Published online Dec 15, 2017. doi: 10.4251/wjgo.v9.i12.475
Peer-review started: June 5, 2017
First decision: July 3, 2017
Revised: July 8, 2017
Accepted: September 4, 2017
Article in press: September 4, 2017
Published online: December 15, 2017
Processing time: 198 Days and 5.4 Hours
To compare the effectiveness of laparoscopic complete mesocolic excision (CME) with central vascular ligation (L-CME) with its open (O-CME) counterpart.
We conducted an electronic search of the PubMed/MEDLINE, Excerpta Medica Database, Web of Science Core Collection, Cochrane Center Register of Controlled Trails, Cochrane Database of Systematic Reviews, SciELO, and Korean Journal databases from their inception until May 2017. We considered randomized controlled trials (RCTs) and controlled clinical trials (CCTs) that included patients with colonic cancer comparing L-CME and O-CME. Primary outcomes included the quality of the resected specimen (lymph nodes retrieved, complete mesocolic plane excision, tumor to arterial high tie, resected mesocolon surface). Secondary outcomes included the three-year and five-year overall and disease-free survival rates, recurrence of the disease, surgical data, and postoperative morbidity and mortality. Two authors of the review screened the methodological quality of the eligible trials and independently extracted data from individual studies.
A total of one RCT and eleven CCTs (four from Europe and seven from Asia) met the inclusion criteria for the current meta-analysis. These studies involved 1619 patients in L-CME and 1477 patients in O-CME. The L-CME was associated with the same quality of the resected specimen, with no differences regarding the retrieved lymphnodes (MD = -1.06, 95%CI: -3.65 to 1.53, P = 0.42), and tumor to high tie distance (MD = 14.26 cm, 95%CI: -4.30 to 32.82, P = 0.13); the surface of the resected mesocolon was higher in the L-CME group (MD = 11.75 cm2, 95%CI: 9.50 to 13.99, P < 0.001). The L-CME was associated with a lower rate of blood transfusions (OR = 0.45, 95%CI: 0.27 to 0.75, P = 0.002), faster recovery of gastrointestinal function, and less postoperative overall complication rate. The L-CME approach was associated with a statistical significant better three-year overall (OR = 2.02, 95%CI: 1.31 to 3.12, P = 0.001, I2 = 28%) and disease-free (OR = 1.45, 95% CI: 1.00 to 2.10, P = 0.05, I2 = 0%) survival.
The laparoscopic approach offers the same quality of the resected specimen as the open approach in complete mesocolic excision with central vascular ligation for colon cancer. The laparoscopic complete mesocolic excision with central vascular ligation is superior in all perioperative results and at least non-inferior in long-term oncological outcomes.
Core tip: The laparoscopic complete mesocolic excision with central vascular ligation was associated with the same quality of the resected specimen, with no differences regarding the retrieved lymphnodes, and tumor to high tie distance; the surface of the resected mesocolon was higher in the laparoscopic group. Laparoscopy was associated with a lower rate of blood transfusions, faster recovery of gastrointestinal function, and less postoperative overall complication rate. The laparoscopic approach was associated with a statistical significant better three-year overall and disease-free survival.
- Citation: Negoi I, Hostiuc S, Negoi RI, Beuran M. Laparoscopic vs open complete mesocolic excision with central vascular ligation for colon cancer: A systematic review and meta-analysis. World J Gastrointest Oncol 2017; 9(12): 475-491
- URL: https://www.wjgnet.com/1948-5204/full/v9/i12/475.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i12.475
Complete mesocolic excision (CME) with central vascular ligation (CVL) represents an extension to the colonic cancer of the already standardized resection for rectal cancer. It adheres to the same guiding principle that sharp surgical dissection, following embryological planes, with central vascular ligation, should improve oncological outcomes[1].
Hohenberger et al[2] (2007) published the technical details of a new concept termed CME and central ligation for colonic cancer. During CME with CVL for right-sided tumors, the ileocolic and right colic vessels should be ligated at their origin from the superior mesenteric artery. Transverse colon tumors require transection of the middle colic artery at its origin. Left-sided tumors require transection of the inferior mesenteric artery (IMA) at its origin from the aorta[3]. Using CME and CVL, Hohenberger et al[4] reported a reduction of the local five-year recurrence rate from 6.5% to 3.6% and an increase in the cancer-related five-year survival rate from 82.1% to 89.1%. This specimen-oriented technique is associated with the removal of more tissue compared with standard surgery, a wider distance from the tumor to the high vascular tie (131 mm vs 90 mm, P < 0.0001), a longer length of large bowel (314 mm vs 206 mm, P < 0.0001), a wider area of removed mesentery (19657 mm2vs 11829 mm2, P < 0.0001) and a greater lymph node yield (30 vs 18, P < 0.0001)[5]. These differences may partially explain the higher reported survival rates with CME and CVL.
One should note the similarities between D3 lymphadenectomy, recommended as a standard of care for stage II and III colon cancer in Eastern countries, and Western CME[3,6]. The Japanese nomenclature includes D1 as pericolic (close to the bowel wall), D2 as intermediate (along the feeding artery), and D3 as main (at the origin of the feeding artery) lymph nodes. For right-sided tumors, a D3 lymphadenectomy requires the transection of the feeding arteries next to their origin from the superior mesenteric artery. In left-sided cancers, a D3 lymphadenectomy requires transection of the IMA close to its aortic origin[7].
Current evidence is consistent with a faster postoperative recovery for laparoscopic colectomies compared with the open approach; the former is not associated with any negative impact regarding local recurrence and survival rates. Therefore, according to the latest National Comprehensive Cancer Network guidelines, the laparoscopic approach is preferred given access to a surgeon with experience in advanced minimally invasive procedures[8].
The objective of this systematic review and meta-analysis is to summarize the current evidence regarding laparoscopic CME (L-CME) and to compare its effectiveness with its open (O-CME) counterpart.
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement[9]. Electronic search, study selection, data extraction, and quality assessment was performed independently by two reviewers.
We conducted an electronic search to identify all published randomized controlled trials (RCTs) and controlled clinical trials (CCTs) using the following databases: United States National Library of Medicine - National Institutes of Health PubMed/MEDLINE, Excerpta Medica Database, Web of Science Core Collection, Cochrane Center Register of Controlled Trails (CENTRAL), Cochrane Database of Systematic Reviews, SciELO, and Korean Journal databases from their inception until May 2017. We did not use any language restrictions. The most recent search in PubMed was performed in May 2017.
We constructed the search strategy using various combinations of terms related to CME or D3 lymphadenectomy using an open or laparoscopic approach to colon cancer. We used in different combinations the following key words: colon, cancer, complete mesocolic excision, central vascular ligation, D3 lymphadenectomy, minimally invasive, laparoscopy, open, surgery, colectomy and resection. These words were identified as truncated words in the title, abstract, or in the medical subject headings (MeSH). We additionally used electronic and manual cross-referencing to find other relevant sources. The search strategy used in PubMed/Medline was: [colon (MeSH Terms)] OR colonic (Title/Abstract) OR lower intestinal (Title/Abstract) OR large bowel (Title/Abstract) AND cancer (MeSH Terms) OR neoplasia (Title/Abstract) OR neoplasm (Title/Abstract) OR tumor (Title/Abstract) AND laparoscopy(MeSH Terms) OR minimally invasive (Title/Abstract) OR laparoscopic (Title/Abstract) AND complete mesocolic excision (Title/Abstract) OR central vascular ligation (Title/Abstract) OR D3 lymphadenectomy (Title/Abstract).
Study eligibility criteria: We considered RCTs and CCTs comparing open with laparoscopic CME or D3 lymphadenectomy as eligible for inclusion if they included patients with colonic cancer.
Primary outcome: Quality of the resected specimen (lymph nodes retrieved, complete mesocolic plane excision, tumor to arterial high tie, resected mesocolon surface).
Secondary outcomes: Three-year and five-year overall and disease-free survival rates, recurrence of the disease, surgical data (operation time, length of the abdominal incision, conversion rate), intraoperative complications, blood loss, postoperative complications (anastomotic leakage, wound infections, overall complications), length of hospital stay, thirty-day mortality, immunologic response, quality of life, and cost.
Two authors[10] (Negoi and Hostiuc) assessed the methodological quality of the eligible trials and independently extracted data from individual studies using a data-extraction form. We extracted the following data: Year of publication, source, title, first author, contact address, criteria for patient inclusion and exclusion, sample size, baseline characteristics, and patient characteristics including mean age, sex ratio, location of the tumor, number of patients assigned to each treatment group, and details of the intervention regimens. We registered the following outcomes: One-, three- and five-year overall and disease-free survival rates, number of removed lymph nodes, length of the resected colon, resection of the mesocolic plane, operation time, length of hospital stay, number and frequency of postoperative complications, and quality of life.
To assess the risk of bias, we used the Cochrane Collaboration tool for RCTs. This tool grades the random sequence generation, allocation concealment, blinding of participant and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases[11]. To evaluate the non-randomized trials, we used the methodological index of non-randomized studies (MINORS)[12]. We scored all of the 12 methodological items for non-randomized comparative studies as follows: 0 - not reported; 1 - reported but inadequate; or 2 - reported and adequate. The global ideal score for comparative (non-comparative) studies was 24.
For statistical analysis, we used Review Manager Software 5.3.5 (The Nordic Cochrane Centre, Copenhagen, Denmark)[11] provided by the Cochrane Collaboration and OpenMetaAnalyst[13] with metaphor package[14] as statitstical softwares. We selected the mean difference (MD) as an effect measure for continuous data and the odds ratio (OR) for dichotomous data; we also reported the 95%CI. In cases of continuous data presented as median and range, we estimated the mean and standard deviation according to the methods described by Hozo et al[15]. We used Chi-square and I2 statistics to assess the studies’ heterogeneity and explain the total variation observed between the studies that be generated by the differences between the trials rather than the sampling error (chance). An I2 value ≤ 25% indicates less heterogeneity, an I2 value > 25% but ≤ 75% indicates a moderate heterogeneity, and I2 values > 75% indicate higher heterogeneity[16]. We explored the reasons behind the statistical heterogeneity using sensitivity analyses and the exclusion of specific studies. We used fixed-effect model analysis for outcomes with low heterogeneity. If we found clinical heterogeneity between included studies due to differences with respect to eligibility criteria (study population), the type of surgical technique, and lacking or differing definitions of outcomes, we performed meta-analysis by applying a random-effect model (the DerSimonian-Laird method)[17]. We used
Begg’s funnel plot and Egger’s test for assessing publication bias[18]. The statistical significance was defined as P < 0.1 in Egger’s test and P < 0.05 for the other statistical tests. To correct possible publication bias, we performed trim and fill analysis[19]. The statistical methods of this study were reviewed by Sorin Hostiuc from the Department of Legal Medicine and Bioethics, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
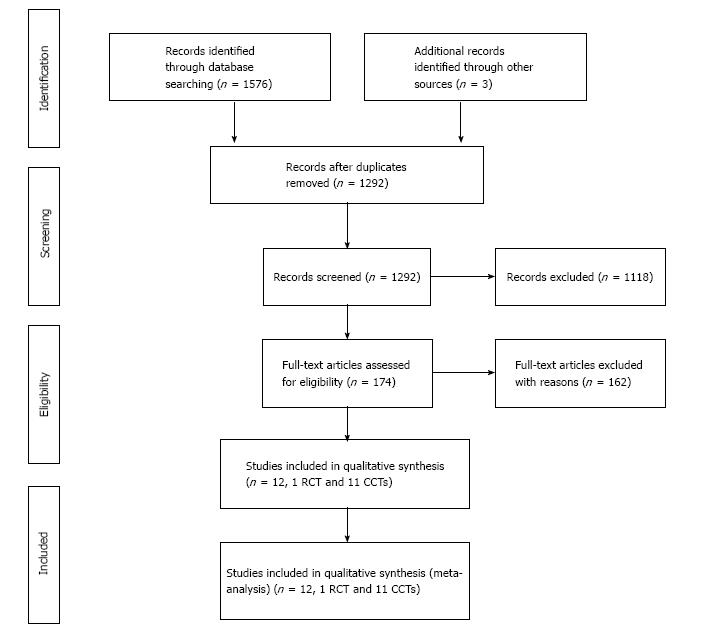
Results of the search: The initial electronic and manual literature searches revealed 174 full-text articles. A total of one RCT (from Japan)[20,21] and eleven CCTs (four from Europe and seven from Asia)[22-32] met the inclusion criteria for the qualitative and quantitative (meta-analysis) synthesis; these studies involved 1619 patients in L-CME and 1477 patients in O-CME. Eleven studies were published in English and one in Chinese. The reasons for exclusion in each stage of the process are shown in Figure 1.
Included studies: The characteristics of the included studies are summarized in Table 1. All of the studies were published between 2012 and 2016, the RCT being published in 2014. The sample size of the studies ranged from 23 to 533 patients. The CME or D3 lymphadenectomy was defined as dissection along the Told’s fascia space and a high (apical or central) ligation of the feeding vessel. Colonic mobilization was conducted using a medial-to-lateral or a lateral-to-medial approach according to the surgeon’s preference. For right-sided tumors, the vascular pedicles were divided at their origin together with removal of the draining lymph nodes along the border of the superior mesenteric vein. For left-sided tumors, removal of the central lymph nodes from the origin of the inferior mesenteric artery was performed with high ligation or with preservation of the left colic artery. In the JCOG 0404 study, the accredited surgeons had completed more than 30 laparoscopic and 30 open colorectal resections[20]. In all of the other studies, the procedures were performed or supervised by colorectal surgeons. Conversion to laparotomy was defined as the extension of the abdominal incision more than eight cm or as the inability to complete the dissection fully laparoscopically. The reported rate of conversion to laparotomy was between 2.82% and 7.6%[20,22-32]. Transverse colon cancers were excluded from the JCOG 0404 study[20]. Storli et al[30] performed 9 (7.3%) transverse colectomies in the open approach but none in the laparoscopic group. In a second paper, Storli et al[22] published their experience regarding CME only in transverse colon cancer. Gouvas et al[31] managed all of the transverse colon cancers using an extended right hemicolectomy. Munkedal et al[25] excluded all cancers in the transverse colon or flexures from their analysis. Bae et al[26], Han et al[27], and Zhao et al[28] managed all cases by a right or extended right hemicolectomy. All studies exhibited remarkable similar exclusion criteria: Stage IV disease and emergency surgery. All of the studies described the technique of laparoscopic CME. Perioperative care was not described in most trials.
| Ref. | Country of origin | Study type | Study period | Female(number, L/O) | Mean age (yr, L/O) | Intervention (L-CME) right/transverse/left location of the tumor | Control (O-CME) right/transverse/left location of the tumor | Adjuvant chemotherapy |
| Kim et al[23], 2016 | South Korea | Case control, unicentre, prospective database | 2008-2013 | 62/44 | 69/67 | 116/0/0 | 99/0/0 | L-CME = 68 pts (58.62%), O-CME = 78 pts (78.78%), recommended to all stage II and III |
| Storli et al[22], 2016 | Norway | Prospective non RT, unicentre | 2007-2014 | 22/13 | 73/23 | 0/33/0 | 0/23/0 | L-CME = 8 (61.5%), O-CME = 5 (62.5%), all stage III below 75 yr |
| Huang et al[24], 2015 | China | Case control, unicentre | 2012-2013 | 20/21 | 56/55 | 53/0/0 | 49/0/0 | NR |
| Yamamoto et al[20], 2014 | Japan | RCT, multicentre | 2004-2009 | 248/215 | 64/64 | 144/0/389 | 156/0/368 | NR, recommended for all stage III |
| Munkedal et al[25], 2014 | Denmark | Prospective nonRT, unicentre | 2008-2011 | 30/38 | 69.1/72.9 | 30/0/53 | 41/0/38 | NR |
| Bae et al[27], 2014 | South Korea | Case control, unicentre | 2006-2008 | 40/38 | 64/65 | 73/12/0 | 76/9/0 | All stage III and II with poor prognosis |
| Han et al[26], 2014 | China | Case control, unicentre | 2003-2010 | 94/67 | 67/65 | 177/0/0 | 147/0/0 | NR, recommended for high risk stage II and stage III |
| Zhao et al[28], 2014 | China | Case control, multicentre | 2000-2009 | 53/44 | 61.3/64.5 | 89/30/0 | 65/36/0 | NR, recommended for high risk stage II and stage III |
| Cong et al[29], 2014 | China | Case control, unicentre | 2008-2011 | 53/45 | 61.5/62.3 | 96/0/0 | 82/0/0 | NR |
| Storli et al[30], 2013 | Norway | Prospective nonRT, unicentre | 2007-2010 | 49/60 | 71.9/73.1 | 50/18/60 2 pts - multiple | 35/44/42 | All stage III below 75 yr |
| Gouvas et al[31], 2012 | Greece | Prospective nonRT, multicentric | 2006-2010 | 19/17 | 62.1/66.3 | 7/9/33 | 9/9/23 | NR |
| Sun et al[32], 2012 | China | Case control, unicentre | 2000-2008 | 58/45 | 60.1/61.9 | 49/7/91 | 43/9/74 | NR, according to stage |
The patient demographics and baseline clinical data were similar between the treatment groups; the L-CME group exhibited a mean age of 69.91 years, and the O-CME group exhibited a mean age of 65.41 years. Women comprised 46.20% and 41.23% of the L-CME and O-CME patients, respectively. None of the studies were blinded, and all of the studies were powered to demonstrate the non-inferiority of the laparoscopic approach.
Excluded studies: We excluded all studies in which the surgical technique did not comply with CME or D3 lymphadenectomy principles[33-42]. We also excluded studies based on the hand-assisted laparoscopic technique[43,44]. Due to the probability of overlapping patients, we have excluded first report of Kim et al[45] which includes only T4 patients.
The risk of bias in the one Japanese RCT was low in all domains[20]. Although blinding of patients and medical personnel was not performed in either trial, the endpoints were considered to be objective, particularly when they were supported by photos. The prospective and retrospective non-randomized studies had good MINORS scores, although the risk of selection, performance, and detection bias was high (Table 2). As expected, the prospective observational studies[22-23,25,30,31] had a higher methodological quality comparing with the retrospective studies[24,26-29].
| Quality evaluation criteria | Kim et al[23], 2016 | Storli et al[22], 2016 | Storli et al[30], 2016 | Huang et al[24], 2015 | Munkedal et al[25], 2014 | Bae et al[27], 2014 | Han et al[26], 2014 | Zhao et al[28], 2014 | Cong et al[29], 2014 | Gouvas et al[31], 2012 | Sun et al[32], 2012 |
| Clear stated aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patients | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 |
| Prospective data collection | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 |
| Endpoints appropriate to the study aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Unbiased assessment of study end-point | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 |
| Appropriate follow-up period | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Loss to follow-up less than 5% | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 1 |
| Prospective calculation of the study size | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adequate group control | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 |
| Contemporary groups | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Baseline equivalence | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Adequate statistical analysis | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Total | 21 | 22 | 22 | 18 | 22 | 20 | 20 | 18 | 15 | 22 | 17 |
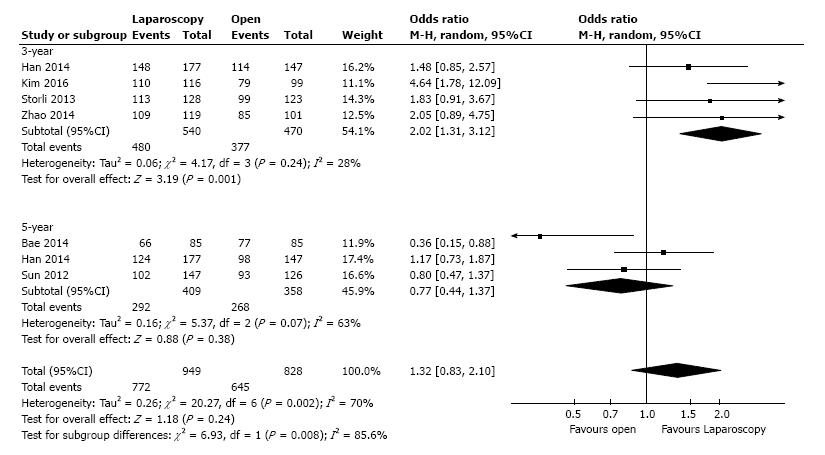
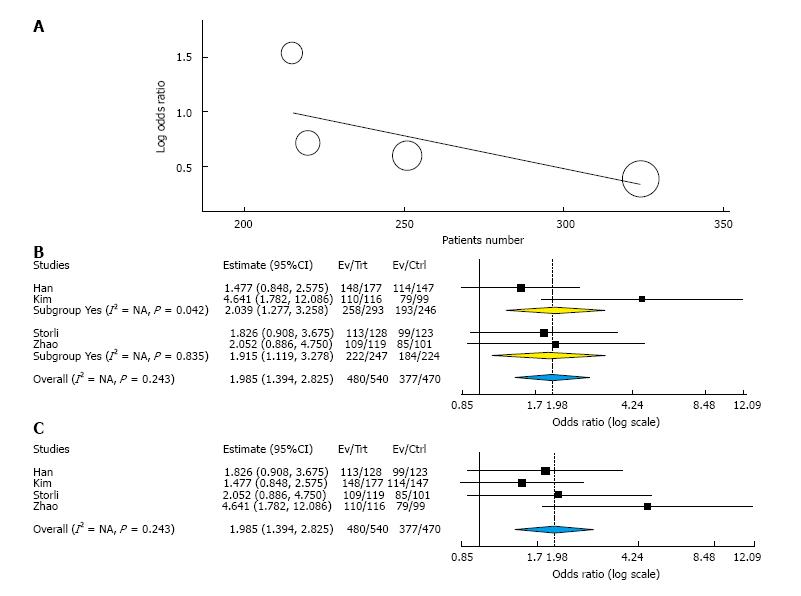
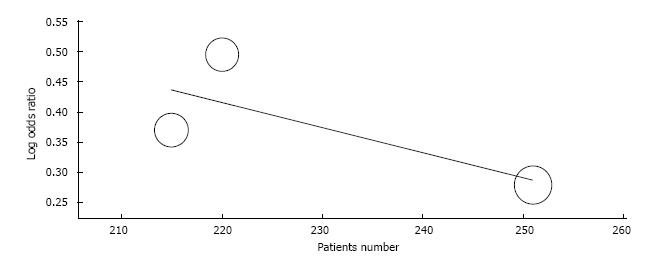
Overall survival: Three-year overall survival was reported by four studies, including 1010 patients (Table 3). The laparoscopic approach was associated with a statistical significant better three-year overall survival, with an OR of 2.02 (95%CI: 1.31 to 3.12, P = 0.001, I2 = 28%). The five-year overall survival was reported by three studies, with a high heterogeneity between them (I2 = 63%). The combined data revealed no statistical significant differences between the L-CME and O-CME (OR = 0.77, P = 0.38, 95%CI: 0.44 to 1.37) (Figure 2). Meta-regression of studies on three-year overall survival according to the number of included patients revealed a trend, although not statistical significant (omnibus P = 0.127), for decreasing of the size of the effect with increasing the number of patients (Figure 3A). The subgroup analysis of studies that include or not only right sided colon cancers, reveled statistical significant results irrespective of that (P = 0.003 and P = 0.018, respectively) (Figure 3B). On the other hand, the cumulative meta-analysis showed a progressively increasing of the size effect while experience is accumulating (Figure 3C).
| Outcome or subgroups | No. of Studies | Participants | Statistical method (95%CI) | Effect estimate (95%CI) | P value | Heterogeneity P, I2 (%) |
| Survival and recurrences | ||||||
| Overall survival | 6 | 1777 | OR (M-H, random) | 1.32 (0.83, 2.10) | 0.24 | 0.002, 70 |
| Three-year | 4 | 1010 | OR (M-H, random) | 2.02 (1.31, 3.12) | 0.001 | 0.24, 28 |
| Five-year | 3 | 767 | OR (M-H, random) | 0.77 (0.44, 1.37) | 0.38 | 0.07, 63 |
| Disease free survival | 4 | 856 | OR (M-H, random) | 1.15 (0.70, 1.87) | 0.58 | 0.09, 54 |
| Three-year | 3 | 686 | OR (M-H, random) | 1.45 (1.00, 2.10) | 0.05 | 0.89, 0 |
| Five-year | 1 | 170 | OR (M-H, random) | 0.50 (0.24, 1.05) | 0.07 | NA |
| Local recurrences | 5 | 1233 | OR (M-H, fixed) | 0.67 (0.38, 1.17) | 0.16 | 0.60, 0 |
| One-year | 2 | 466 | OR (M-H, fixed) | 0.52 (0.20, 1.35) | 0.18 | 0.30, 7 |
| Five-year | 3 | 767 | OR (M-H, fixed) | 0.77 (0.38, 1.54) | 0.46 | 0.52, 0 |
| Distant recurrences | 4 | 1018 | OR (M-H, random) | 0.98 (0.61, 1.58) | 0.94 | 0.17, 40 |
| Three-year | 1 | 251 | OR (M-H, random) | 1.28 (0.54, 3.03) | 0.58 | NA |
| Five-year | 3 | 767 | OR (M-H, random) | 0.90 (0.48, 1.69) | 0.75 | 0.10, 57 |
| Port site metastasis | 2 | 494 | OR (M-H, fixed) | 1.52 (0.20, 11.42) | 0.69 | 0.55, 0 |
| Quality of the resected specimen | ||||||
| Lymphnodes retrieved | 10 | 2647 | MD (IV, random) | -1.06 (-3.65, 1.53) | 0.42 | < 0.001, 92 |
| RCTs | 1 | 1057 | MD (IV, random) | 1.00 (-0.34, 2.34) | 0.14 | NA |
| NRCTs | 9 | 1590 | MD (IV, random) | -1.32 (-4.42, 1.78) | 0.40 | < 0.001, 92 |
| Lymphnodes retrieved | 10 | 2647 | MD (IV, random) | -1.06 (-3.65, 1.53) | 0.42 | < 0.001, 92 |
| < 100 patients | 4 | 478 | MD (IV, random) | -3.18 (-8.69, 2.33) | 0.26 | < 0.001, 85 |
| > 100 patients | 6 | 2169 | MD (IV, random) | 0.29 (-1.64, 2.21) | 0.77 | < 0.001, 83 |
| Lymphnodes retrieved | 10 | 2647 | MD (IV, random) | -1.06 (-3.65, 1.53) | 0.42 | < 0.001, 92 |
| Europe | 4 | 559 | MD (IV, random) | -3.33 (-8.31, 1.64) | 0.19 | < 0.001, 90 |
| Asia | 6 | 2088 | MD (IV, random) | 0.56 (-1.33, 2.46) | 0.56 | < 0.001, 77 |
| Tumor to arterial high tie (mm) | 2 | 252 | MD (IV, random) | 14.26 (-4.30, 32.82) | 0.13 | < 0.001, 92 |
| Resected mesocolon surface (cm2) | 2 | 252 | MD (IV, fixed) | 11.75 (9.50, 13.99) | < 0.001 | 0.55, 0 |
| Complete mesocolic plane excision | 1 | 90 | OR (M-H, fixed) | 0.77 (0.20, 2.96) | 0.71 | NA |
| Operative data | ||||||
| Duration of surgery | 7 | 2266 | MD (IV, random) | 26.26 (5.06, 47.46) | 0.02 | < 0.001, 94 |
| Incision length (cm) | 2 | 1159 | MD (IV, random) | -14.01 (-14.35, -13.66) | < 0.001 | 0.89, 0 |
| Blood loss (mL) | 5 | 1868 | MD (IV, random) | -52.11 (-78.57, -25.65) | < 0.001 | < 0.001, 89 |
| Transfusion requirement | 2 | 1272 | OR (M-H, random) | 0.45 (0.27, 0.75) | 0.002 | 0.54, 0 |
| Intraoperative morbidity | 1 | 1057 | OR (M-H, fixed) | 2.12 (0.95, 4.72) | 0.07 | NA |
| Postoperative course | ||||||
| Time to first flatus (d) | 4 | 1771 | MD (IV, random) | -0.90 (-1.46, -0.34) | 0.002 | < 0.001, 97 |
| Time to liquid diet (d) | 5 | 1031 | MD (IV, random) | -1.84 (-2.93, -0.74) | 0.001 | < 0.001, 98 |
| Short-term morbidity and mortality | ||||||
| Thirty-day overall morbidity | 7 | 2144 | OR (M-H, fixed) | 0.57 (0.46, 0.71) | < 0.001 | 0.76, 0 |
| RCTs | 1 | 1057 | OR (M-H, fixed) | 0.66 (0.49, 0.89) | 0.006 | NA |
| NRCTs | 6 | 1087 | OR (M-H, fixed) | 0.49 (0.36, 0.68) | < 0.001 | 0.89, 0 |
| Wound complications | 8 | 2322 | OR (M-H, fixed) | 0.43 (0.30, 0.61) | < 0.001 | 0.80, 0 |
| Postoperative bleeding | 4 | 1662 | OR (M-H, fixed) | 1.20 (0.46, 3.12) | 0.71 | 0.75, 0 |
| Pneumonia | 5 | 867 | OR (M-H, random) | 0.61 (0.20, 1.84) | 0.38 | 0.21, 32 |
| Anastomotic leakage | 8 | 2471 | OR (M-H, fixed) | 0.82 (0.54, 1.25) | 0.36 | 0.77, 0 |
| Need for reoperation | 2 | 1113 | OR (M-H, fixed) | 0.59 (0.28, 1.23) | 0.16 | 0.79, 0 |
| Thirty-day mortality | 6 | 2237 | OR (M-H, fixed) | 0.42 (0.16, 1.12) | 0.07 | 0.98, 0 |
| Hospital stay (d) | 9 | 2573 | MD (IV, random) | -4.07 (-5.87, -2.28) | < 0.001 | < 0.001, 91 |
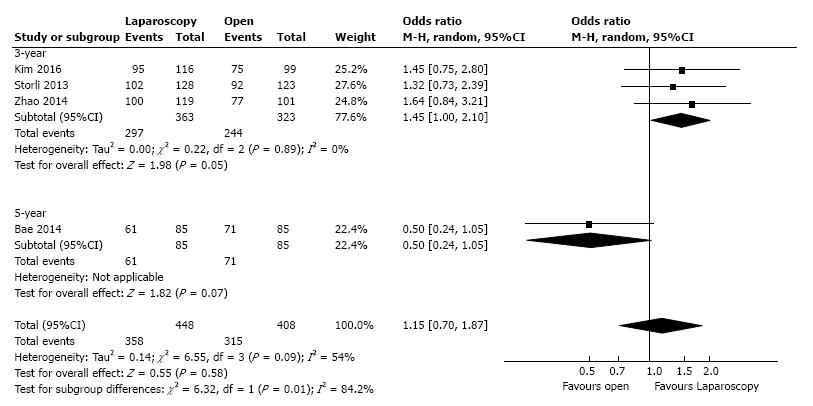
Three studies, with a total of 686 patients, reported the three-year DFS with a low heterogeneity between them (I2 = 0%). However, to adjust for possible methodological differences we used the random-effects model, which revealed that the laparoscopic approach is associated with a statistical significant better three-year DFS (OR = 1.45, 95%CI: 1.00 to 2.10, P = 0.05) (Figure 4). Meta-regression of studies on three-year overall survival according to the number of the included patients revealed a trend, although not statistical significant (Omnibus P = 0.718), for decreasing of the size of the effect with increasing the number of patients (Figure 5).
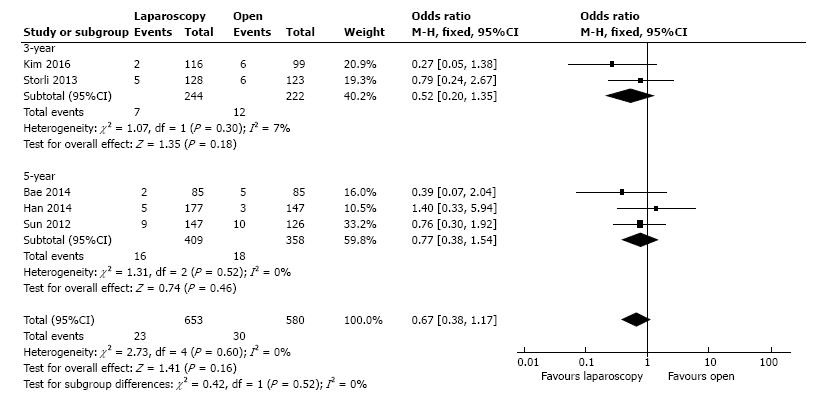
The local recurrence rate was presented by five studies, including 1233 patients. In the fixed-effect meta-analysis there were no statistical significant differences between L-CME and O-CME (OR = 0.67, 95%CI: 0.38 to 1.17, P = 0.16, I2 = 0%) (Figure 6).
The distant recurrence rate was presented by four studies, with a moderate heterogeneity between them (I2 = 40%). In the random-effects meta-analysis there were no statistical significant differences between the two groups (OR = 0.98, 95%CI: 0.61 to 1.58, P = 0.94). Using Egger’s test, no publication bias was found for local (t = 0.22, P = 0.42) or distant recurrences (t = 0.38, P = 0.36).
The port size metastasis rate was reported by two studies including 494 patients, with a low heterogeneity between them (I2 = 0%). In the fixed-effect analysis model there was no difference regarding the port size metastasis rate between laparoscopic and open CME (OR = 1.52, 95%CI: 0.20 to 11.42, P = 0.69).
Standardized evaluation of the resected specimen and grading its quality are objective measures that predict recurrence rate and survival. These data are correlated with the accuracy of the surgical technique.
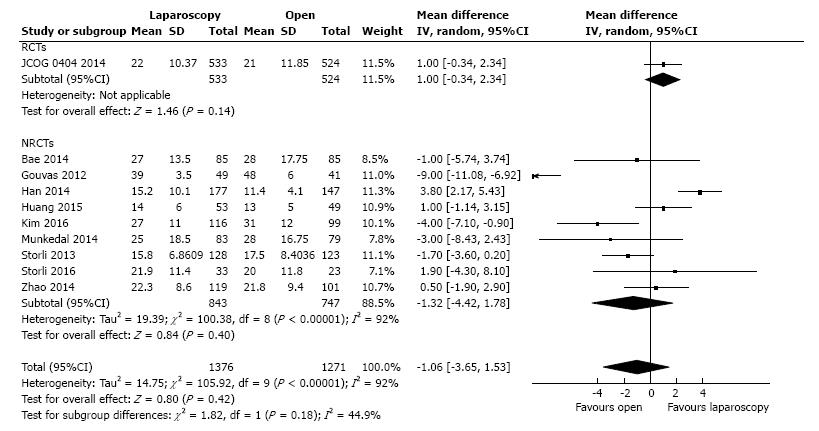
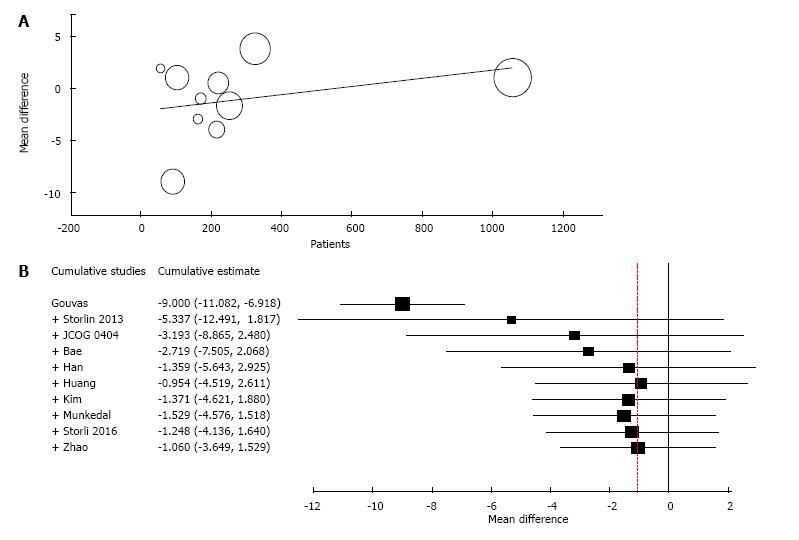
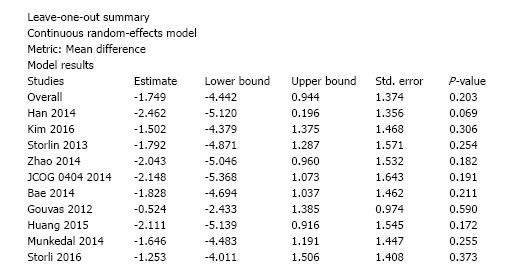
Ten studies reported the number of retrieved lymph nodes for 1376 L-CME patients and 1271 O-CME patients. There was a high heterogeneity between the studies (I2 = 92%). In the random-effects model, we found no statistically significant mean difference between L-CME and O-CME (MD = -1.06, 95%CI: -3.65 to 1.53, P = 0.42) (Figure 7). In order to address the observed heterogeneity, we performed subgroup analysis according to the number of included patients (less or more than 100 patients in each group) and the geographical location of the study (Europe and Asia). The subgroup analysis revealed a high heterogeneity between studies with less than (I2 = 85%) or more (I2 = 83%) than 100 patients into laparoscopy or open group. The results remained with no statistical significance into the two subgroups. Studies coming from Europe showed a high heterogeneity (I2 = 90%) and with no differences regarding the number of retrieved lymphnodes (P = 0.19). Studies published in Asia had also a high heterogeneity (I2 = 77%), and no statistical significant difference between L-CME and O-CME (P = 0.56). Meta-regression of retrieved lymphnodes according to the number of patients revealed that the equivalence between laparoscopic and open approach is stronger with the increased experience in laparoscopic approach (number of the included patients - omnibus P = 0.314, Figure 8A; and year of publishing of the study, Figure 8B).
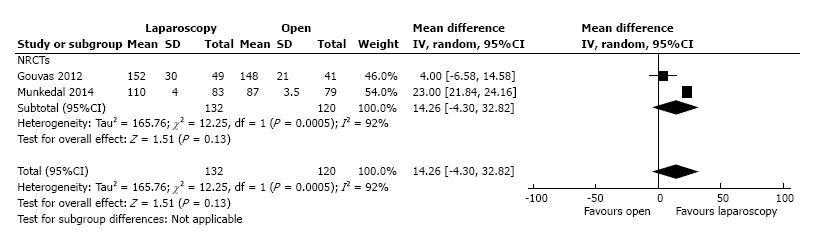
The mean distance from the tumor to the arterial high tie was reported by two studies that included 132 patients in the L-CME group and 120 patients in the O-CME group; we noted high heterogeneity among the studies (I2 = 92%). Using the random-effects model, we did not find any statistically significant difference between the L-CME and O-CME groups (MD = 14.26 cm, 95%CI: -4.30 to 32.82, P = 0.13) (Figure 9).
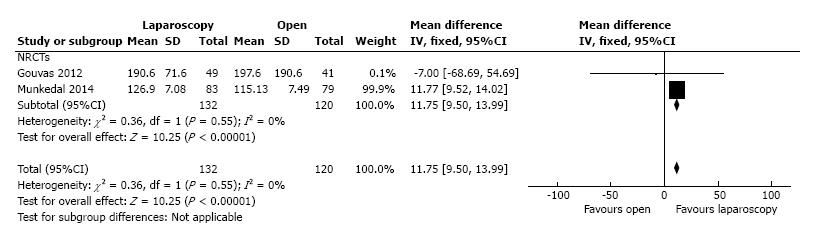
The surface of the resected mesocolon was reported by two studies with 132 patients in the L-CME group and 120 patients in the O-CME group. The surface of the resected mesocolon was larger in the L-CME group (MD = 11.75 cm2, 95%CI: 9.50 to 13.99, P < 0.001) (Figure 10).
One study reported the rate of complete mesocolic plane excision, with no statistically significant difference between the laparoscopic and open approach (OR = 0.77, 95%CI: 0.20 to 2.96).
The duration of surgery was reported by seven studies, with a high heterogeneity between data (I2 = 94%). The L-CME group had a longer duration of surgery with a mean difference of 26.26 min (95%CI: 5.06 to 47.46, P = 0.02). Using Egger’s test, no publication bias was found (t = 0.71, P = 0.26).
The incision length was reported by two studies, including 586 patients in the L-CME group and 573 patients in the O-CME group. Patients from the laparoscopic group had a shorter incision, with a mean difference of 14.01 cm (95%CI: -14.35 to -13.66, P < 0.001).
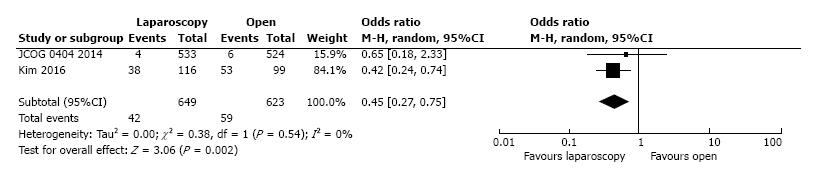
Intraoperative blood-loss data were presented by five studies, with 964 and 904 patients in the L-CME and O-CME, respectively. Due to the high heterogeneity of the data (I2 = 89%) we have used the random-effect analysis. The laparoscopic approach was associated with statistical significant lower intraoperative bleeding, with a mean difference of 52.11 mL (95%CI: -78.57 to -25.65, P < 0.001). Using Egger’s test, no publication bias was found (t = 0.17, P = 0.44). Should be noted the clinical significance of lower intraoperative blood loss associated with laparoscopic approach, which was translated in a lower need for transfusion rate (OR = 0.45, 95%CI: 0.27 to 0.75, P = 0.002). Two studies, including a total of 1272 patients, reported the need for blood transfusions, with a low heterogeneity between them (I2 = 0%) (Figure 11).
The time to first flatus was reported by four studies, including 914 and 857 patients in the L-CME and O-CME, respectively. In the random-effects meta-analysis the laparoscopic approach was associated with a shorter time interval to first flatus, with a mean difference of 0.90 d (95%CI: -1.46 to -0.34, P = 0.002, I2 = 97%).
The time to liquid diet was reported by five studies, with a high heterogeneity between them (I2 = 98%). The time to liquid diet was shorter for the L-CME patients, with a mean difference of 1.84 d (95%CI: -2.93 to -0.74, P = 0.001).
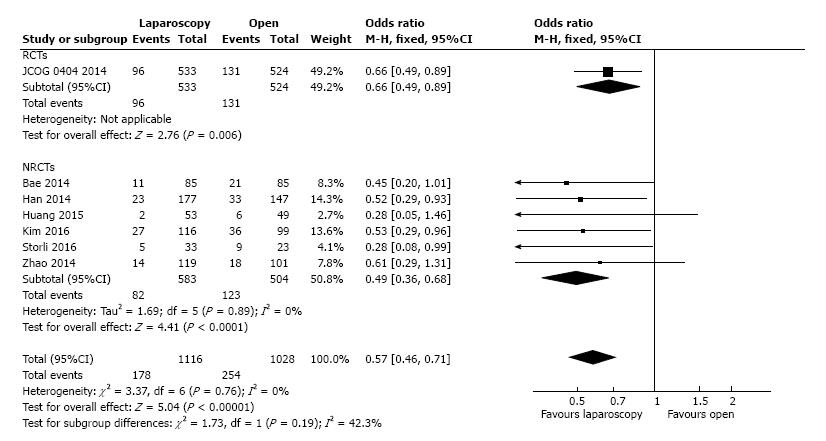
Seven studies presented the postoperative overall morbidity, and these studies included 1116 patients in the L-CME group and 1028 patients in the O-CME group. There was low statistical heterogeneity among the studies (I2 = 0%). The L-CME procedure was associated with a lower postoperative morbidity (OR = 0.57, 95%CI: 0.46 to 0.71, P < 0.001) (Figure 12).
Wound complications, reported by eight studies, were significantly less frequent in the L-CME group (OR = 0.43, 95%CI: 0.30 to 0.61, P < 0.001). There was no statistical heterogeneity among the studies (I2 = 0%).
There was no difference between the two groups regarding postoperative bleeding (OR = 1.20, 95%CI: 0.46 to 3.12, P = 0.71), anastomotic leakage (OR = 0.82, 95%CI: 0.0.54 to 1.25, P = 0.36), need for reoperation (OR = 0.59, 95%CI: 0.28 to 1.23, P = 0.16), and pulmonary complications (OR = 0.61, 95%CI: 0.20 to 1.84, P = 0.38).
The 30-d mortality was reported by six studies with 1158 patients in the L-CME group and 1079 patients in the O-CME group. There was low heterogeneity among the studies (I2 = 0%). In the fixed-effects meta-analysis we observed no statistically significant difference between the L-CME and O-CME groups (OR = 0.42, 95%CI: 0.16 to 1.12).
Nine studies, with 1340 and 1233 patients in the L-CME and O-CME, respectively reported the hospital stay. There was a high heterogeneity between the studies (I2 = 91%). In the random-effects meta-analysis we found a statistical significant lower hospital stay for laparoscopic group, with a mean difference of 4.07 d (95%CI: -5.87 to -2.28, P < 0.001).
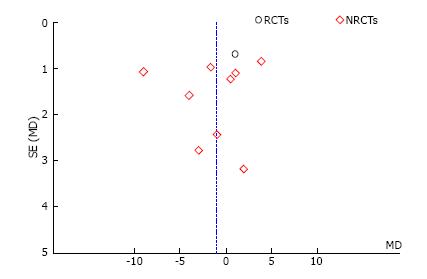
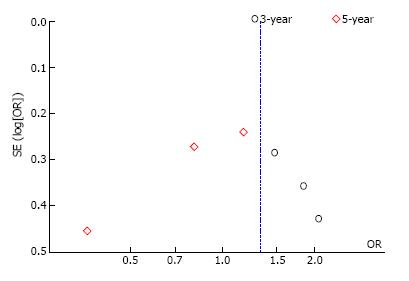
We conducted sensitivity analysis to assess statistical heterogeneity based on excluding specific studies with a high risk of bias (Figure 13). There were no relevant changes in the overall effects of the quantitative synthesis. Our analysis of the funnel plots reveals no significant asymmetries for the studied outcomes (Figures 14 and 15).
Our meta-analysis revealed that laparoscopic CME with CVL for colon cancer offers the same quality of the resected specimen as the open approach, being superior in all perioperative results and at least non-inferior in long-term oncological outcomes. Although not addressed the complete mesocolic excision or D3 lymphadenectomy technique, the equivalence of laparoscopy in terms of resected lymphnodes was showed in four large, multicenter, studies-Clinical Outcomes of Surgical Therapy (COST), Conventional vs Laparoscopic-Assisted Surgery in Colorectal Cancer (CLASSIC), Colon Cancer Laparoscopic or Open Resection I (COLOR I), and the Australasian Randomized Clinic Study Comparing Laparoscopic and Conventional Open Surgical Treatments for Colon Cancer (ALCCaS); the mean number of resected lymph nodes was 10.13 in the laparoscopic group and 10.14 in the open group[40-42]. An RCT from Taiwan comparing open with laparoscopic left-sided D2 resections for stage II or III colon cancer reported 16 ± 3 dissected lymph nodes in its laparoscopic group and 16 ± 6 in its open group[33]. The long-term oncological outcomes between the L-CME and O-CME groups were also comparable; there were no differences regarding the local and distant recurrence rate, the three- and five-year overall rates and the disease-free survival rates. In our study, the three-year overall and disease-free survival were superior in the laparoscopic group; however, should be noted the extensive experience in laparoscopy of the reporting centers. In Barcelona study, the laparoscopic approach was associated with a slight increase in survival rate, a faster postoperative recovery, and a shorter in-hospital stay duration[38]. In the COLOR study, 1248 patients were randomized for open or laparoscopic colon resection[46]. After a median follow-up of 53 mo, the combined three-year, disease-free survival rate was 74.2% in the laparoscopic group and 76.2% in the open group (P = 0.70). The combined three-year overall survival rate was 81.8% in the laparoscopic group and 84.2% in the open group (P = 0.45). The authors concluded that a difference in the three-year, disease-free survival rate could not be ruled out due to limitations of the study[46]. In the CLASSIC trial, 794 patients with colorectal cancer were randomized for open or laparoscopic resection[47]. An analysis of the subgroup of patients with colon cancer, 140 in the open group and 273 in the laparoscopic group, did not reveal any differences in terms of three-year overall survival rates (P = 0.51). After a median follow-up of 62.9 mo, there were no statistically significant differences in overall survival and disease-free survival rates[48]. In the COST study, 872 patients were randomized to receive an open or laparoscopic colectomy[49]. The 3- and 5-year follow-ups revealed no differences regarding recurrence rate and overall survival rates[49,50].
We found a longer duration of surgery in the laparoscopic group. However, all the perioperative outcomes, such as blood loss, need for transfusion, incision length, wound complications, and thirty-day overall morbidity were less frequent in the laparoscopic group. In the COST, CLASSIC, COLOR I, and ALCCaS trials, the mean duration of surgery was 145-180 compared to 95-135 min, the hospital stay was 5-10 vs 6-11 d, the 30-d morbidity was 21%-38% vs 20%-45%, and the 30-d mortality was 0.5%-4.0% vs 0.7%-5.0% in the laparoscopic and open groups, respectively[40-42,49]. Liang et al[33] found a longer operative time for left-sided resections (224.4 ± 44.8 min vs 184.0 ± 30.6 min), less blood loss (54 ± 12 mL vs 240 ± 34 mL), a shorter wound incision (10.6 ± 1.6 cm vs 18 ± 3.1 cm) for the laparoscopic approach, but there were no statistically significant differences regarding total postoperative complications (20 vs 29, P = 0.15).
Our meta-analysis showed that patients from the laparoscopic group had a shorter hospital stay and a shorter recovery time to regain gastrointestinal function. This result is consistent with the current evidence that supported earlier recovery of bowel functioning and oral diet with an in-hospital stay duration 1.7 d shorter in the laparoscopic group[51]. The studies included in the current meta-analysis did not evaluate how surgery affected immune functioning. According to Liang et al[33], the postoperative proinflammatory response, evaluated by C-reactive protein and the erythrocyte sedimentation rate and postoperative immunosuppression and assessed by alteration of lymphocyte counts and the CD4+/CD8+ ratio, was significantly less in the laparoscopic group (P < 0.001).
An important concern regarding laparoscopic colon surgery is the reproducibility of results given the nature of multicenter, specialized centers and the heterogeneity of general surgeons. All surgical procedures from the studies included in this meta-analysis were performed by highly experienced or accredited surgeons. An analysis of the short-term outcomes of colon and rectal laparoscopic resections in Sydney South West Area Health Service revealed a lower morbidity (28.8% vs 54.4%, P < 0.001), fewer transfusions (0.4 units vs 0.7 units, P = 0.0028), a longer operative time (24.1 min, P < 0.0001) and a shorter length of stay (7 vs 10 d, P = 0.0011) for laparoscopic procedures[52]. Dobbins et al[53] published the results of laparoscopic resections for colon and rectal cancer from all of the public and private hospitals in New South Wales, Australia. The laparoscopic colon resections were associated with a reduced rate of extended stay (OR = 0.60, 95%CI: 0.49-0.72) and 28-d readmissions (OR = 0.86, 95%CI: 0.74-0.99). Survival benefits for laparoscopy, regarding cancer-specific survival, were observed in higher-caseload hospitals but not in lower-caseload hospitals[53].
The current meta-analysis has as a main limitation the clinical heterogeneity of the included studies, and caution should be exercised when interpreting its results. This meta-analysis involves several types of study designs, including retrospective, prospective, and RCT. There is an increased heterogeneity of the tumor localization on the colon, with the transverse colon cancers being excluded from the analysis in two studies, while the others included them into the right/extended right hemicolectomy group. Excepting the one randomized controlled trial, the experience in minimally invasive surgery of the surgeons from the laparoscopic group is not quantified, although all procedures were performed or supervised by trained colorectal surgeons. However, using random-effects meta-analysis, with subgroup analysis and meta-regression, we limited the variance of the included outcomes.
In summary, the current data suggest that the laparoscopic approach offers the same quality of resected specimens as the open approach in CME with CVL for colon cancer while maintaining all of the short-term benefits of a minimally invasive approach. Although a specimen-oriented surgical dissection in colon cancer via a laparoscopic approach is challenging, the magnification and predisposition to details of a minimally invasive technique are associated with a lower postoperative morbidity.
Complete mesocolic excision with central vascular ligation represents an extension to the colonic cancer of the already standardized resection for rectal cancer. It adheres to the same guiding principle that sharp surgical dissection, following embryological planes, with central vascular ligation, should improve oncological outcomes. The technical details of this new concept were published in 2007.
A high-level evidence that laparoscopic approach offers the same quality of the resected specimen as open surgery for complete mesocolic excision with central vascular ligation for colon cancer is lacking.
Current evidence is consistent with a faster postoperative recovery for laparoscopic colectomies compared with the open approach; the former is not associated with any negative impact regarding local recurrence and survival rates. This study reveals that laparoscopy offers the same quality of the resected specimen as the open approach in complete mesocolic excision with central vascular ligation for colon cancer. The laparoscopic complete mesocolic excision with central vascular ligation is superior in all perioperative results and at least non-inferior in long-term oncological outcomes.
Due to all advantages of laparoscopy, the teaching and mentoring of minimally invasive techniques for colon resections should be accentuated, in order to increase the proportion of laparoscopic over open procedures.
During complete mesocolic excision with central vascular ligation for right-sided tumors, the ileocolic and right colic vessels should be ligated at their origin from the superior mesenteric artery, medial (patient left-hand side) to the superior mesenteric vein. Transverse colon tumors require transection of the middle colic artery at its origin. Left-sided tumors require transection of the inferior mesenteric artery at its origin from the aorta.
This is an interesting meta-analysis and review of a highly debatable topic in surgery, the consensus about laparoscopic vs open surgery in high ligation.
| 1. | Negoi I, Hostiuc S, Runcanu A, Negoi RI, Beuran M. Superior mesenteric artery first approach versus standard pancreaticoduodenectomy: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2017;16:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Hohenberger W, Merkel S, Weber K. [Lymphadenectomy with tumors of the lower gastrointestinal tract]. Chirurg. 2007;78:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol. 2012;30:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 316] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 4. | Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009;11:354-364; discussion 364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1153] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 5. | West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 547] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 6. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 607] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 7. | Feng H, Zhao XW, Zhang Z, Han DP, Mao ZH, Lu AG, Thasler WE. Laparoscopic Complete Mesocolic Excision for Stage II/III Left-Sided Colon Cancers: A Prospective Study and Comparison with D3 Lymph Node Dissection. J Laparoendosc Adv Surg Tech A. 2016;26:606-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | NCCN. Colon Cancer, Version 2.2017. NCCN Clin Pract Guidel Oncol. 2017;. |
| 9. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13819] [Article Influence: 812.9] [Reference Citation Analysis (3)] |
| 10. | Negoi I, Hostiuc S, Paun S, Negoi RI, Beuran M. Extralevator vs conventional abdominoperineal resection for rectal cancer-A systematic review and meta-analysis. Am J Surg. 2016;212:511-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | The Cochrane C. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011;. |
| 12. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 6169] [Article Influence: 268.2] [Reference Citation Analysis (0)] |
| 13. | Byron W, Dahabreh I, Trikalinos A, Lau J, Trow P, Schmid C, 49 (2012): 5. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J Statistical Software. 2012;49:1-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 511] [Article Influence: 36.5] [Reference Citation Analysis (1)] |
| 14. | Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Statistical Software. 2010;36:1-48. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7317] [Cited by in RCA: 7427] [Article Influence: 464.2] [Reference Citation Analysis (0)] |
| 15. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 7290] [Article Influence: 347.1] [Reference Citation Analysis (1)] |
| 16. | Chootrakool H, Shi JQ, Yue R. Meta-analysis and sensitivity analysis for multi-arm trials with selection bias. Stat Med. 2011;30:1183-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 17. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 31238] [Article Influence: 781.0] [Reference Citation Analysis (1)] |
| 18. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 441] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7948] [Cited by in RCA: 9575] [Article Influence: 368.3] [Reference Citation Analysis (0)] |
| 20. | Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, Sugihara K, Watanabe M, Moriya Y, Kitano S; Japan Clinical Oncology Group Colorectal Cancer Study Group. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014;260:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 21. | Nakajima K, Inomata M, Akagi T, Etoh T, Sugihara K, Watanabe M, Yamamoto S, Katayama H, Moriya Y, Kitano S. Quality control by photo documentation for evaluation of laparoscopic and open colectomy with D3 resection for stage II/III colorectal cancer: Japan Clinical Oncology Group Study JCOG 0404. Jpn J Clin Oncol. 2014;44:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Storli KE, Eide GE. Laparoscopic Complete Mesocolic Excision versus Open Complete Mesocolic Excision for Transverse Colon Cancer: Long-Term Survival Results of a Prospective Single Centre Non-Randomized Study. Dig Surg. 2016;33:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Kim IY, Kim BR, Choi EH, Kim YW. Short-term and oncologic outcomes of laparoscopic and open complete mesocolic excision and central ligation. Int J Surg. 2016;27:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Huang JL, Wei HB, Fang JF, Zheng ZH, Chen TF, Wei B, Huang Y, Liu JP. Comparison of laparoscopic versus open complete mesocolic excision for right colon cancer. Int J Surg. 2015;23:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Munkedal DL, West NP, Iversen LH, Hagemann-Madsen R, Quirke P, Laurberg S. Implementation of complete mesocolic excision at a university hospital in Denmark: An audit of consecutive, prospectively collected colon cancer specimens. Eur J Surg Oncol. 2014;40:1494-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Han DP, Lu AG, Feng H, Wang PX, Cao QF, Zong YP, Feng B, Zheng MH. Long-term outcome of laparoscopic-assisted right-hemicolectomy with D3 lymphadenectomy versus open surgery for colon carcinoma. Surg Today. 2014;44:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol. 2014;21:2288-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Zhao LY, Chi P, Ding WX, Huang SR, Zhang SF, Pan K, Hu YF, Liu H, Li GX. Laparoscopic vs open extended right hemicolectomy for colon cancer. World J Gastroenterol. 2014;20:7926-7932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 29. | Cong J, Chen C, Feng Y, Ma M, Xia Z, Liu D. Comparison of short-term outcomes between laparoscopic and open complete mesocolic excision/D3 radical operation for stage II/III right hemicolon carcinoma. Chin J Clin Oncol. 2014;41:1591-1596. [DOI] [Full Text] |
| 30. | Storli KE, Søndenaa K, Furnes B, Eide GE. Outcome after introduction of complete mesocolic excision for colon cancer is similar for open and laparoscopic surgical treatments. Dig Surg. 2013;30:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Gouvas N, Pechlivanides G, Zervakis N, Kafousi M, Xynos E. Complete mesocolic excision in colon cancer surgery: a comparison between open and laparoscopic approach. Colorectal Dis. 2012;14:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Sun YW, Chi P, Lin HM, Lu XR, Huang Y, Xu ZB, Huang SH. [Comparison of efficacy between laparoscopic versus open complete mesocolic excision for colon cancer]. Zhonghua Weichang Waike Zazhi. 2012;15:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Liang JT, Huang KC, Lai HS, Lee PH, Jeng YM. Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann Surg Oncol. 2007;14:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Kaiser AM, Kang JC, Chan LS, Vukasin P, Beart RW Jr. Laparoscopic-assisted vs. open colectomy for colon cancer: a prospective randomized trial. J Laparoendosc Adv Surg Tech A. 2004;14:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Ramacciato G, D’Angelo F, Aurello P, Nigri G, Valabrega S, Pezzoli F, Ravaioli M, Cescon M, Cucchetti A, Lauro A. [Right hemicolectomy for colon cancer: a prospective randomised study comparing laparoscopic vs. open technique]. Chir Ital. 2008;60:1-7. [PubMed] |
| 36. | King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH, Kipling RM, Kennedy RH. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg. 2006;93:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 37. | King PM, Blazeby JM, Ewings P, Kennedy RH. Detailed evaluation of functional recovery following laparoscopic or open surgery for colorectal cancer within an enhanced recovery programme. Int J Colorectal Dis. 2008;23:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 38. | Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1901] [Cited by in RCA: 1831] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 39. | Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 439] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 40. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2326] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 41. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy AM; COlon cancer Laparoscopic or Open Resection Study Group (COLOR). Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1713] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 42. | Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens JH, Stevenson AR. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (1)] |
| 43. | Kang JC, Chung MH, Chao PC, Yeh CC, Hsiao CW, Lee TY, Jao SW. Hand-assisted laparoscopic colectomy vs open colectomy: a prospective randomized study. Surg Endosc. 2004;18:577-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Chung CC, Ng DC, Tsang WW, Tang WL, Yau KK, Cheung HY, Wong JC, Li MK. Hand-assisted laparoscopic versus open right colectomy: a randomized controlled trial. Ann Surg. 2007;246:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 45. | Kim IY, Kim BR, Kim YW. The short-term and oncologic outcomes of laparoscopic versus open surgery for T4 colon cancer. Surg Endosc. 2016;30:1508-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1077] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 47. | Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1121] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 48. | Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 49. | Clinical Outcomes of Surgical Therapy Study Group. Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2543] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 50. | Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H; Clinical Outcomes of Surgical Therapy Study Group. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655-662; discussion 662-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Mathis KL, Nelson H. Controversies in laparoscopy for colon and rectal cancer. Surg Oncol Clin N Am. 2014;23:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | McKay GD, Morgan MJ, Wong SK, Gatenby AH, Fulham SB, Ahmed KW, Toh JW, Hanna M, Hitos K. Improved short-term outcomes of laparoscopic versus open resection for colon and rectal cancer in an area health service: a multicenter study. Dis Colon Rectum. 2012;55:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Dobbins TA, Young JM, Solomon MJ. Uptake and outcomes of laparoscopically assisted resection for colon and rectal cancer in Australia: a population-based study. Dis Colon Rectum. 2014;57:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Horesh N, Gurkan A S- Editor: Cui LJ L- Editor: A E- Editor: Lu YJ