Published online Apr 15, 2017. doi: 10.4251/wjgo.v9.i4.184
Peer-review started: October 13, 2016
First decision: November 14, 2016
Revised: December 14, 2016
Accepted: January 2, 2017
Article in press: January 3, 2017
Published online: April 15, 2017
Processing time: 181 Days and 8.5 Hours
To estimate Helicobacter pylori (H. pylori) recurrence rate in Latin America, a region with a significant H. pylori prevalence and gastric cancer burden.
PubMed, LILACS, SciELO, Cochrane databases and abstracts from relevant meetings were reviewed. Information collected included: Participants’ characteristics, recruitment strategy, diagnostic modality, treatment arms, follow-up and recurrence rates. Recurrence was calculated using 100-patients-year rates, and data were pooled using a random effects model. The I2 statistic assessed between study heterogeneity. Meta-regression analyses evaluated for effect modifying variables.
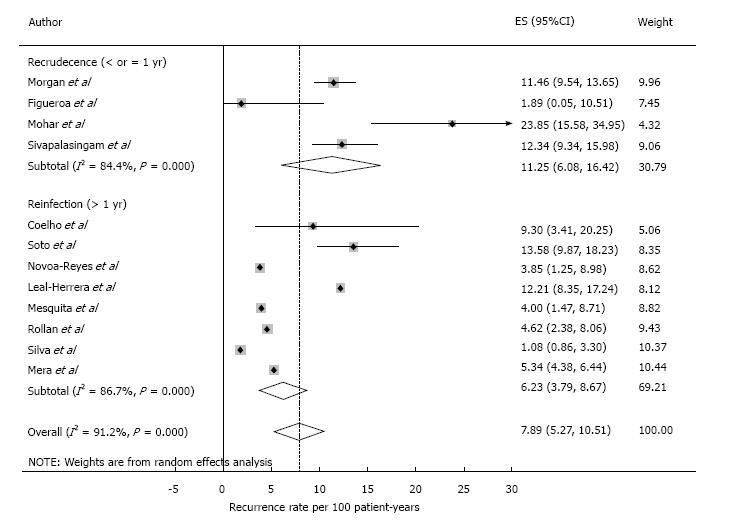
Literature search yielded 163 articles. Twelve studies involving 4848 patients from 9 countries met inclusion criteria. Four hundred and thirty-two reinfections were recorded in 5487 person-years of follow-up. Pooled analysis showed a recurrence rate of 7.9 cases per 100 person-years (95%CI: 5.3-10.5). Meta-regression revealed that neither the antibiotic schema, a second antibiotic course, nor the diagnostic modality had an impact on the observed risk of recurrence. The recurrence rate in the first year after treatment, predominantly recrudescence, was 11.2 (6.1-16.4) per 100 patient years. Recurrence in subsequent years, was only 6.2 (3.8-8.7).
H. pylori recurrence rates in Latin America are significant, and with geographic variability, yet are acceptable based upon the current literature for consideration of large scale intervention trials. Further research in Latin America is warranted to evaluate the efficacy, cost-effectiveness, and potential adverse outcomes of proposed eradication programs.
Core tip: Latin America has a high burden of gastric cancer mortality, with significant geographical variability, which offers the opportunity for prevention trials and interventions. Recent trials and meta-analysis show that Helicobacter pylori (H. pylori) eradication reduces the risk of gastric adenocarcinoma. H. pylori reinfection rates in Latin America are similar to those seen in Asian trials. Recurrent cases occur mostly within the first year suggesting treatment failure (re-growth), not reinfection. These findings were not significantly modified by diagnostic modality, the antibiotics selected, retreatment, or the time check for eradication success. Eradication programs are a potentially attractive strategy for gastric cancer prevention in Latin America.
- Citation: Corral JE, Mera R, Dye CW, Morgan DR. Helicobacter pylori recurrence after eradication in Latin America: Implications for gastric cancer prevention. World J Gastrointest Oncol 2017; 9(4): 184-193
- URL: https://www.wjgnet.com/1948-5204/full/v9/i4/184.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i4.184
Gastric cancer is the third most common cause of cancer mortality globally, and the leading infection-associated cancer[1,2]. Of the 989000 gastric cancer cases in the world in 2008, 78% (770000) were estimated to be attributed to Helicobacter pylori (H. pylori) chronic infection[3]. Gastric cancer has a marked geographic variability[4,5]. Latin America has a particularly high burden of prevalent H. pylori infection and gastric cancer incidence and mortality[6-8]. Estimated age-standardized mortality rates for males per 100000 are elevated in Honduras (22.3), Costa Rica (16.8), Peru (18.2), Chile (15.0), and Ecuador (20.7)[5,9]. A concentration of incident gastric cancer is observed in the mountainous regions along the Pacific littoral, including in lower incidence countries (e.g., Mexico), which may offer the opportunity for focused prevention trials and interventions[5].
Recent trials and a meta-analysis suggest that screening and eradication of H. pylori can reduce the risk of gastric cancer[10,11]. The Shangdong Intervention Trial, the largest randomized clinical trial to date, had a 53% H. pylori cumulative recurrence rate at 7 years, yet demonstrated a significant reduction in gastric cancer at 14.8 years (OR = 0.6, 95%CI: 0.4-0.9)[10]. Trial participants were principally middle-aged Asian adults, and the generalizability of results to other populations is uncertain[12]. Two subsequent meta-analyses confirmed the findings, while noting that the results were primarily driven by trials conducted in Asia[11,13]. The International Agency for Cancer Research (IARC) has recently called for the design and study of large scale interventions for gastric cancer prevention in high incidence regions of the world, including Latin America[12].
The H. pylori infection recurrence rate after eradication therapy is the critical determinant of the efficacy of an H. pylori eradication program designed to reduce the burden of gastric cancer. This review aims to estimate the reinfection rate of H. pylori after completion of antibiotic treatment in Latin America based upon existing literature. We present overall recurrence rates which includes both recrudescence (also called re-growth: Same strain, dominant in the first year after eradication) and reinfection (new strain, dominant in subsequent years), as the majority of studies do not genotype H. pylori strains.
Review methods and reporting were performed according to the PRISMA guidelines[14]. Literature databases PubMed (United States National Library of Medicine), LILACS (Latin America and the Caribbean Literature on Health Sciences), SciELO (Scientific Electronic Library Online) and Cochrane (the Cochrane Collaboration) were included as well as the abstracts from three major gastroenterology and infectious disease meetings [Digestive Disease Week (DDW), American College of Gastroenterology Scientific Meeting (ACG), and ID Week (IDW)]. Studies evaluating H. pylori reinfection in the 20 countries comprising Latin America, as defined by the United Nations Educational Scientific and Cultural Organization[15], published in any language up to November 1st 2014 were included.
The search was performed in PubMed using the following sequence: H. pylori (MeSH term) AND [Recurrence (MeSH) or Recrudescence (MeSH) or Reinfection (not MeSH term)] AND (MeSH terms Latin America or Central America or South America or Argentina or Bolivia or Brazil or Colombia or Costa Rica or Cuba or Chile or Dominican Republic or Ecuador or El Salvador or Guatemala or Honduras or Mexico or Nicaragua or Panama or Paraguay or Peru or Puerto Rico or Uruguay or Venezuela). No other filters or limits were used. Analogous strategies were used to search the other two databases and the meetings’ abstracts. Three additional meta-analyses relevant to the study were reviewed for further references[16-18].
Three investigators (Juan E Corral, Corey W Dye and Douglas R Morgan) independently reviewed titles and abstracts for selection of potentially relevant articles. For journal manuscripts, full text articles were retrieved for further review. Titles that could not be associated with an abstract were excluded from review. A priori, studies with a sample smaller than 50 patient-years (PYs) and studies reporting same populations as other previously registered were excluded from meta-analysis. Citations of retrieved articles were reviewed for studies that may have been missed or were absent from our database queries. Authors were not contacted to provide additional information.
The following information was abstracted from each article: Year of publication, first author, country, information regarding participants (age, recruitment strategy), treatment arms (number of arms, medications used and duration in each arm), follow-up details (duration, intervals of appointment), diagnostic modality and recurrence rates. The interval of possible recurrence started with the last day of antibiotic regimen treatment, and ended with the day of follow-up H. pylori diagnostic testing; the last day of treatment was chosen to optimally account for eradication regimens of varying duration. In a given study, if there was more than one follow-up H. pylori diagnostic test for recurrence, each testing result was documented independently. The earliest time interval to consider infection recurrence and to be included in the review was 6 mo.
The quality of data (risk of bias) was assessed recording 5 variables, using the same methodology as Camargo et al[17] Antibiotic strategy was recorded in detail (medications and length of treatment) and was also scored as an ordinal variable [0 = only one antibiotic without a proton pump inhibitor (PPI), ranitidine or bismuth; 1 = either one antibiotic and a PPI or two antibiotics but no PPI (ranitidine or bismuth allowed); 2 = includes two antibiotics and a PPI (regardless of scheme, for example, triple, quadruple, sequential)].
All treatment arms in each study were reviewed individually. Cases were allocated in two groups: The patients that received antibiotics and those that received either placebo or an antacid medication (PPI, H2 blocker or bismuth) but without antibiotics. Only antibiotic arms were included in meta-analysis. The number of patients with a negative test immediately after treatment (range 4-8 wk after antibiotic course) were recorded for the intention to eradicate analysis. The patients compliant with subsequent H. pylori testing were analyzed for our main analysis, and per our protocol, in this group, the patients lost to follow-up between eradication test (post antibiotics) and subsequent testing were not included. We also documented whether a second antibiotic course was offered for those patients with persistent infection or not.
We used a random-effects model to summarize recurrence rates. Summary reinfection rates and corresponding 95%CIs were calculated using the Poisson distribution. Forest plot graphs were created with 95%CIs. Given the relevance of differentiating between recrudescence (re-growth) and reinfection, subgroup analysis was performed for studies that looked for H. pylori recurrence at one year or less (< 53 wk cutoff) and those with longer follow-up[16]. Pooled recurrence rates were calculated for different points in time for Latin America, starting at the first six months after completing antibiotics and for all subsequent years where data was available.
A secondary analysis was conducted with four additional comparisons: Recurrence 3 years after eradication, recurrence in studies enrolling children compared to studies restricted to adults, antibiotics regimens with high (> 75%) or low (≤ 75%) eradication success, and studies that assessed recurrence with endoscopy and biopsy compared to other diagnostic methods.
Meta-regression analyses were performed to evaluate for five effect modifying variables: Study population (community volunteers, patients with duodenal ulcers, dyspepsia, or intestinal metaplasia), H. pylori diagnostic modality (with or without urea breath test), quality of antibiotic treatment (0 to 2 points), possibility of a second antibiotic course, and length of follow-up (in years). Between-study heterogeneity was quantified using the I2 statistic. Finally, publication bias was investigated by visual inspection of funnel plots. All statistical analyses were performed with Stata version SE 11.2 (Stata-Corp, College Station, TX, United States). The study plan and results were reviewed by a biomedical statistician (Robertino Mera).
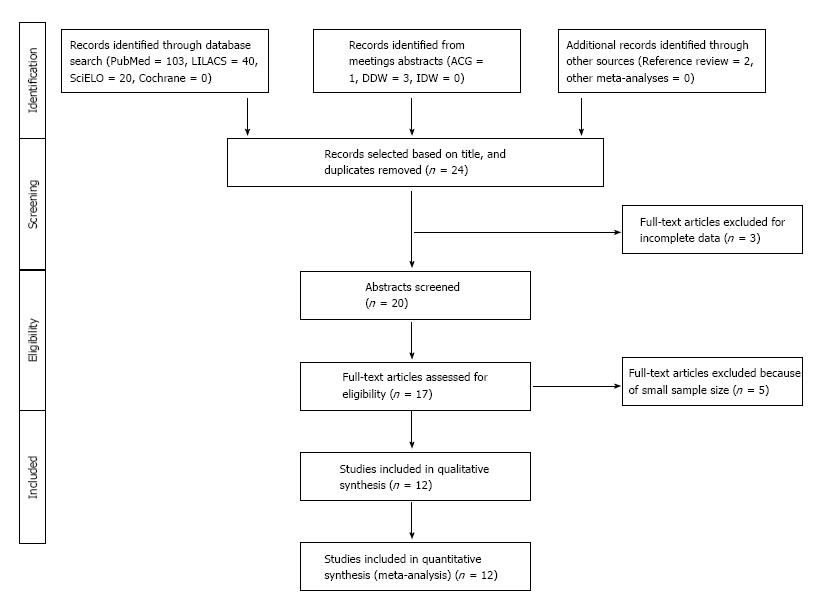
The literature search resulted in 164 articles from the following sources: PubMed (104), LILACS (40), SciELO (20), and Cochrane (0) (Figure 1). Four abstracts were considered relevant from our review of conference reports (ACG 1, DDW 3, IDW 0). Two additional articles were identified after screening the references of manuscripts found in first review. After excluding 139 irrelevant or duplicate publications, 25 full text articles were retrieved for further evaluation, of which 7 were excluded because of incomplete data or duplicate samples and six additional per protocol for their small sample size (< 50 person-years of follow-up).
In summary, 12 studies from 9 countries met criteria for inclusion, which were published between 1991 and 2014 (Table 1). Ten studies included only adults, and an additional 2 studies included both adults and children. Eleven manuscripts were written in English and one in Spanish. Time to evaluate H. pylori eradication success ranged from 4 to 13 wk after last day of antibiotics (3 studies reported percentage of successful treatment “after randomization” without further details). Follow-up ranged from 6 mo to 16 years. These twelve studies encompassed 4848 patients [4685 patients received a treatment regimen that included antibiotics, 163 were assigned to a placebo group or other treatment arm without antibiotics (only anti-acids)]. In these studies, the mean eradication rate after initial treatment was 72.2%, with a range of 30.2% to 100%. Reinfection rates ranged from 1.8% to 85.4%. In total, there were 432 reinfection events recorded in 5487 PYs follow-up in patients with sufficient data to calculate recurrence rates. Of the 4848 patients, 2172 (44.8%) did not complete follow-up diagnostic testing (Table 2).
| Ref. | Year | Country | Patients enrolled or randomized | Mean age ± SD (age range) | Patient population | Treatment arm(s) | Antibiotic duration (d) | Second antibiotic treatment | Eradi-cation success rate | Wait time (wk) | Diagnostic method(s) | Follow-up, yr | Study design and quality[17] |
| Morgan et al[22] | 2013 | 6 countriesa | 1463 | (21-65) | Community populations | 3 options: | Variable: | PPI + M + Bis + Tetrab | Total 77.4% | 6-8 | 13C, CagA IgG | 1 | 5 |
| PPI + A + C | 14 | 82.20% | |||||||||||
| PPI + A/PPI + A + M | 5/5 | 76.50% | |||||||||||
| PPI + A + C + M | 5 | 73.60% | |||||||||||
| Silva et al[34] | 2010 | Brazil | 150 | 46.7 (16-85) | Duodenal ulcer | PPI + A + C | 7 | PPI + Tetra + Furazolidone | 92.50% | 13 | 14C, H (RUT, PCR) | 5 | 3 |
| Mesquita et al[35] | 2005 | Brazil | 50 | 49 ± 14 (> 18) | Duodenal ulcer | H2 + Bis + C | 14 | NA | 100% | 13 | H (RUT, H and E) | 3 | 2 |
| Coelho et al[36] | 1991 | Brazil | 48 | 40.4 (adults) | Duodenal ulcer | A + M + Furaz | 5 | NA | 60.40% | 8.5 | 14C | 1.5 | 2 |
| Rollan et al[37] | 2000 | Chile | 111 | 38 (16-75) | Duodenal ulcer | 2 options: H2 + A + M PPI + A + Tinidazole | 14 14 | Cross-over | Total 75.7% 79% 73% | 4-6 | 14C, H (RUT, Warthin-S, PCR) | 3 | 3 |
| Figueroa et al[38] | 1996 | Chile | 57 | 49.1 (16-65) | Duodenal ulcer | PPI + A + M + Bis | 28 | NA | 80.70% | 4 | H (RUT, Gram, Clt) | 1 | 5 |
| Novoa-Reyes et al[39] | 2014 | Peru | 140 | 48.9 ± 12.3 (18-85) | Non-ulcer dispepsia | PPI + A + C | 10 | NA | 72.10% | 4 | 14C, H (H and E) | 2 | 3 |
| Soto et al[40] | 2003 | Peru | 235 | 37 ± 8.7 (18-55) | Non-ulcer dispepsia | PPI + A + C | 14 | NA | 85.50% | 4 | 14C, H (Warthin-S, Clt) | 1.5 | 5 |
| Leal-Herrera et al[41] | 2003 | Mexico | 467 | (> 5)c | Non-ulcer dispepsia | PPI + A + C | 14 | NA | 30.20% | 4-6 | 14C, H (Giemsa, Clt, PCR), Serology | 2 | 4 |
| Mohar et al[42] | 2002 | Mexico | 131 | 51.4 ± 9.3 (> 40) | Healthy volunteers | PPI + A + C | 7 | NA | 76.30% | 6 | H (H and E, Elisa), CagA IgG | 1 | 4 |
| Sivapa- lasingam et al[43] | 2014 | Bolivia | 848 | (> 6 mo)d | Community populations | PPI + A + C | 10 | “Triple therapy” | 64.00% | 6 | 13C, CagA IgG | 1 | 3 |
| Mera et al[19,44] | 2005 | Colombia | 976 | 50.8 (29-69) | Intestinal metaplasia | Variable (the majority A + M + Bis) | 14 | NA | 51.60% | 156 | 13C, H (H and E, Steiner) | 16 | 5 |
| Ref. | Patients that received antibiotics | Patients present at f/u appointment | Recurrent cases total | Crude reinfection rate1 | Follow-up (yr) | Year patients (present at f-u appointment) | Recurrence rate per 100 PY (95%CI) |
| Morgan et al[22] | 1133 | 1091 | 125 | 11.46 | 1 | 1091 | 11.46 (9.54-13.65) |
| Silva et al[34] | 147 | 112 | 10 | 8.98 | 5 | 557 | 1.80 (0.86-3.30) |
| Mesquita et al[35] | 50 | 50 | 6 | 12.00 | 3 | 150 | 4.00 (1.47-8.71) |
| Coelho et al[36] | 29 | 43 | 6 | 13.95 | 1.5 | 64.5 | 9.30 (3.41-20.25) |
| Rollan et al[37] | 84 | 96 | 12 | 12.50 | 3 | 260 | 4.62 (2.39-8.06) |
| Figueroa et al[38] | 47 | 53 | 1 | 1.89 | 1 | 53 | 1.89 (0.05-10.52) |
| Novoa-Reyes et al[39] | 101 | 65 | 5 | 7.69 | 2 | 130 | 3.85 (1.25-8.98) |
| Soto et al[40] | 201 | 216 | 44 | 20.37 | 1.5 | 324 | 13.58 (9.87-18.23) |
| Leal-Herrera et al[41] | 141 | 131 | 32 | 24.43 | 2 | 262 | 12.21 (8.35-17.24) |
| Mohar et al[42] | 183 | 109 | 26 | 23.85 | 1 | 109 | 23.85 (15.58-34.95) |
| Sivapalasingam et al[43] | 543 | 462 | 57 | 12.34 | 1 | 462 | 12.34 (9.34-15.98) |
| Mera et al[19,44] | 679 | 126 | 108 | 85.37 | 16 | 2024 | 5.34 (4.38-6.44) |
| Total | 3338 | 2554 | 432 | 16.92 | 5487 | 7.89 (5.27-10.51) |
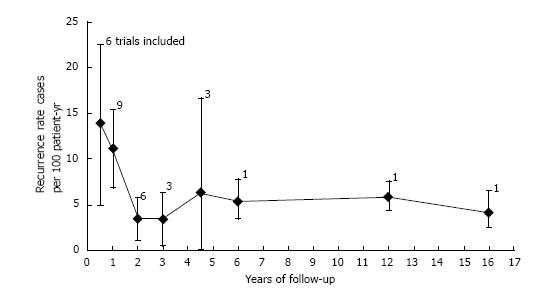
Pooled analysis showed an overall recurrence rate of 7.9 cases per 100 PYs (95%CI: 5.3-10.5) (Figure 2). Analysis on an intention to eradicate basis (those with a negative test immediately after treatment) had a recurrence rate of 7.1 (4.7-9.6) per 100 PYs. The recurrence rate in the first year after treatment, postulated to be predominantly recrudescence, was 11.2 (6.1-16.4) per 100 PYs (all 12 studies included), while recurrence in subsequent years, an estimate of reinfection, was 6.2 (3.8-8.7) per 100 PYs. The cumulative reinfection rate at the 5 and 7 year time points were 36.2 and 48.6 per 100 PYs, respectively. Recurrence rates from different countries were combined for the first 6 years, 12 and 16 years (only one study had follow-up beyond 5 years[19]). Recurrence rate were higher in the first six months and decreased afterwards. Data from year 4 and 5 were combined as they had few PY follow-up (132 and 98, respectively). After the first year, reinfection rates ranged from 3.4 per 100 PYs in year 2 to 6.3 per 100 PYs in the combined 4-5 year period (Figure 3).
In a secondary analysis, the reinfection rate was lower when using a 3-year time cutoff; with an estimated rate of 3.8 (95%CI: 1.6-6.1) cases per 100 PYs. Recurrence rates were two times higher in studies that enrolled children compared to those that only enrolled adults 12.3 (95%CI: 9.6-14.9) vs 6.9 (95%CI: 4.2-9.6) cases per 100 PYs. There was no significant difference in recurrence rates among trials with high or low initial eradication success [7.8 (95%CI: 3.4-12.3) vs 8.4 (95%CI: 4.6-12.1), respectively]. Recurrence rates were higher in studies that evaluated eradication by endoscopy, 11.6 (95%CI: 9.9-13.3), compared to those that used non-invasive diagnostic methods, 6.6 (95%CI: 4.0-9.1).
In the meta-regression, neither the study population, the method used to detect H. pylori, the initial antibiotic strategy, the use of a second antibiotic course, nor the length of follow up had a significant impact on the observed risk of reinfection. As anticipated, the recurrence rates decreased after the first year by 40%, but this was not statistically significant after including all five variables (P = 0.6).
Risk of bias according to Camargo scale ranged from 2 to 5 points. Even though reviewed studies used various methods to asses H. pylori recurrence, 10 (83%) used at least two different methods, including 9 (75%) that used urea breath tests. Most studies lost points because of sampling techniques or because they failed to describe salient patient characteristics. The I2 for the model was 90% and adjusted R2 was -38.8%. Funnel plot showed asymmetry towards higher recurrence rates, with a lack of missing studies with low sample size (high SD) where the rates are lower. The top of the funnel plot demonstrated a low risk for publication bias.
H. pylori recurrence after eradication is a critical determinant of the efficacy of potential gastric cancer prevention programs utilizing antibiotic treatment. This measure may be more important than the choice of initial antibiotic regimen and bacterial resistance rates, and is likely to differ by global region[19-21]. Latin America populations have high colonization rates of H. pylori, as well as a significant burden of gastric adenocarcinoma. Our meta-analysis estimates a recurrence rate of 7.9 cases per 100 PYs in Latin America, 11.2 in year-one and 6.2 in subsequent years. This overall rate is higher than the estimated global recurrence rate of 4.5 (95%CI: 4.2-4.8), but significantly lower than that reported for resource-limited nations 8.7 (7.8-9.6) and 13.0 (6.0-21.0) observed in two independent meta-analysis[16,18].
Is there a maximum H. pylori infection recurrence threshold for potential intervention programs? In the Shangdong trial reported by Ma et al[10] with healthy volunteers in East Asia, H. pylori eradication significantly reduced incident gastric cancer compared to placebo after 14.8 years of follow up [OR 0.6 (0.4-0.9), P = 0.3]. These results have been supported by recent meta-analyses[11]. In the Shangdong study, omeprazole and amoxicillin comprised both the treatment and the retreatment regimen, and only 47% of subjects were H. pylori negative at the 7-year post-eradication point. In general terms, this may suggest a 50% threshold at the 5 to 7 year time point as a minimum eradication efficacy target. Our estimated 5-year and 7-year reinfection rates in the current meta-analysis are lower or at least similar to the 7-year reinfection rate observed in the Shangdong study: 36.2% and 48.6%, vs 53%, respectively. Thus, H. pylori screening and eradication in asymptomatic populations may be an attractive strategy for gastric cancer prevention in Latin America. Further research to evaluate feasibility, cost-effectiveness, acceptance, and adverse consequences of eradication programs in the region is needed. For example, the 1-year recurrence analysis in the large 6-country H. pylori eradication trial in Latin America suggested that potential programs may need to be tailored based upon region, gender and age of the participants[22]. Uncertainties about H. pylori screening and treatment have to be answered and significant challenges are foreseen before such programs can be implemented at a population level (Table 3).
| Components | Challenges and considerations | Implementation approaches |
| Public policy | Lack of awareness among the Ministries of Health, stakeholders, and the public | Large scale education campaigns for cancer and gastric cancer Joint initiatives with international stakeholders: WHO, IARC, PAHO, UICC, NCI, and CDC |
| Economic investment | Cost of H. pylori eradication program Economics of growing gastric cancer burden | Conduct CEAs at the country and regional level. The CEAs may differ for HICs and LMICs |
| Program design | Uncertainties and regional variation for target age, screening approach, treatment regimen, and follow-up | Pilot-test eradication campaigns and perform community implementation trials Adapt evidence from cost-effectiveness models and available epidemiologic data. Incorporate screening into existing public health practices (e.g., cervical cancer) |
| Appropriate technologies | Technical difficulties in H. pylori testing Consistent eradication confirmation norms Management of high risk patients | Develop economic, point-of-care H. pylori testing Coordinate endoscopy protocols for high risk patients (e.g., premalignant lesions) Implement information networks to coordinate eradication programs, health centers, and endoscopy centers |
| Adherence measures | Poor compliance with H. pylori eradication regimen, leading to treatment failure and increased infection recrudescence | Consider medication side effect profiles Pre-regimen counseling for common side effects Consider adherence measures, usual (e.g., direct observed therapy), or novel (e.g., cell phone contact) |
| H. pylori recurrence | Elevated reinfection rate may affect program efficacy and feasibility | Improve living conditions to reduce potential environmental sources of reinfection Consider the family or the village as the intervention target |
| Potential overall program risks and unknowns | Alteration of the human microbiome Induction of antibiotic resistance Potential increased risk for certain diseases (e.g., allergic diseases, esophageal cancers) Unknown role(s) of H. pylori as a component of the human microbiome: Commensal and pathogen, which may be strain and/or age dependent | H. pylori eradication programs should be considered investigational, with use of rigorous methodology and long term surveillance Monitoring of antibiotic resistance and microbiome profiles Global antibiotic stewardship programs (e.g., OTC antibiotic use, veterinary use) |
| Parallel research agendas | Incorporate evolving approaches and technologies | Develop novel biomarkers for host risk and H. pylori virulence Develop biomarkers for premalignant lesions (e.g., intestinal metaplasia) to facilitate endoscopy surveillance Incorporate endoscopy technologies, including advanced imaging and low-cost approaches |
| H. pylori Vaccination | Unknown long-term effectiveness and side effects Lack of data showing impact in clinical outcomes | Evaluate long-term (> 3 yr) effectiveness in other centers, populations and countries[23] Complete regulatory evaluations, collect additional safety data and approval by national agencies. Phase IV studies |
H. pylori infection recurrence represents the combination of recrudescence and reinfection, and different strategies may be required to effectively reduce these component rates. Recrudescence or re-growth, usually occurs during the first year after treatment at a rate primarily driven by antibiotic treatment failure, in the setting of a false negative test immediately after treatment. This common scenario may be difficult to distinguish from reinfection with the same strain from a family member in the same household. Reinfection, is the principal component of recurrence after the first year, and persists at a lower but steady state. Molecular analysis comparing pre- and post-treatment strains of patients have shown that 80% of recurrent cases are genetically identical, whereas differing strains were found in only a minority of the cases[23]. This suggests that the majority of initial recurrent cases are a product of treatment failure, or reinfection with a strain common to close contacts or family members. In this meta-analysis, recurrence rates significantly decreased after the first year and remained stable in subsequent intervals, ranging from 3.4% to 5.8% per 100 PYs.
Strategies aiming to reduce these two types of recurrence should be different. The first scenario requires a clinical approach where cost-effective antibiotic selection and medication compliance measures are crucial, whereas the second involves a broader public health strategy. Reducing reinfection rate is complex as it involves improving living conditions and reducing potential environmental sources of reinfection, including consideration of interventions at the family or the village levels, and possibly vaccination[24]. In this approach, children become a challenging target group with higher therapeutic failure and higher reinfection as seen in most studies (including this meta-analysis)[25-27].
Our results are significantly influenced by two trials: The study by Morgan et al[22] as the largest trial with 1463 PYs follow up, and the study by Mera et al[19] as the cohort with the longest follow-up time. The Mera study was the only cohort followed for more than 5 years; subjects with preneoplastic gastric lesions were enrolled from a geographically circumscribed region of Colombia, and thus, the results may not be generalizable to the remainder of Latin America. Of note, the Caribbean was not represented, where the higher African ancestry, different diets and other environmental exposures may affect generalization. In this review, we observed geographic variability in H. pylori recurrence rates, as had been previously described[22]. This likely represented both regional H. pylori ecology differences, as well as socioeconomic differences in the study populations. Improved socioeconomic status in subsequent birth cohorts may help explain lower acquisition rates. For example, Chile and Peru are countries with divergent development rates, yet similar ethnography and comparable H. pylori prevalence rates-lower reinfection rates are observed in Chile[16,28]. One likely explanation is that in Chile, the generation ≥ 40 years who contracted H. pylori in their childhood and remains colonized, coexists with younger generations that have grown in improved living conditions with reduced H. pylori prevalence. This paradox of high prevalence but low reinfection rates has been previously described in Japanese patients with peptic ulcer disease[29].
In our meta-regression analysis, the findings were not significantly modified by any of the evaluated factors: Study population, H. pylori diagnostic modality, the antibiotic strategy selected, retreatment (a second antibiotic course), or the time interval to check for H. pylori eradication success. Antibiotic selection varied among different studies, but half of them used the standard triple therapy regimen. This 14-d regimen has been proven to be superior to sequential and concomitant therapy in Latin America post-eradication time, but not at the 1-year time point[22,30]. Diagnostic modalities were appropriate, and 8 out of 12 studies used two methods to diagnose H. pylori, wherein one of them was the urea breath test[31]. Studies that used endoscopy-based diagnostic methods noted higher recurrence rates, which may be an incidental finding, related to occasional iatrogenic infection, or reflect the improved sensitivity of this approach[32]. One limitation of this analysis was the study designs which were not able to differentiate whether cases were secondary to reinfection or recrudescence by molecular fingerprinting. Finally, heterogeneity was significant and there is a possibility of publication bias[33]. The Forrest plot suggested missing studies with low sample size (wide standard deviation) wherein the recurrence rates may be lower, with the exception of the Peru study, but this is attributed to the inclusion criteria of at least 50 PYs of follow-up.
The meta-analysis of studies in Latin America suggests that the H. pylori recurrence rate in the first year is 11.2 (95%CI: 6.1-16.4) per 100 person-years, and 6.2 (95%CI: 3.8-8.7) per 100 person-years in subsequent years, or approximately 50% at 7 years. Overall, the reinfection rates are lower than initially reported, making H. pylori screening and eradication a reasonable strategy for gastric cancer prevention programs in Latin America, within the context of well-designed clinical trials[18]. Further research is needed to evaluate the feasibility, cost-effectiveness, and the potential adverse outcomes (e.g., microbiome effects, antibiotic resistance) of eradication programs, while in parallel, to explore novel biomarkers and eradication strategies.
Authors would like to thank Leonardo J Tamariz for assisting with statistical analysis.
Recent trials and systematic reviews suggest that Helicobacter pylori (H. pylori) eradication may reduce the risk of gastric adenocarcinoma if the 5 to 7-year recurrence rate is less than 50%.
There is limited evidence of H. pylori recurrence after treatment outside of Asia.
The authors’ estimated 5-year and 7-year recurrence rates are similar to the recurrence rates observed in the literature, including the Shangdong study. Recurrent cases occur mostly within the first year suggesting treatment failure.
H. pylori screening and eradication in asymptomatic populations with chronic gastritis may be an attractive strategy for gastric cancer prevention in Latin America. Further research is needed to evaluate the feasibility, cost-effectiveness, and the potential adverse outcomes (e.g., microbiome effects, antibiotic resistance and stewardship) of eradication programs in the region.
This manuscript is well written and clinically interesting. Results are presented clearly and conclusions are supported by results.
| 1. | de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11891] [Article Influence: 792.7] [Reference Citation Analysis (6)] |
| 3. | Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 732] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 4. | International Agency of Cancer Research SoCI. GLOBOCAN 2008 Fact Sheet. Available from: http://globocan.iarc.fr/factsheet.asp. |
| 5. | Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, Dominguez R, Morgan D. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control. 2013;24:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Coelho LG, León-Barúa R, Quigley EM. Latin-American Consensus Conference on Helicobacter pylori infection. Latin-American National Gastroenterological Societies affiliated with the Inter-American Association of Gastroenterology (AIGE). Am J Gastroenterol. 2000;95:2688-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 2000] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 8. | Goss PE, Lee BL, Badovinac-Crnjevic T, Strasser-Weippl K, Chavarri-Guerra Y, St Louis J, Villarreal-Garza C, Unger-Saldaña K, Ferreyra M, Debiasi M. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013;14:391-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 9. | Corral JE, Delgado Hurtado JJ, Domínguez RL, Valdez de Cuéllar M, Balmore Cruz C, Morgan DR. The descriptive epidemiology of gastric cancer in Central America and comparison with United States Hispanic populations. J Gastrointest Cancer. 2015;46:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 361] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 11. | Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 451] [Article Influence: 37.6] [Reference Citation Analysis (1)] |
| 12. | Herrero R, Parsonnet J, Greenberg ER. Prevention of gastric cancer. JAMA. 2014;312:1197-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Ford AC, Forman D, Hunt R, Yuan Y, Moayyedi P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev. 2015;7:CD005583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 18060] [Article Influence: 1062.4] [Reference Citation Analysis (1)] |
| 15. | The State of Education in Latin America and the Caribbean Guartanteeing Quality Education for All. France: United Nations Educational, Scientific and Cultural Organization 2007; . |
| 16. | Gisbert JP. The recurrence of Helicobacter pylori infection: incidence and variables influencing it. A critical review. Am J Gastroenterol. 2005;100:2083-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Camargo MC, García A, Riquelme A, Otero W, Camargo CA, Hernandez-García T, Candia R, Bruce MG, Rabkin CS. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol. 2014;109:485-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 18. | Niv Y, Hazazi R. Helicobacter pylori recurrence in developed and developing countries: meta-analysis of 13C-urea breath test follow-up after eradication. Helicobacter. 2008;13:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 19. | Mera RM, Piazuelo MB, Bravo LE, Camargo CA, Bravo JC, Delgado AG, Romero-Gallo J, Yepez MC, Realpe JL, Mora Y. SB, Morgan DR, Peek RM, Wilson KT, Correa P. The gastric precancerous cascade A 16-year follow-up of a cohort of Colombian subjects with gastric precancerous lesions. Digestive Disease Week - DDW 2014. Chicago: American Gastroenterology Association - AGA 2014; . |
| 20. | Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244-1252. [PubMed] |
| 21. | Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004;53:34-37. [PubMed] |
| 22. | Morgan DR, Torres J, Sexton R, Herrero R, Salazar-Martínez E, Greenberg ER, Bravo LE, Dominguez RL, Ferreccio C, Lazcano-Ponce EC. Risk of recurrent Helicobacter pylori infection 1 year after initial eradication therapy in 7 Latin American communities. JAMA. 2013;309:578-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Peitz U, Hackelsberger A, Malfertheiner P. A practical approach to patients with refractory Helicobacter pylori infection, or who are re-infected after standard therapy. Drugs. 1999;57:905-920. [PubMed] |
| 24. | Zeng M, Mao XH, Li JX, Tong WD, Wang B, Zhang YJ, Guo G, Zhao ZJ, Li L, Wu DL. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 25. | Rowland M, Kumar D, Daly L, O’Connor P, Vaughan D, Drumm B. Low rates of Helicobacter pylori reinfection in children. Gastroenterology. 1999;117:336-341. [PubMed] |
| 26. | Kato S, Abukawa D, Furuyama N, Iinuma K. Helicobacter pylori reinfection rates in children after eradication therapy. J Pediatr Gastroenterol Nutr. 1998;27:543-546. [PubMed] |
| 27. | Alarcón T, José Martínez-Gómez M, Urruzuno P. Helicobacter pylori in pediatrics. Helicobacter. 2013;18 Suppl 1:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Khalifa MM, Sharaf RR, Aziz RK. Helicobacter pylori: a poor man’s gut pathogen? Gut Pathog. 2010;2:2. [PubMed] [DOI] [Full Text] |
| 29. | Adachi M, Mizuno M, Yokota K, Miyoshi M, Nagahara Y, Maga T, Ishiki K, Inaba T, Okada H, Oguma K. Reinfection rate following effective therapy against Helicobacter pylori infection in Japan. J Gastroenterol Hepatol. 2002;17:27-31. [PubMed] |
| 30. | Greenberg ER, Anderson GL, Morgan DR, Torres J, Chey WD, Bravo LE, Dominguez RL, Ferreccio C, Herrero R, Lazcano-Ponce EC. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet. 2011;378:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 31. | Neil GA, Suchower LJ, Ronca PD, Skoglund ML. Time of Helicobacter pylori eradication assessment following treatment. Helicobacter. 1997;2:13-20. [PubMed] |
| 32. | Tytgat GN. Endoscopic transmission of Helicobacter pylori. Aliment Pharmacol Ther. 1995;9 Suppl 2:105-110. [PubMed] |
| 33. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48684] [Article Influence: 2116.7] [Reference Citation Analysis (4)] |
| 34. | Silva FM, Navarro-Rodriguez T, Barbuti RC, Mattar R, Hashimoto CL, Eisig JN. Helicobacter pylori reinfection in Brazilian patients with peptic ulcer disease: a 5-year follow-up. Helicobacter. 2010;15:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Mesquita MA, Lorena SL, Zeitune JM, Montes CG, Guerrazzi F, Santos JO, Carvalho AF, Almeida JR. Recurrence of Helicobacter pylori infection after eradication therapy in Brazilian patients with peptic ulcer. J Clin Gastroenterol. 2005;39:447. [PubMed] |
| 36. | Coelho LG, Passos MC, Chausson Y, Costa EL, Maia AF, Brandao MJ, Rodrigues DC, Castro LP. Duodenal ulcer and eradication of Helicobacter pylori in a developing country. An 18-month follow-up study. Scand J Gastroenterol. 1992;27:362-366. [PubMed] |
| 37. | Rollan A, Giancaspero R, Fuster F, Acevedo C, Figueroa C, Hola K, Schulz M, Duarte I. The long-term reinfection rate and the course of duodenal ulcer disease after eradication of Helicobacter pylori in a developing country. Am J Gastroenterol. 2000;95:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Figueroa G, Acuña R, Troncoso M, Portell DP, Toledo MS, Albornoz V, Vigneaux J. Low H. pylori reinfection rate after triple therapy in Chilean duodenal ulcer patients. Am J Gastroenterol. 1996;91:1395-1399. [PubMed] |
| 39. | Novoa Reyes I, Caravedo Martínez M, Huerta-Mercado Tenorio J, De los Ríos Senmache R, Pinto Valdivia J, Bussalleu Rivera A. [Recurrence rate of Helicobacter pylori infection two years after successful eradication in Peruvian patients presenting with postprandial distress syndrome]. Rev Gastroenterol Peru. 2014;34:15-21. [PubMed] |
| 40. | Soto G, Bautista CT, Roth DE, Gilman RH, Velapatiño B, Ogura M, Dailide G, Razuri M, Meza R, Katz U. Helicobacter pylori reinfection is common in Peruvian adults after antibiotic eradication therapy. J Infect Dis. 2003;188:1263-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Leal-Herrera Y, Torres J, Monath TP, Ramos I, Gomez A, Madrazo-de la Garza A, Dehesa-Violante M, Muñoz O. High rates of recurrence and of transient reinfections of Helicobacter pylori in a population with high prevalence of infection. Am J Gastroenterol. 2003;98:2395-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Mohar A, Ley C, Guarner J, Herrera-Goepfert R, Figueroa LS, Halperin D, Parsonnet J. Eradication rate of Helicobacter pylori in a Mexican population at high risk for gastric cancer and use of serology to assess cure. Am J Gastroenterol. 2002;97:2530-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Sivapalasingam S, Rajasingham A, Macy JT, Friedman CR, Hoekstra RM, Ayers T, Gold B, Quick RE. Recurrence of Helicobacter pylori infection in Bolivian children and adults after a population-based “screen and treat” strategy. Helicobacter. 2014;19:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Romano M, Tepes B, Zhang L S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ