Published online Apr 15, 2017. doi: 10.4251/wjgo.v9.i4.166
Peer-review started: October 19, 2016
First decision: November 22, 2016
Revised: December 7, 2016
Accepted: February 8, 2017
Article in press: February 10, 2017
Published online: April 15, 2017
Processing time: 174 Days and 16.7 Hours
To evaluate whether a high risk macroscopic appearance (Type IV and giant Type III) is associated with a dismal prognosis after curative surgery, because its prognostic relevance remains elusive in pathological stage II/III (pStage II/III) gastric cancer.
One hundred and seventy-two advanced gastric cancer (defined as pT2 or beyond) patients with pStage II/III who underwent curative surgery plus adjuvant S1 chemotherapy were evaluated, and the prognostic relevance of a high-risk macroscopic appearance was examined.
Advanced gastric cancers with a high-risk macroscopic appearance were retrospectively identified by preoperative recorded images. A high-risk macroscopic appearance showed a significantly worse relapse free survival (RFS) (35.7%) and overall survival (OS) (34%) than an average risk appearance (P = 0.0003 and P < 0.0001, respectively). A high-risk macroscopic appearance was significantly associated with the 13th Japanese Gastric Cancer Association (JGCA) pT (P = 0.01), but not with the 13th JGCA pN. On univariate analysis for RFS and OS, prognostic factors included 13th JGCA pStage (P < 0.0001) and other clinicopathological factors including macroscopic appearance. A multivariate Cox proportional hazards model for univariate prognostic factors identified high-risk macroscopic appearance (P = 0.036, HR = 2.29 for RFS and P = 0.021, HR = 2.74 for OS) as an independent prognostic indicator.
A high-risk macroscopic appearance was associated with a poor prognosis, and it could be a prognostic factor independent of 13th JGCA stage in pStage II/III advanced gastric cancer.
Core tip: In this study, we for the first time clarify the clinicopathological relevance of the macroscopic high risk patients with pathological stage II/III gastric cancer who underwent curative surgery with postoperative S1 adjuvant chemotherapy in Japan.
- Citation: Yamashita K, Ema A, Hosoda K, Mieno H, Moriya H, Katada N, Watanabe M. Macroscopic appearance of Type IV and giant Type III is a high risk for a poor prognosis in pathological stage II/III advanced gastric cancer with postoperative adjuvant chemotherapy. World J Gastrointest Oncol 2017; 9(4): 166-175
- URL: https://www.wjgnet.com/1948-5204/full/v9/i4/166.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i4.166
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer-related deaths worldwide[1]. Advanced gastric cancer with depth of invasion of T2 or beyond continues to show unsatisfactory survival outcomes despite progress in multidisciplinary therapy, especially for postoperative S1 adjuvant therapy[2,3], while early gastric cancer is largely a curable disease[4,5]. Among advanced gastric cancers, macroscopic features and patient age were recently proven to be simple but the most potent independent prognostic factors[6]. Type IV and large type III gastric cancer have the most dismal prognosis[6-8].
The gastric cancer section of the Japan Clinical Oncology Group (JCOG) has also classified advanced gastric cancer into macroscopic high risk and average risk to conduct clinical trials to propose novel multimodal treatment strategies. Giant type III (designated as 8 cm in length or greater) and type IV gastric cancer are being proposed as high-risk gastric cancer with dismal prognoses, for which neoadjuvant chemotherapy of cisplatin/S1 (CS) may be promising as a novel therapeutic strategy[9]. This strategy may be successful because of the clinical success of neoadjuvant chemotherapy for gastric cancer in the Western world, where neoadjuvant chemotherapy with epirubicin/cisplatin/5-fluorouracil (ECF) improved progression free survival (PFS) and overall survival (OS) better than surgery alone in aggressive advanced gastric cancer; gastric cancer in Western countries has shown a more aggressive phenotype than in Eastern countries[10].
Macroscopic features have been repeatedly reported to be a prognostic factor independent of stage as earlier described[6,7], but there have been no investigations of their relevance in advanced gastric cancer patients with pathological stage II/III who underwent curative gastrectomy together with postoperative S1 adjuvant chemotherapy. In this study, the clinicopathological relevance of macroscopic high-risk with pathological stage II/III gastric cancer in patients who underwent curative surgery with postoperative S1 adjuvant chemotherapy was examined for the first time.
Between January 1, 2000, and December 31, 2010, 1673 patients underwent gastrectomy for gastric adenocarcinoma in the gastrointestinal surgery division, Kitasato University Hospital. A total of 396 patients with 13th Japanese Gastric Cancer Association (JGCA) stage II/III advanced gastric cancer underwent curative gastrectomy with D1-D2 lymph node dissection, and 67 underwent neoadjuvant chemotherapy or postoperative chemotherapy other than S-1 as previously reported[11-13]. Advanced gastric cancer was defined as pathological T2 (13th JGCA stage) or beyond, and pT1 gastric cancers were excluded from this study even when they were pathological stage II. Older age, defined as 67 years of age or older was used from a prognostic point of view from the previous reports[11]. Among the 329 patients with pStage II/III, 172 agreed to undergo adjuvant S-1 therapy after curative resection. The 172 patients who underwent adjuvant S-1 chemotherapy after surgery for at least one day were registered in the S-1 group. The clinicopathological features of the 172 patients in this study were investigated.
We participated in the ACTS-GC trial[2], and started postoperative adjuvant use of S-1 for pStage II/III gastric cancer from October, 2001. Since 2007, when the interim analysis of the trial results was disclosed and recommended annual S-1 therapy after curative operation[2], we recommended S-1 postoperative adjuvant therapy to patients with 13th JGCA pStage II/III advanced gastric cancer.
Among the 172 patients, D1 lymph node dissection (n = 26) was performed for various reasons: preoperative diagnosis of clinical T1 cancer (n = 12), omitted D2 dissection in the operative views (surgical T1) during the surgery (n = 4), omitted D2 dissection because of systemic complications (n = 6), surgery of remnant stomach cancer (n = 3); and elderly (n = 1).
The dose of S-1 was determined based on body surface area: < 1.25 m2 (80 mg daily); ≥ 1.25 m2 but < 1.50 m2 (100 mg daily); ≥ 1.50 m2 (120 mg daily). The adjuvant S-1 chemotherapy regimen was administered for 4 wk followed by 2 wk of rest. This 6-wk cycle was repeated during the first year after surgery. Toxicity of chemotherapy was assessed using Common Toxicity Criteria of the National Cancer Institute, version 4.0 (NCI-CTC)[14]. If patients had hematologic toxic effects of grade 3 or 4 or nonhematologic toxic effects of grade 2, 3 or 4, their daily dosage was reduced, or their treatment was postponed or stopped according to each physician’s judgment.
In the present study, the 13th JGCA stage classifications were used[15], because ACTS-GC was established based on this staging system. In the 13th edition, the T category is classified into four categories: T1, the depth of invasion is mucosal or submucosal; T2, the depth of invasion is muscularis propria or subserosa; T3, the depth of invasion is serosa exposed; and T4, the depth of invasion is infiltrating into other organs. On the other hand, the status of lymph node metastasis is classified into four categories according to the anatomical classification of the involved lymph nodes. The descriptions are as follows: N0, no evidence of lymph node metastasis; N1, metastasis within the first tier of lymph nodes; N2, metastasis within the second tier of lymph nodes (extra-perigastric regional lymph nodes); and N3, metastasis to the third tier of lymph nodes (extra-regional lymph nodes). The latest (7th) UICC TNM stage is shown for reference purposes.
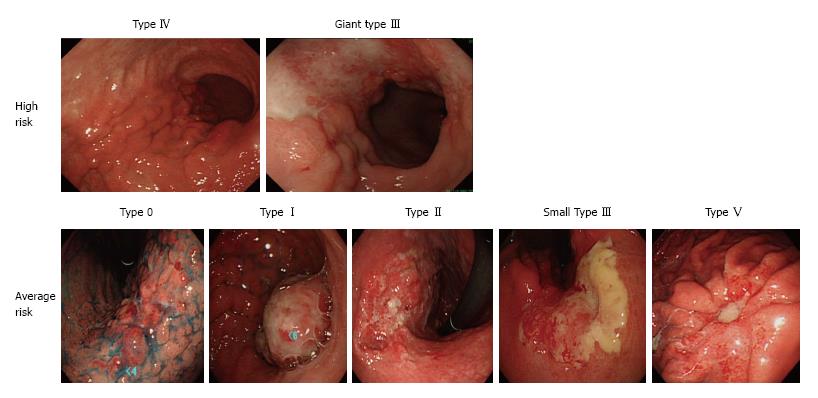
Macroscopic features were retrospectively determined by gastro-endoscopy based on the 13th JGCA classification[15] in combination with computed tomography (CT). Type 0, mucosal or submucosal; Type I, polypoid; Type II, fungating, ulcerated with sharp raised margins; Type III, ulcerated with poorly defined infiltrative margins; Type IV, infiltrative, predominantly intramural lesion, poorly demarcated; Type V, unclassified features. Representative tumors were shown in Figure 1. Giant type III was defined by its maximal diameter (8 cm or greater) assessed by upper gastrointestinal (UGI) barium contrast series as recently described[8].
All histologic and clinicopathological factors were assessed independently and blindly by any of 20 well trained histopathologists. Lymphatic invasion (ly) and vascular invasion (v) were defined as ly0, 1, 2, and 3 and v0, 1, 2, and 3 by infiltrative grade. Histologically, there are two major types of gastric adenocarcinoma (Lauren’s classification). In this study, cancers were classified into diffuse type (por1, por2, sig, muc) and intestinal type (pap, tub1, tub2).
Cumulative 5-year OS was estimated by the Kaplan-Meier method, and statistical differences were tested by the log rank test. OS was measured from the date of surgery to the date of death or the last follow-up. Fatal cases in the analysis of OS included those who died from causes other than gastric cancer. Cumulative 5-year relapse free survival (RFS) was estimated by the Kaplan-Meier method, and statistical differences were tested by the log-rank test. RFS was measured from the date of surgery to the date of recurrence or the last follow-up. Deaths from other reasons were not defined as events for RFS.
Blood tests and physical examinations were done every 3 mo and imaging examinations were performed every 6 mo. Blood tests included a complete blood count and serum biochemistry including tumor markers such as CEA, CA19-9, and CA125. Diagnosis of recurrences was based on clinical reports of radiologists with reference to clinical findings (symptoms and blood test) or histological findings.
The median observation was 56 mo (range, 11 to 122 mo). Variables that had prognostic potential on univariate analysis (P < 0.05) were subjected to multivariate analysis with a Cox proportional hazards regression model. A value of P < 0.05 was considered significant. All statistical analyses were done with JMP, version 11 (SAS Institute, Cary, NC).
Prognosis of advanced gastric cancer patients with pathological stage II/III who underwent curative surgery followed by S1 postoperative adjuvant chemotherapy
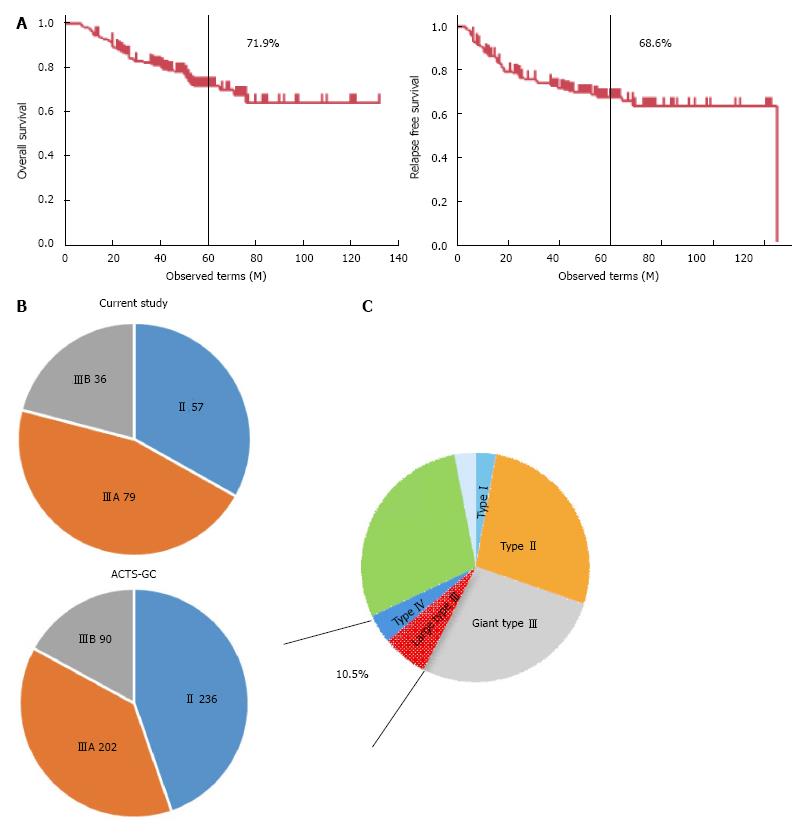
The prognosis of gastric cancer patients with pathological stage II/III who underwent curative gastrectomy followed by S1 adjuvant chemotherapy was investigated first. Pathological stage II/III cases did not include those with pathological stage II T1 gastric cancer. Five-year OS and 5-year RFS were 71.9% and 68.6%, respectively (Figure 2A). These survival rates are almost the same as the survival outcomes in the ACTS-GC trial (71.7% and 65.4%)[3]. On the other hand, the stage distribution included a lower rate of stage II gastric cancer and a higher rate of stage III gastric cancer than in the ACTS-GC trial (Figure 2B). These findings indicated that the patient population treated in our institute included more advanced gastric cancer than the ACTS-GC trial.
Classification of macroscopic features in pathological stage II/III advanced gastric cancer
Retrospective diagnosis with regard to the macroscopic features of gastric cancer was done by review of the recorded gastroscopic images in combination with the CT scan images (if primary tumors were visible on CT scan images, they were considered type I to IV macroscopic features, not type 0 macroscopic features). Among the type III macroscopic features, maximal tumor size was assessed by UGI series, and tumors with size of 8 cm or beyond were defined as giant type III gastric cancers as previously described[8]. As a result, high risk macroscopic features (type IV and giant type III) were identified in 18 cases (10.5%) (Figure 2C).
Multivariate Cox proportional hazards model for RFS identified macroscopic high risk as an independent prognostic factor in pathological stage II/III gastric cancer
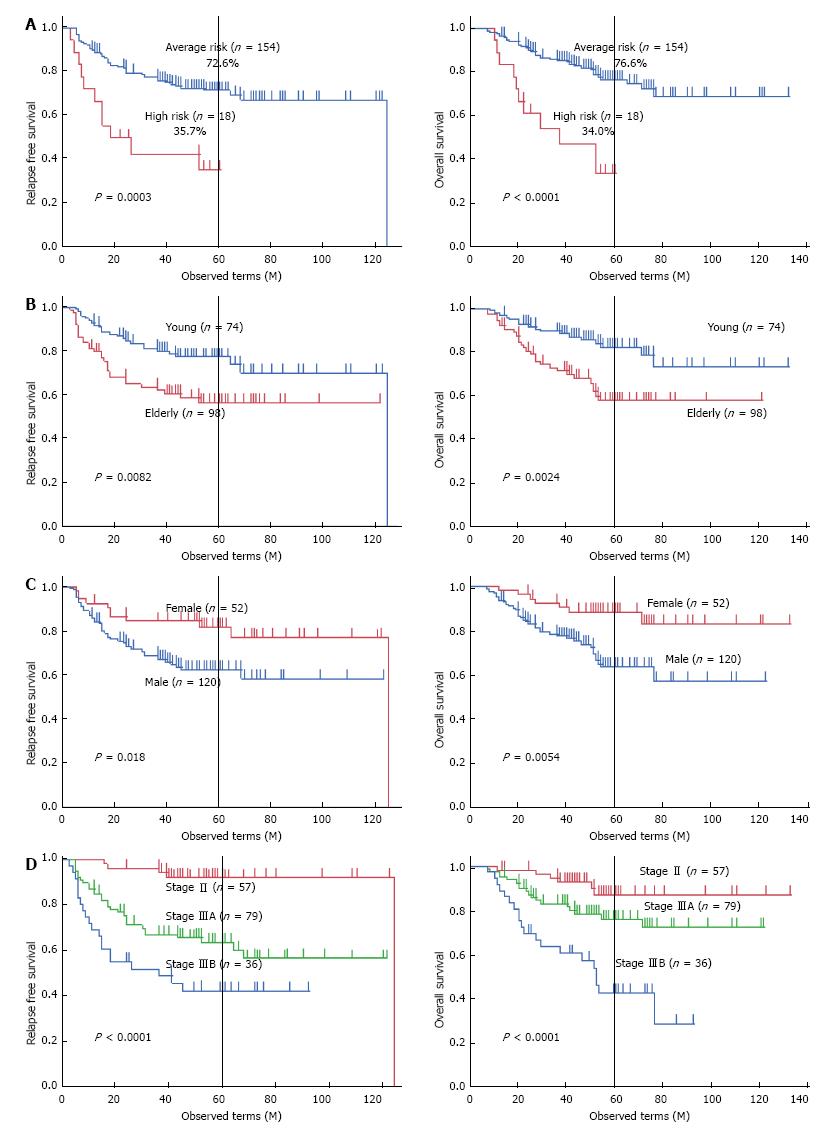
RFS was compared with regard to various clinicopathological factors including macroscopic features (Table 1). There was a significant difference in RFS (P = 0.0003) between macroscopic high risk gastric cancer and average risk gastric cancer (Figure 3A). Five-year RFS of macroscopic high-risk gastric cancer was 35.7%, while that of average-risk gastric cancer was 72.6%. Other negative prognostic factors were older age (P = 0.0082), male sex (P = 0.018), 13th JGCA pT (P = 0.019), 13th JGCA pN (P = 0.0043), and 13th JGCA stage (P < 0.0001). These significant prognostic factors for RFS excluding TNM factor components were applied to a multivariate Cox proportional hazards model, which identified the 13th JGCA stage (P < 0.0001), macroscopic high risk (P = 0.036), sex (P = 0.031), and age (P = 0.029) as independent prognostic factors as shown in Table 2. Kaplan-Meier survival curves are shown in terms of age (left panel of Figure 3B), sex (left panel of Figure 3C), and 13th JGCA stage (left panel of Figure 3D).
| Clinicopaghological factors | Classification | Number | Univariate analysis (5-yr RFS) | Univariate analysis (P value) | 5-yr OS | P value |
| Age | Young | 74 | 77.40% | 0.0082 | 82.30% | 0.0024 |
| Elderly | 98 | 56.90% | 58.10% | |||
| Sex | Male | 120 | 62.50% | 0.018 | 63.50% | 0.0054 |
| Female | 52 | 81.90% | 83.10% | |||
| Tumor location | Upper | 54 | 59.60% | 0.12 | 81.10% | 0.027 |
| Middle | 74 | 68.40% | 76.10% | |||
| Lower | 44 | 80.50% | 59.20% | |||
| Method | Total | 100 | 67.40% | 0.51 | 69.00% | 0.18 |
| Distal | 72 | 70.00% | 76.00% | |||
| Lymphadenectomy | D1 | 10 | 68.60% | 0.95 | 56.00% | 0.53 |
| D1+ | 16 | 64.30% | 79.60% | |||
| D2 | 146 | 69.00% | 72.20% | |||
| Laparoscopic | Yes | 25 | 77.30% | 0.16 | 77.30% | 0.2 |
| No | 147 | 67.00% | 70.90% | |||
| Splenectomy | Yes | 51 | 61.50% | 0.2 | 65.80% | 0.31 |
| No | 121 | 71.50% | 74.50% | |||
| Transfusion | Yes | 23 | 63.90% | 0.58 | 68.00% | 0.37 |
| No | 149 | 69.30% | 72.70% | |||
| 13th JGCA pT | T2 | 65 | 80.30% | 0.019 | 83.00% | 0.021 |
| T3 | 105 | 61.90% | 65.80% | |||
| T4 | 2 | 50.00% | 50.00% | |||
| 13th JGCA pN | N0 | 24 | 90.50% | 0.0043 | 89.40% | 0.022 |
| N1 | 82 | 74.00% | 77.30% | |||
| N2 | 66 | 54.30% | 59.10% | |||
| 13th JGCA pStage | II | 57 | 92.10% | < 0.0001 | 86.80% | < 0.0001 |
| IIIA | 79 | 63.90% | 76.00% | |||
| IIIB | 36 | 43.00% | 42.80% | |||
| Lauren histology | Intestinal | 60 | 63.60% | 0.23 | 68.30% | 0.27 |
| Diffuse | 112 | 71.30% | 74.20% | |||
| INF | Alpha | 13 | 76.90% | 0.83 | 84.60% | 0.53 |
| Beta | 75 | 69.90% | 77.50% | |||
| Gamma | 84 | 66.40% | 67.50% | |||
| Lymphatic invasion | ly0 | 9 | 100.00% | 0.2 | 100.00% | |
| ly1 | 45 | 74.80% | 77.10% | 0.19 | ||
| ly2 | 62 | 63.70% | 71.20% | |||
| ly3 | 56 | 63.90% | 63.60% | |||
| Vascular invasion | v0 | 16 | 87.10% | 0.055 | 85.60% | 0.043 |
| v1 | 55 | 69.90% | 65.20% | |||
| v2 | 55 | 72.80% | 83.50% | |||
| v3 | 46 | 55.70% | 62.70% | |||
| Macroscopic feature | High risk | 18 | 35.70% | 0.0003 | 34.00% | < 0.0001 |
| Average risk | 154 | 72.60% | 76.60% |
| Clinicopaghological factors | Classification | Number | Multivariate analysis for PFS (Hazard ratio) | Multivariate analysis for OS (95%CI) | P value | Hazard ratio | 95%CI | P value |
| Age | Young | 74 | Reference | 0.029 | Reference | 0.008 | ||
| Elderly | 98 | 1.83 | 1.07-3.20 | 2.35 | 1.25-4.58 | |||
| Sex | Male | 120 | Reference | 1.06-4.34 | 0.031 | Reference | 0.87-4.91 | 0.11 |
| Female | 52 | 2.05 | 1.93 | |||||
| Tumor location | Upper | 54 | 2.84 | 1.24-7.19 | 0.013 | |||
| Middle | 74 | 1.73 | 0.70-4.66 | 0.24 | ||||
| Lower | 44 | Reference | ||||||
| 13th JGCA pStage | II | 57 | Reference | Reference | ||||
| IIIA | 79 | 6.17 | 2.42-20.83 | < 0.0001 | 2.25 | 0.93-6.27 | 0.08 | |
| IIIB | 36 | 8.48 | 3.11-29.70 | < 0.0001 | 4.81 | 1.79-14.72 | 0.002 | |
| Vascular invasion | v0 | 16 | Reference | |||||
| v1 | 55 | 1.46 | 0.38-9.55 | 0.62 | ||||
| v2 | 55 | 0.71 | 0.17-4.80 | 0.68 | ||||
| v3 | 46 | 1.34 | 0.33-9.01 | 0.71 | ||||
| Macroscopic feature | High risk | 18 | 2.29 | 1.06-4.63 | 0.036 | 2.74 | 1.17-6.15 | 0.021 |
| Average risk | 154 | Reference | Reference |
Multivariate Cox proportional hazards model for OS identified macroscopic high risk as an independent prognostic factor in pathological stage II/III gastric cancer
OS was compared with regard to various clinicopathological factors including macroscopic features (Table 1). There was significant difference in OS (P < 0.0001) between macroscopic high risk gastric cancer and average risk gastric cancer (Figure 3A). Five-year OS of macroscopic high risk gastric cancer was 34.0%, while that of average-risk gastric cancer was 76.6%. Other negative prognostic factors were older age (P = 0.0024), male sex (P = 0.0054), tumor location (P = 0.027), 13th JGCA pT (P = 0.021), 13th JGCA pN (P = 0.022), 13th JGCA stage (P < 0.0001), and vascular permeation (P = 0.043). These significant prognostic factors for OS excluding each TNM factor components were applied to the multivariate Cox proportional hazards model, which identified the 13th JGCA stage (P = 0.0015), macroscopic high risk (P = 0.021), age (P = 0.0082), and tumor location (P = 0.013) as independent prognostic factors as shown in Table 2. Each TNM factor was excluded, because these 3 factors are confounders for stage definition. Kaplan-Meier survival curves are shown in terms of age (right panel of Figure 3B), sex (right panel of Figure 3C), and 13th JGCA stage (right panel of Figure 3D).
Clinicopathological features of macroscopic high risk among pathological stage II/III gastric cancer patients who underwent standard treatment
Clinicopathological backgrounds with regard to the negative prognostic factors were then compared between the high-risk group and the average-risk group (Table 3). The macroscopic high-risk group included more patients with higher pathological T (P = 0.0025), and higher 13th JGCA pathological stage (P = 0.0004), while there were no significant differences in pN distribution and lymph node dissection level between the macroscopic high-risk group and the average-risk group. In our previous reports, lymph node dissection level was proven not to affect prognosis in these 172 cases[11].
| Clinicopaghological factors | Classification | Number | High risk gastric cancer n = 18 | Average risk gastric cancer n = 154 | P value |
| Age | Young | 74 | 8 | 66 | 0.26 |
| Elderly | 98 | 10 | 88 | ||
| Sex | Male | 120 | 13 | 107 | 0.81 |
| Female | 52 | 5 | 47 | ||
| Lymphadenectomy | D1 | 26 | 4 | 22 | 0.37 |
| D2 | 146 | 14 | 132 | ||
| Tumor location | Upper | 54 | 3 | 51 | 0.32 |
| Middle | 74 | 9 | 65 | ||
| Lower | 44 | 6 | 38 | ||
| 13th JGCA pT | T2 | 65 | 1 | 64 | 0.0025 |
| T3 | 105 | 17 | 88 | ||
| T4 | 2 | 0 | 2 | ||
| 13th JGCA pN | N0 | 24 | 2 | 22 | 0.11 |
| N1 | 82 | 5 | 77 | ||
| N2 | 66 | 11 | 55 | ||
| 13th JGCA pStage | II | 57 | 3 | 54 | 0.0004 |
| IIIA | 79 | 4 | 76 | ||
| IIIB | 36 | 11 | 25 | ||
| 7th UICC pT | T2 | 29 | 0 | 29 | 0.02 |
| T3 | 36 | 1 | 35 | ||
| T4a | 105 | 17 | 88 | ||
| T4b | 2 | 0 | 2 | ||
| 7th UICC pN | N0 | 24 | 2 | 22 | 0.08 |
| N1 | 45 | 1 | 44 | ||
| N2 | 40 | 4 | 36 | ||
| N3 | 63 | 11 | 52 | ||
| 7th UICC pStage | IIA | 13 | 0 | 13 | < 0.0001 |
| IIB | 37 | 2 | 35 | ||
| IIIA | 46 | 2 | 44 | ||
| IIIB | 38 | 3 | 35 | ||
| IIIC | 38 | 11 | 27 | ||
| Vascular invasion | v0 | 16 | 1 | 15 | 0.94 |
| v1 | 55 | 6 | 49 | ||
| v2 | 55 | 6 | 49 | ||
| v3 | 46 | 5 | 41 |
Recurrent cases were seen in 11 out of 18 cases with macroscopic high risk (Table 4). The 11 cases were composed of 7 giant type III gastric cancers and 4 type IV gastric cancers. Giant type III gastric cancer tended to have extra-regional lymph node recurrences, while type IV gastric cancer had peritoneal dissemination. We recently reported RTKs expression in gastric cancer, and HER3 and EGFR were of prognostic relevance in pathological stage II/III advanced gastric cancer[12]. The expression patterns of RTKs such as EGFR, HER2, HER3, IGF1R and EphA2 are also included in Table 4 from the previous studies[12]. Among the 11 recurrent cases, 9 showed strong expression (2+/3+) of EGFR, and 10 showed positive immunostaining (1+/2+) for HER3, which were both remnant independent prognostic factors in pathological stage II/III advanced gastric cancer[12].
| Case | Age | Sex | 13th JGCA pT | 13th JGCA pN | 13th JGCA pStage | Macroscopic features | EGFR | HER2 | HER3 | IGF1R | EphA2 | Initial recurrences |
| 1 | 74 | M | 3 | 2 | IIIB | Giant type III | 2+ | 1+ | 2+ | 2+ | 1+ | #16 LN |
| 2 | 62 | M | 3 | 2 | IIIB | Giant type III | 2+ | 0+ | 1+ | 2+ | 0+ | #20 LN |
| 3 | 79 | F | 3 | 2 | IIIB | Giant type III | 2+ | 1+ | 1+ | 0+ | 1+ | #13 LN |
| 4 | 68 | M | 3 | 2 | IIIB | Giant type III | 3+ | 1+ | 1+ | 1+ | 0+ | #16,13 |
| 5 | 68 | M | 3 | 2 | IIIB | Giant type III | 3+ | 0+ | 1+ | 1+ | 1+ | #13 LN |
| 6 | 45 | M | 3 | 2 | IIIB | Giant type III | 2+ | 3+ | 2+ | 0+ | 2+ | #13 LN |
| 7 | 69 | M | 3 | 2 | IIIB | Giant type III | 3+ | 3+ | 1+ | 1+ | 2+ | liver |
| 8 | 71 | M | 3 | 1 | IIIA | Type IV | 2+ | 0+ | 1+ | 1+ | 0+ | #13 LN |
| 9 | 69 | F | 3 | 1 | IIIA | Type IV | 2+ | 0+ | 2+ | 1+ | 0+ | Peritoneum |
| 10 | 69 | M | 3 | 1 | IIIA | Type IV | 1+ | 2+ | 0+ | 1+ | 1+ | Peritoneum |
| 11 | 59 | F | 3 | 2 | IIIB | Type IV | 1+ | 1+ | 1+ | 1+ | 0+ | Peritoneum |
This study reported for the first time the outcomes of macroscopic high-risk gastric cancer (giant type III and type IV) treated by “local” standard therapy in Japan (or partly in some Asian countries) in stage II/III advanced gastric cancer. The ACTS-GC trial demonstrated that postoperative S1 chemotherapy could improve the prognosis of pathological stage II/III advanced gastric cancer[2,3], but there has been no report on the prognosis of macroscopic high risk gastric cancer patients with pathological stage II/III who underwent standard treatment. In this study, 5-year RFS and OS of the macroscopic high-risk group were 35.7% and 34.0%, respectively, and the prognosis of gastric cancer patients with macroscopic high-risk was significantly poorer than that of those with average risk (72.6% and 76.6%, respectively). These results suggest that the present S1 postoperative chemotherapy is not sufficient to control such high risk disease, and novel therapeutic strategies are needed.
In the Western world, perioperative ECF chemotherapy has been shown to improve survival of gastric cancer patients when, ECF chemotherapy was compared to surgery alone[10]. Gastric cancer with ECF chemotherapy showed 5-year OS of 36.3%, compared to 23.0% for surgery alone. This outcome is totally different from average-risk advanced gastric cancer in the Eastern world, with an OS of 60%-70% of OS, whereas it is similar to gastric cancer with macroscopic high-risk. In the present cases, gastric cancer patients who were peritoneal cytology test-positive were excluded, because it represents stage IV in Japan, while the MAGIC trial may have included cytology test positive cases. In any case, the MAGIC trial demonstrated that potent preoperative chemotherapy has a great clinical potential in aggressive gastric cancer. In Japan, preoperative neoadjuvant chemotherapy was evaluated to validate the actual clinical effects including improvement of prognosis in very limited gastric cancer such as macroscopic high risk gastric cancer, namely giant type III and type IV gastric cancer[9]; CS (cisplatin/S1) neoadjuvant chemotherapy was proposed as an effective regimens in gastric cancer with macroscopic high risk, and 5-year survival was recently reported to be around 30% in JCOG0210. This is inferior to our standard therapy results, likely because peritoneal cytology test negativity was not mandatory to register in JCOG0210.
Neoadjuvant therapy is a promising therapeutic strategy for giant type III and type IV gastric cancer. We have developed a docetaxel/cisplatin/S1 (DCS) chemotherapeutic regimen in metastatic gastric cancer[16], and KDOG1001 was developed to validate the clinical effect of DCS NAC in aggressive gastric cancer including giant type III and type IV. We are registering patients in this clinical phase II trial for such high-risk patients, and registration has almost been completed. DCS was recently compared to CS in neoadjuvant settings in high-risk gastric cancer with bulky N2 disease in JCOG1002, and detailed results of the clinical outcomes will be available soon. The first report of patients with high-risk advanced gastric cancer who underwent CS neoadjuvant chemotherapy should appear in April, 2017. Such potent chemotherapy would have a promising potential to improve the prognosis of aggressive gastric cancer.
Another therapeutic strategy we can propose in such aggressive gastric cancer is long-term postoperative adjuvant S1 chemotherapy[17,18]. Gastric cancer that was cytology test-positive (CY1) or type IV showed a dismal prognosis, but detailed prognostic analysis showed that there were long-term survivors among the patients who underwent long-term postoperative adjuvant S1 chemotherapy. Okuyama et al[19] actually showed that 2-year administration of postoperative chemotherapy showed a better prognosis than 1-year administration in gastric cancer. This strategy might be very promising due to its easy feasibility, and should be considered as another therapeutic option. Giant type III and type IV gastric cancers are unique in their recurrence patterns, because minimal residual peritoneal disease is fundamental with regard to disease progression[8,18]. This means that minimal residual disease of the peritoneum should be a primary therapeutic target. S1 is more effective against peritoneal disease than against other distant metastases such as liver metastases[2] due to unknown mechanisms, thus, long-term S1 administration may be a reasonable rationale in macroscopic high-risk gastric cancer.
We previously identified HER3 immunostaining positive (defined as +1/+2 immunostaining) as an independent prognostic factor, and HER3 could be a promising therapeutic target[12]. HER2 immunostaining (defined as +3 immunostaining) is the well-established molecular target in far advanced gastric cancer using trastuzumab[20], but HER2-positive cases are infrequently found in recurrent gastric cancer[12]. Even in high-risk advanced gastric cancer, HER2-positive cases were infrequently seen (Table 4), while HER3-positive cases were frequently found. Moreover, EGFR-positive (defined as +2/+3) together with HER3-positive showed a dismal prognosis in advanced gastric cancer with pathological stage II/III[12], and EGFR-positive together with HER3-positive was found in 9 of 11 recurrent cases among the high-risk advanced gastric cancer patients in this study. We are now investigating in vitro efficacy for tumor reduction by using cetuximab together with HER3 antibody. The combination treatments could have potential in the recurrent cases of high-risk gastric cancer.
The limitations of this study were that it was a single-center study, and the follow-up period was insufficient for definitive conclusions. Moreover, the sample size was small, especially for high-risk advanced gastric cancer. If this result is validated in a larger sample size in the near future, the conclusions would be strengthened. Moreover, this study only collected patients who underwent curative surgery plus adjuvant S1 chemotherapy, we didn’t mention if these results can be seen from other patients with advanced gastric cancer.
In conclusion, this study demonstrated for the first time that macroscopic high-risk gastric cancer showed a poorer prognosis than average risk gastric cancer, and a novel therapeutic strategy should be urgently developed in order to improve outcomes in such cases in the near future.
High risk macroscopic appearance (giant type III and type IV) is known to show dismal prognosis in advanced gastric cancer, however it remains elusive whether it is true or not in advanced gastric cancer who underwent curative gastrectomy and the latest evidenced postoperative S1 adjuvant chemotherapy.
This study investigated whether the high risk macroscopic appearance could be an independent prognostic factor in advanced gastric cancer who underwent curative gastrectomy and postoperative adjuvant chemotherapy.
Macroscopic appearance can be preoperatively diagnosed, and it could be designated as a kind of preoperative surrogate marker for prognosis.
Macroscopic appearance is a good candidate for promising therapeutic strategy of neoadjuvant chemotherapy, the novel method in East Asia, if it is true.
The size of the giant type III gastric cancer is defined as 8 cm or beyond in the preoperative imaging such as endoscopy, upper gastrointestinal series, and/or computed tomography.
Yamashita et al presented a study title as “Macroscopic appearance of Type IV and giant Type III is a high risk for a poor prognosis in pathological stage II/III advanced gastric cancer with postoperative adjuvant chemotherapy”. The study has some new and interesting findings which authors believe they add some contribution to the literature. Authors were well summarized results, they have novel findings and discussion was pretty good.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86. |
| 2. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1980] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 3. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1119] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 4. | Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Validation of staging systems for gastric cancer. Gastric Cancer. 2008;11:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Yamashita K, Sakuramoto S, Nemoto M, Shibata T, Mieno H, Katada N, Kikuchi S, Watanabe M. Trend in gastric cancer: 35 years of surgical experience in Japan. World J Gastroenterol. 2011;17:3390-3397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 6. | Yamashita K, Sakuramoto S, Katada N, Kikuchi S, Watanabe M. Simple prognostic indicators using macroscopic features and age in advanced gastric cancer. Hepatogastroenterology. 2014;61:512-517. [PubMed] |
| 7. | Yamashita K, Hosoda K, Katada N, Moriya H, Mieno H, Higuchi K, Sasaki T, Katada C, Sakuramoto S, Tanabe S, Koizumi W, Kikuchi S, Watanabe M. Survival outcome of Borrmann type IV gastric cancer potentially improved by multimodality treatment. Anticancer Res. 2015;35:897-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Hosoda K, Yamashita K, Katada N, Moriya H, Mieno H, Sakuramoto S, Kikuchi S, Watanabe M. Preoperative tumor size is a critical prognostic factor for patients with Borrmann type III gastric cancer. Surg Today. 2015;45:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4739] [Article Influence: 237.0] [Reference Citation Analysis (7)] |
| 11. | Ema A, Yamashita K, Sakuramoto S, Wang G, Mieno H, Nemoto M, Shibata T, Katada N, Kikuchi S, Watanabe M. Lymph node ratio is a critical prognostic predictor in gastric cancer treated with S-1 chemotherapy. Gastric Cancer. 2014;17:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Ema A, Yamashita K, Ushiku H, Kojo K, Minatani N, Kikuchi M, Mieno H, Moriya H, Hosoda K, Katada N. Immunohistochemical analysis of RTKs expression identified HER3 as a prognostic indicator of gastric cancer. Cancer Sci. 2014;105:1591-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Ema A, Waraya M, Yamashita K, Kokubo K, Kobayashi H, Hoshi K, Shinkai Y, Kawamata H, Nakamura K, Nishimiya H. Identification of EGFR expression status association with metastatic lymph node density (ND) by expression microarray analysis of advanced gastric cancer. Cancer Med. 2015;4:90-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. USA: Department of Health and Human Services, National Institutes of Health, National Cancer Institute 2009; . |
| 15. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma. 2nd English ed. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 964] [Reference Citation Analysis (0)] |
| 16. | Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, Takagi S, Azuma M, Ae T, Ishido K. A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601). Cancer Chemother Pharmacol. 2012;69:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Yamashita K, Ushiku H, Katada N, Hosoda K, Moriya H, Mieno H, Kikuchi S, Hoshi K, Watanabe M. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol. 2015;41:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Yamashita K, Hosoda K, Katada N, Moriya H, Mieno H, Higuchi K, Sasaki T, Katada C, Sakuramoto S, Tanabe S. Survival outcome of Borrmann type IV gastric cancer potentially improved by multimodality treatment. Anticancer Res. 2015;35:897-906. [PubMed] |
| 19. | Okuyama T, Korenaga D, Edagawa A, Itoh S, Oki E, Kawanaka H, Ikeda Y, Kakeji Y, Tateishi M, Tsujitani S. Prognostic effects of oral anti-cancer drugs as adjuvant chemotherapy for 2 years after gastric cancer surgery. Surg Today. 2012;42:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5537] [Article Influence: 346.1] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bilir C, Jagric T, Sitarz R, Wang WB S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ