307
- URL: https://www.wjgnet.com/1948-5204/full/v9/i7/300.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i7.300
Colorectal cancer (CRC) is the third most common malignancy and the fourth leading cause of cancer-related deaths worldwide. Five-year overall survival (OS) remains unsatisfactory due to local recurrence or metastasis[1]. Predictors that may determine overall survival and patient outcome are extremely important, as the capability to distinguish patients more likely to acquire poor outcome following surgery would enable surgical and chemotherapeutic treatment personalized appropriately for each individual case[2]. To date, prognostic factors, such as tumor, node, and metastasis (TNM) stage, cell differentiation grade, Dukes’ stage, tumor type, have commonly been applied[3]. The TNM stage system is a gold standard for clinical management as it aids in directing the clinician towards appropriate treatment selection and also predicts the patient’s prognosis. However, what the current system lacks is the ability to predict the response and outcome individually[4]. For this reason the development of economical and readily available prognostic markers for risk determination in CRC treatment, is a must to thereby deliver a more personalized form of cancer care[5]. Recent studies have clearly shown the relationship between host inflammatory response to tumor carcinogenesis and the important role it plays in cancer development, progression and metastasis[6,7].
Since the first observation by Virchow who described the link between inflammation and tumorigenesis, the impact of inflammation on cancer development has been shown in an extensive number of tissues through processes that involve genomic destabilization and initiation of invasion and metastasis[8]. Inflammation is now widely accepted to be a key element of disease advancement and survival in CRC[9]. The local inflammation that is caused by the tumor is mirrored in a systemic inflammatory response (SIR) that may be straightforwardly measured preoperatively[2]. Many studies have shown the SIR to be a key factor in determining outcomes and survival in CRC, by measuring various circulating markers of systemic inflammation[9]. Peripheral blood neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and prognostic nutritional index (PNI) which have been shown to be indicators of systematic inflammatory response, are widely used as valuable predictors for prognosis of cancer patients. However, the results of studies concerning the relationship of these biomarkers and prognosis of CRC patients remained inconsistent[10,11]. For this reason the aim of this study was to evaluate the prognostic significance the indicators of systematic inflammatory response by using PLR, NLR, LMR and PNI in patients undergoing curative resection of colon cancer[2].
Two hundred and sixty-three patients with histologically proven CRC who, on the basis of laparotomy findings and pre-operative abdominal computed tomography, were considered to have undergone potentially curative resection between 2010 and 2016 at Ankara Numune Training and Research Hospital were retrospectively analyzed and clinicopathological characteristics included age, sex, tumor type, grade, size and localization, the number of metastatic and total number of lymph nodes removed, vascular and perineural invasion of the tumor, TNM stages, tumor marker levels (CEA, CA19-9, AFP, CA-125, CA15-3), complete blood counts, albumin levels, overall survival (months), NLR, PLR, LMR and PNI ratios were recorded. We included blood count data available within 1 mo of surgical resection for this study. According to the following exclusion criteria: (1) emergency surgery; (2) death within 30 d of surgery; (3) clinical evidence of infection or other inflammatory conditions, such as inflammatory bowel disease or rheumatoid arthritis; (4) lack of preoperative complete blood counts and biochemical analysis; and (5) metastatic disease at diagnosis, 80 patients were excluded from the study. The records of 183 patients were retrospectively reviewed and analyzed from the electronic database. Measurements of a white cell count, absolute neutrophil, lymphocyte and monocyte counts and albumin were recorded within 1 mo before surgery. Tumors were staged using the conventional tumor, node, metastasis (TNM) staging system, 7th Edition, 2010 (AJCC, 2010). Patients were seen every 3 mo for the first 2 years, every 6 mo for the next 3 years, and once annually thereafter. NLR was determined by dividing the absolute neutrophil count by the absolute lymphocyte count; PLR by dividing the absolute platelet count by the absolute lymphocyte count; LMR by dividing the absolute lymphocyte count by the absolute monocyte count and PNI by the formula: Serum albumin (g/L) + 5 × total lymphocyte count × 109/L[12]. The primary outcome measure was overall survival.
All of the patients included in the present study were elective resections. Of the 183 colorectal cancer patients, 111 (60.7%) were men and 72 (39.3%) were women. The mean patient age was 63.5 ± 13.1 years (range 33-89 years). Diagnosis was adenocarcinoma in all cases. Two patients (1.1%) were in stage 0, 20 patients were in stage 1 (10.9%), 23 patients were in stage 2A (12.6%), 22 patients were in stage 2B (12%), 54 patients were in stage 2C (29.5%), 3 patients were in stage 3A (1.6%), 10 patients were in stage 3B (5.5%), 49 patients were in stage 3C (26.8%). Only 23 patients (12.6%) had Tis, T1 or T2 lesions, and 120 patients (65.6%) were lymph node negative. All patients underwent surgical resection, and 45 (24.6%) had lymphovascular invasion. Tumor size ranged from 10 to 140 mm, with a median size of 50 mm. One hundred and eighteen (64.5%) patients received adjuvant therapy following resection of the primary tumor. Thirty-day mortality rate following resection was 3.8% (n = 7). The median value of neutrophil was 5.1 × 106/mL (range 3.9-67.85), lymphocyte was 1.7 × 106/mL (range 1.3-2.22), platelet was 273 × 106/mL (range 219.5- 354), monocyte was 0.6 × 106/mL (range 0.47-0.8), and albumin was 39 g/L (range 34-43). The median 5-year OS rate was 61%. The relationship between clinicopathological characteristics, NLR, PLR, LMR, PNI, CA19-9 and overall survival in patients undergoing potentially curative resection for CRC is shown in Table 1. The data were tested for normality by the Kolmogorov- Smirnov test. Normally distributed variables were expressed as mean ± SD and non-parametric variables were expressed as median (interquartile range). Categorical variables were presented as numbers and percentages. A receiver operating characteristics (ROC) curve was generated to calculate the optimal cutoff value of inflammation parameters. OS was the study end point. Survival analysis was drawn using the Kaplan-Meier method, and the differences were compared using the log-rank test. Univariate and multivariate analysis using a Cox proportional hazards model was used to test independent significance. A two-tailed P-value < 0.05 was considered to be statistically significant. Statistical analyses were performed using the SPSS 21.0 software (IBM Corporation, Armonk, NY, United States). Regarding overall survival, on univariate analysis the following variables were significantly associated with poor outcome following resection: T-stage (P = 0.037), lymph node invasion (P = 0.037), cancer stage (P = 0.034), CEA (P = 0.042), CA 19-9 (P = 0.004), and PNI (P = 0.001). To evaluate the independent prognostic value, multivariate Cox proportional hazard analysis to control for other prognostic factors was used. Using cancer-specific death as an end point for NLR, PLR, LMR, PNI and CA19-9 the optimal cut off values were calculated by ROC analysis. In analysis of all patients, a cutpoint of 3.3 for the LMR, 35 for the PNI, 3.5 for the NLR, 180 for the PLR and 17 for the CA19-9 was found respectively. Regarding overall survival, on multivariate analysis high CA 19-9 (HR = 1.001, 95%CI: 1.00-1.002, P = 0.012) and low PNI (HR = 0.938, 95%CI: 0.891-0.987, P = 0.014) were the only variables independently associated with shortened overall survival. Patients with a PNI < 35 had a median OS of 52.25 mo. In contrast, patients with an PNI > 35 had a median OS of 66 mo. Patients with a CA19-9 < 17 had a median OS of 66 mo and in patients with a CA19-9 > 17 had a median OS of 53.76 mo. Kaplan- Meier survival curves demonstrating the associations of the PNI and CA19-9 with overall survival are shown in Figure 1.
| Clinicopathologic characteristics | Univariate analysis, HR (95%CI) | P value | Multivariate analysis, HR (95%CI) | P value |
| Sex | 0.798 | |||
| Tumor location | 0.685 | |||
| The depth of invasion | 0.877 | |||
| Perineural invasion | 0.931 | |||
| T-stage | 1.404 (1.021-1.930) | 0.037 | ||
| Lymph Node | 0.234 (0.080-0.683) | 0.037 | ||
| Cancer Stage | 1.173 (1.012-1.359) | 0.034 | ||
| CEA | 1.005 (1.00-1.009) | 0.042 | ||
| CA19-9 | 1.001 (1.00-1.002) | 0.040 | 1.001 (1.00-1.002) | 0.012 |
| NLR | 0.135 | |||
| LMR | 0.127 | |||
| PLR | 0.064 | |||
| PNI | 0.906 (0.865-0.948) | 0.001 | 0.938 (0.891- 0.987) | 0.014 |
In the present study, we report a longitudinal comparison of the four-systemic inflammation-based prognostic scores and CA19-9 in patients with resectable CRC. The our knowledge this is this first time these 5 markers have been used. Our findings demonstrate that the preoperative PNI and CA19-9 are independent predictors of OS for patients with CRC undergoing curative surgical resection. In the present study PNI and CA19-9 are superior to NLR, PLR and LMR in predicting OS as inflammatory markers. To our knowledge this is the first study in which PNI, LMR, NLR, PLR and CA19-9 were compared as preoperative inflammatory and tumor markers to predict the OS for the patients who have undergone curative resection for colorectal cancer.
The association between cancer progression and systemic inflammatory response and markers of inflammation on malignancy progression and survival been explored extensively[13]. There are several reports that have shown factors specific to the individual, such as loss of weight, performance status, and a systemic inflammatory response, to be important indicators of the outcome of clinical treatment[14,15]. Both biochemical and hematological markers have been used in oncological malignancies to enumerate the impact of SIR upon outcomes such as the elevation in C-reactive protein (CRP) concentration, increased white cell, neutrophil and platelet counts, and hypoalbuminemia[16]. Although studies have shown that a relationship exists between tumor progression and systemic inflammation, the exact mechanism remains unclear[17].The role different leukocytic infiltrates may play can differ in and around neoplasms. While granulocytes promote tumor development, adaptive immune cells such as T lymphocytes induce an antitumor response[18]. In colorectal cancer, the lymphocytes play a major role in human immune response, whereas systematic inflammation significantly depressed cellular immunity, resulting in a significantly decrease of CD4+ T lymphocytes and an increasing of CD8+ suppressor T lymphocytes[1]. A chronically inflamed microenvironment can effect cell proliferation which can lead to the cells losing their ability to control growth resulting in hyperproliferation and tumorigenesis[19]. Consequently, the inflammatory response plays a critical role in carcinogenesis and a series of inflammatory cells and innate immune system signaling molecules are involved in tumor progression, such as neutrophil, lymphocyte, platelet and monocyte. Thus, NLR, PNI, PLR and LMR that represent systematic inflammatory response are potential prognostic factors for CRC[20].
Zahorec[21] was the first to report the relationship between NLR and disease severity as a prognostic factor in critically ill patients. Studies evaluating the relationship between NLR and CRC have also shown it to be a strong prognostic factor. For patients with CRC it is presumed that NLR is a combined indicator of both inflammation and the immune status. However, it has not been clearly described whether there is an association between elevated NLR and poor oncologic outcome[22,23]. There are studies which have confirmed the potential prognostic utility of the NLR in patients with CRC and other solid tumors[24,25]. A study by He et al aimed to evaluate the prognostic and predictive value of the NLR and PLR in 243 patients with initially metastatic CRC patients. Their study showed NLR and PLR to be statistically significant poor prognostic factors[26]. Our results do not support those findings. A reason for this could be that those studies that found a negative correlation between NLR and OS assessed patients with locally advanced or metastatic colorectal cancers where inflammation may be more advanced due to the extensive nature of the cancer[27]. Therefore, it is possible that higher levels of NLR are more probable to predict overall poor outcome when the cancer is advanced[28]. Another debate concerning NLR is determining the optimal ratio that has the greatest prognostic significance. The cut-off value for NLR used in the present study is different from that reported in previous studies. In previous reports, different values have been used; however, in our study, the best cut-off value based on the ROC analysis was 3.5[24]. Similarly to NLR evidence for a prognostic role for PLR in colorectal cancer is also conflicting. A study by Kwon et al[22] demonstrated that an elevated PLR was independently associated with decreased overall survival, in CRC patients that underwent curative resection. Comparably Emir et al[33] found a statistically significant association between an elevated PLR and decreased overall 5-year survival in uni and multivariate analysis in 140 patients with resectable CRC[22,29]. In contrast, similar to our study Baranyai et al[30] assessed the PLR in patients with non-metastatic CRC of different stages and liver-only metastatic CRC and found PLR not to be a prognostic factor in either group.
Lymphocytes play a vital role in cytotoxic cell death and cytokine production which inturn prevent proliferation and metastatic activity of malignant cells[31]. Studies concerning lymphocyte ratio also have conflicting results. Some studies have shown that lymphocytes declined in patients with more advanced colon cancer[32]. However, tumor infiltrating lymphocytes have been identified to be associated with a better overall survival in early stage CRC patients[33]. The prognostic value of the novel LMR in malignancy, reporting independently significant associations with poor outcome across a range of cancer types including stage III primary colon cancer[2]. In a study by Stotz et al[34] evaluating the prognostic significance of the preoperative LMR in patients with stage III CRC also establish that a decreased LMR envisaged for shorter disease free survival and OS, and indicated that patients with decreased LMR may not profit from 5-FU-based adjuvant treatment. However, in our study, similar to the studies of Ying et al[1] and Neal et al[2], we found no statistically significant relationship between LMR and OS[1,2]. In previous studies, results were obtained in the form of NLR was superior to PLR in predicting OS[1,22], however similar to Chan et al[32] we found neither the NLR nor the PLR to be independently prognostic when studied together with the LMR.
The nutritional and immunologic conditions of patients apparently effect the postoperative outcomes associated with malignancies. The PNI was also shown recently to be a predictive marker for both postoperative complications and prognosis in patients with CRC[35]. The prognostic nutritional index (PNI), calculated from serum albumin levels and peripheral lymphocyte count, reflects both the nutritional and immune status of the patient[36]. Studies have shown that the PNI is very similar to the systemic inflammation-based prognostic scores (mGPS, NLR, PLR and PI), but the optimal cut-off value remain unclear. Similar to others in this study a significant relationship between poor prognosis of CRC and PNI was confirmed[12,36]. The present result therefore supports the debate that the systemic inflammatory response plays a major role in the relationship between nutritional decline and poor outcome in patients with cancer.
CA19-9 was first defined by Ozawa et al[37] as a monoclonal antibody against human CRC cell line, and it has been admitted as a molecule that contributes to tumor metastasis by mediating the adhesion of tumor cells to the endothelial cells of blood vessels. In the consideration of guideline suggestions and published documents, the function of CA19-9 as a prognostic marker is still a debated subject[38]. Many reports have suggested that CA19-9 might be a clinically valuable prognostic marker in patients with metastatic CRC[39]. In our study, multivariate analysis also indicated that a high preoperative serum CA19-9 level was an independent prognostic factor for poor OS in patients who had undergone curative resection. As far as we know, our study is the second to suggest the predictive power of the preoperative serum CA19-9 level for tumor prognosis in patients with CRC who have undergone curative resection.
The major limitations of the present paper are; its retrospective nature and exclusion of approximately one third of the initial number of patients for various reasons. Furthermore, CRP is not routinely measured prior to CRC resection at our centre, and, therefore, comparison of CRP with the other inflammatory predictors was not possible.
On the other hand being in a single center ensures a more homogeneous evaluation and less loss of data compared with a multicentric study. Besides this, the evaluation of these prognostic factors separately in different disease stages may be a different study subject.
CRC apparently has many molecular markers and studies for new molecular target drugs are presently ongoing, but although these molecular markers may be useful for predicting prognosis, many are limited in terms of the need for resected or biopsied specimens and high prices[35]. Furthermore, patients undergoing CRC surgery all undergo preoperative full blood counts and biochemical evaluation. The PNI and CA19-9 can be easily analyzed from routinely available data and does not require any further expenditure. The evaluation of these prognostic factors separately in different disease stages may also be a valuable different study subject.
In conclusion, this study shows that decrease in the PNI and increase in CA19-9 is associated with poor survival in patients with resectable colon cancer. Further investigation with larger datasets is required to confirm our findings.
In colorectal cancer (CRC) patients, the current survival and risk classification is basically based on the pathology result, and the gold standard is tumor node metastasis (TNM) staging. However, different survival results can be found in different patients with the same stage. For this reason, this prognosis prediction can be improved by using different methods. Cancer progression was also found to be associated not only with the tumor’s local characteristics but also with the systemic host response. Inflammation-based prognostic scores, such as peripheral blood neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and prognostic nutritional index (PNI), produced by components of the systemic inflammatory response were found to be associated with patient survival. The effect of these markers on patient overall survival was investigated in various studies in the literature, but inconsistencies were observed between the results. Studies comparing these inflammatory markers in the same patient population are lacking in the literature..
Patients with metastasis, infection, chronic systemic inflammatory disease, and patients who were operated under emergency conditions were excluded in this study, which may have an effect on the inflammatory response. The authors investigated the prognostic significance of patient survival in patients with curative colorectal cancer resection in a single center by comparing parameters such as NLR, PLR, LMR and PNI together with other clinicopathological features. It has been found that preoperative low PNI and high CA 19-9 values are associated with low survival in patients with resectable colorectal cancer. The PNI has been shown to be superior to other inflammatory markers.
Predictors that may determine overall survival and patient outcome are extremely important, as the capability to distinguish patients more likely to develop poor outcome succeeding surgery would enable surgical and chemotherapeutic treatment tailored appropriately for each individual case. In the present study, authors’ report, for the first time, a longitudinal comparison of the four systemic inflammation-based prognostic scores and CA19-9 in patients with resectable CRC. Their findings demonstrate that the preoperative PNI and CA19-9 are independent predictors of overall survival for patients with CRC undergoing curative surgical resection. In the present study PNI and CA19-9 are superior to NLR, PLR and LMR in predicting OS as inflammatory markers.
The presence of low levels of PNI and high levels of CA19-9, detected during preoperative evaluation in colorectal cancer patients may help them to predict postoperative patient overal survival. Furthermore, patients undergoing CRC surgery all undergo preoperative full blood counts and biochemical evaluation. The PNI and CA19-9 can be easily analyzed from already routinely available data and does not require any further expenditure.
It’s a well-written manuscript about prognostic efficacy of inflammation-based markers in colorectal cancer resection patients. This study is interesting and the objective very clear.
| 1. | Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (2)] |
| 2. | Neal CP, Cairns V, Jones MJ, Masood MM, Nana GR, Mann CD, Garcea G, Dennison AR. Prognostic performance of inflammation-based prognostic indices in patients with resectable colorectal liver metastases. Med Oncol. 2015;32:144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008;61:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Jia J, Zheng X, Chen Y, Wang L, Lin L, Ye X, Chen Y, Chen D, Dettke M. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumour Biol. 2015;36:9319-9325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg. 2009;249:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2125] [Article Influence: 125.0] [Reference Citation Analysis (2)] |
| 7. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48820] [Article Influence: 3254.7] [Reference Citation Analysis (12)] |
| 8. | Virchow R. An Address on the Value of Pathological Experiments. Br Med J. 1881;2:198-203. [PubMed] |
| 9. | McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 1062] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 10. | Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer. 2013;109:395-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Chiang SF, Hung HY, Tang R, Changchien CR, Chen JS, You YT, Chiang JM, Lin JR. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis. 2012;27:1347-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 619] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 13. | Hong C, Wei Y, Jiang J, Zhao C, Liang G, Wang G, Yang H. Associations between lifestyles and neutrophil-lymphocyte and platelet-lymphocyte ratios in colorectal cancer. Asia Pac J Clin Oncol. 2014;10:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, Glare P, Nabal M, Viganò A, Larkin P. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240-6248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 496] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 15. | Graf W, Bergström R, Påhlman L, Glimelius B. Appraisal of a model for prediction of prognosis in advanced colorectal cancer. Eur J Cancer. 1994;30A:453-457. [PubMed] |
| 16. | Malietzis G, Giacometti M, Kennedy RH, Athanasiou T, Aziz O, Jenkins JT. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21:3938-3946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Choi WJ, Cleghorn MC, Jiang H, Jackson TD, Okrainec A, Quereshy FA. Preoperative Neutrophil-to-Lymphocyte Ratio is a Better Prognostic Serum Biomarker than Platelet-to-Lymphocyte Ratio in Patients Undergoing Resection for Nonmetastatic Colorectal Cancer. Ann Surg Oncol. 2015;22 Suppl 3:S603-S613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1664] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 19. | Kozak MM, von Eyben R, Pai JS, Anderson EM, Welton ML, Shelton AA, Kin C, Koong AC, Chang DT. The Prognostic Significance of Pretreatment Hematologic Parameters in Patients Undergoing Resection for Colorectal Cancer. Am J Clin Oncol. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11517] [Article Influence: 479.9] [Reference Citation Analysis (2)] |
| 21. | Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5-14. [PubMed] |
| 22. | Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, Park KJ, Roh MS, Kim SG, Kim HJ. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Kubo T, Ono S, Ueno H, Shinto E, Yamamoto J, Hase K. Impact of the perioperative neutrophil-to-lymphocyte ratio on the long-term survival following an elective resection of colorectal carcinoma. Int J Colorectal Dis. 2014;29:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, Hirakawa K. A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res. 2013;33:3291-3294. [PubMed] |
| 25. | Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, Chen X, Rong R, Zhang B, Xia L. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013;30:439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 27. | Shin JS, Suh KW, Oh SY. Preoperative neutrophil to lymphocyte ratio predicts survival in patients with T1-2N0 colorectal cancer. J Surg Oncol. 2015;112:654-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Jankova L, Dent OF, Chan C, Chapuis P, Clarke SJ. Preoperative neutrophil/lymphocyte ratio predicts overall survival but does not predict recurrence or cancer-specific survival after curative resection of node-positive colorectal cancer. BMC Cancer. 2013;13:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Liu H, DU X, Sun P, Xiao C, Xu Y, Li R. Preoperative platelet-lymphocyte ratio is an independent prognostic factor for resectable colorectal cancer. Nanfang Yike Daxue Xuebao. 2013;33:70-73. [PubMed] |
| 30. | Baranyai Z, Krzystanek M, Jósa V, Dede K, Agoston E, Szász AM, Sinkó D, Szarvas V, Salamon F, Eklund AC. The comparison of thrombocytosis and platelet-lymphocyte ratio as potential prognostic markers in colorectal cancer. Thromb Haemost. 2014;111:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Mallappa S, Sinha A, Gupta S, Chadwick SJ. Preoperative neutrophil to lymphocyte ratio & gt; 5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, Clarke SJ. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann Surg. 2017;265:539-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 33. | Emir S, Aydin M, Can G, Bali I, Yildirim O, Öznur M, Yildiz ZD, Sözen S, Gürel A. Comparison of colorectal neoplastic polyps and adenocarcinoma with regard to NLR and PLR. Eur Rev Med Pharmacol Sci. 2015;19:3613-3618. [PubMed] |
| 34. | Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110:435-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 263] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 35. | Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, Watanabe M, Baba H. Prognostic Nutritional Index Predicts Severe Complications, Recurrence, and Poor Prognosis in Patients With Colorectal Cancer Undergoing Primary Tumor Resection. Dis Colon Rectum. 2015;58:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Gao P, Chen X, Song Y, Shi J, Zhao J, Sun J, Xu Y, Wang Z. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysis. Oncotarget. 2016;7:58543-58552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 37. | Ozawa T, Ishihara S, Nishikawa T, Tanaka T, Tanaka J, Kiyomatsu T, Hata K, Kawai K, Nozawa H, Kazama S. The preoperative platelet to lymphocyte ratio is a prognostic marker in patients with stage II colorectal cancer. Int J Colorectal Dis. 2015;30:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Giessen-Jung C, Nagel D, Glas M, Spelsberg F, Lau-Werner U, Modest DP, Schulz C, Heinemann V, Di Gioia D, Stieber P. Preoperative serum markers for individual patient prognosis in stage I-III colon cancer. Tumour Biol. 2015;36:7897-7906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Ozawa T, Ishihara S, Kawai K, Nozawa H, Yamaguchi H, Kitayama J, Watanabe T. Prognostic Significance of Preoperative Serum Carbohydrate Antigen 19-9 in Patients With Stage IV Colorectal Cancer. Clin Colorectal Cancer. 2016;15:e157-e163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pescatori M S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL