Published online Dec 15, 2017. doi: 10.4251/wjgo.v9.i12.466
Peer-review started: July 14, 2017
First decision: August 7, 2017
Revised: September 17, 2017
Accepted: October 15, 2017
Article in press: October 15, 2017
Published online: December 15, 2017
Processing time: 154 Days and 7.6 Hours
To evaluate the prognostic value of the tumor-stroma ratio (TSR) in rectal cancer.
TSR was determined on hematoxylin and eosin stained histological sections of 154 patients treated for rectal adenocarcinoma without prior neoadjuvant treatment in the period 1996-2006 by two observers to assess reproducibility. Patients were categorized into three categories: TSR-high [carcinoma percentage (CP) ≥ 70%], TSR-intermediate (CP 40%, 50% and 60%) and TSR-low (CP ≤ 30%). The relation between categorized TSR and survival was analyzed using Cox proportional hazards model.
Thirty-six (23.4%) patients were scored as TSR-low, 70 (45.4%) as TSR-intermediate and 48 (31.2%) as TSR-high. TSR had a good interobserver agreement (κ = 0.724, concordance 82.5%). Overall survival (OS) and disease free survival (DFS) were significantly better for patients with a high TSR (P = 0.01 and P = 0.02, respectively). A similar association existed for disease specific survival (P = 0.06). In multivariate analysis, patients without lymph node metastasis and an intermediate TSR had a higher risk of dying from rectal cancer (HR = 5.27, 95%CI: 1.54-18.10), compared to lymph node metastasis negative patients with a high TSR. This group also had a worse DFS (HR = 6.41, 95%CI: 1.84-22.28). An identical association was seen for OS. These relations were not seen in lymph node metastasis positive patients.
The TSR has potential as a prognostic factor for survival in surgically treated rectal cancer patients, especially in lymph node negative cases.
Core tip: The tumor-stroma ratio (TSR) can be determined accurately on routine histopathological sections by different observers. The TSR has potential as a prognostic factor for survival in surgically treated rectal cancer patients, especially in lymph node negative cases. It could therefore be useful in decision making regarding adjuvant treatment in individual patients.
- Citation: Scheer R, Baidoshvili A, Zoidze S, Elferink MAG, Berkel AEM, Klaase JM, van Diest PJ. Tumor-stroma ratio as prognostic factor for survival in rectal adenocarcinoma: A retrospective cohort study. World J Gastrointest Oncol 2017; 9(12): 466-474
- URL: https://www.wjgnet.com/1948-5204/full/v9/i12/466.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i12.466
Colorectal cancer (CRC) is a common form of cancer in both men and women. More than 15000 new patients with a colorectal carcinoma were diagnosed in The Netherlands in 2016[1]. The common form to stage this type of cancer is the TNM staging system of the Union Internationale Contre le Cancer/American Joint Cancer Committee (UICC/AJCC)[2]. This system is also used in decision making about the appliance of (neo)adjuvant (chemo)radiotherapy. Although the TNM staging system is still regarded as the most important prognostic factor[3], it seems insufficiently able to predict the prognosis of the individual patient. This applies in particular to patients with stage II rectal cancer[4]. A part of the patients is overtreated and consequently exposed to a higher risk on therapy related complications, indicating a need for additional prognostic factors.
More recently, some studies have focused on the tumor-host interaction in relation to metastatic invasion. This interaction is enacted in an environment including cancer cells, the stromal tissue, consisting of different cell types like fibroblasts, myofibroblasts, endothelial cells and immune cells, and the extracellular matrix[5]. Mesker et al[6] showed that a high tumor-stroma ratio (TSR), the proportion of carcinoma relative to the proportion of tumor stroma in the histopathological section through the tumor, is an indicator of a better outcome of disease in colon cancer. This is more outspoken for right sided tumors[6]. Similar results were seen in breast cancer, oral squamous cell carcinoma and prostate cancer[7-9]. A high TSR is possibly related to both a longer disease free and overall survival (OS) according to a study on a small number of rectal cancer patients[10]. In this respect, it is meaningful to explore the relevance of the TSR in a larger cohort of patients with rectal adenocarcinoma.
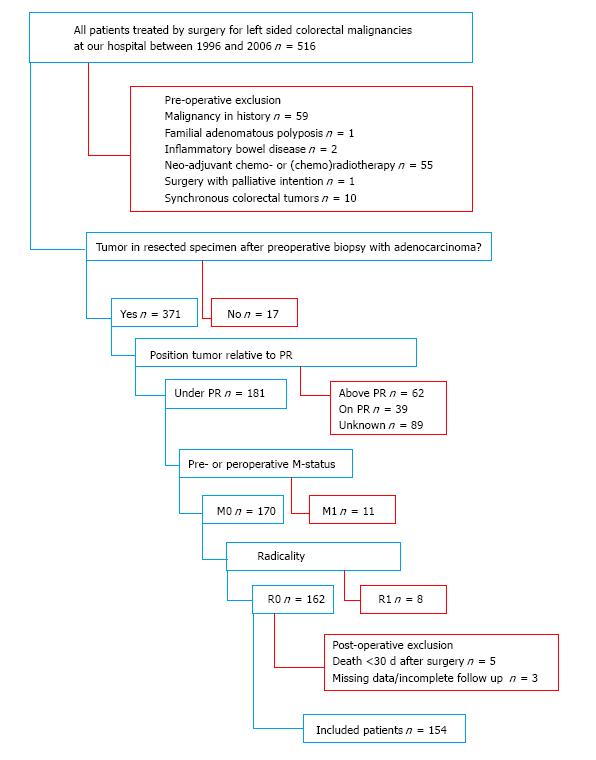
Patients with rectal adenocarcinomas under the peritoneal reflection were identified out of all patients, who underwent surgery for left sided colorectal malignancies at our hospital between 1996 and 2006, by analyzing the histopathological reports. Only patients treated with curative intent were included, i.e., patients without known distant metastases at surgery and radically resected tumors (M0, R0 resections). Patients who received neoadjuvant therapy, with malignancies in the past, other than radically excised basal cell carcinoma of the skin, and cases where no tumor was found in the resected specimen, despite preoperative adenocarcinoma in the biopsy of a suspected abnormality, were excluded. Other exclusion criteria were the presence of synchronous colorectal tumors, Lynch syndrome, familial adenomatous polyposis, and inflammatory bowel diseases. Patients who died within thirty days after surgery, with incomplete follow-up or unavailable histopathological material were also excluded (Figure 1).
Data concerning local recurrences, distant metastases, death, and cause of death were collected from the patient records and by consultation of general practitioners. Furthermore, dates of death were retrieved from the population-based Netherlands Cancer Registry. All data were handled in a coded anonymous fashion according to the Code for proper secondary use of human tissue from the Dutch Federation of Medical Scientific Societies and with respect to the Helsinki Declaration.
TSR was determined on hematoxylin and eosin (H and E) stained histological sections. The section with the most invasive part of the tumor was identified to semiquantitatively assess the carcinoma percentage (CP) in 10% steps. The CP is a derivative of the TSR and is complementary to the percentage of stroma and other components, like mucus. For example, a CP of 20% corresponds to a stroma percentage of 80%, which coincides with a low TSR. The section was viewed with a 5 × objective (50 times magnification). The CPs were determined on all image fields of the entire section with tumor cells in all sides of it (North-East-South-West). Areas with the lowest CP were given more weight in rating the mean CP of the total assessed area, as is common practice in routine pathology in determining tumor differentiation. All sections were assessed separately by two observers (René Scheer and Shorena Zoidze) to allow assessment of reproducibility.
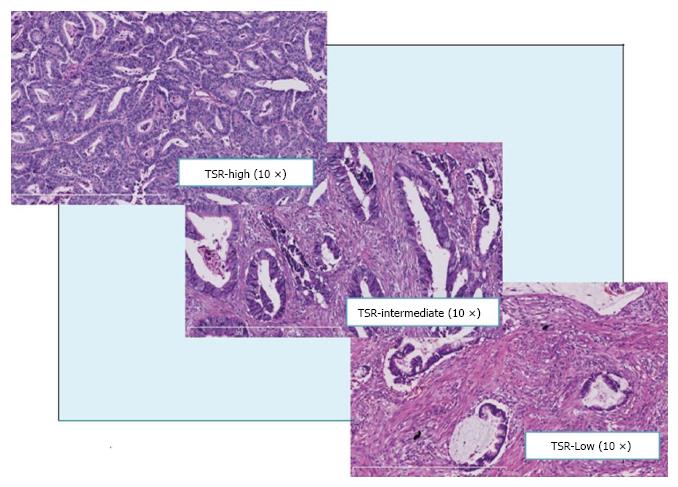
The absolute CPs were categorized for a good clinical reproducibility and clarity reasons into three categories, finally. TSR-low including the CP-values ≤ 30%, TSR-intermediate including the CP-values 40%, 50% and 60%, and TSR-high including the CP-values ≥ 70% (Figure 2). In the results only the categorized TSR are shown for clarity.
In case of a difference of 10% in determined CPs, which lead to a different TSR category, the lowest CP was used for the determination of the final TSR. The sections were reviewed by a third observer (AB) in case of > 10% difference in determined CPs leading to different TSR categories. This third opinion was considered as decisive.
Data were analyzed using SPSS, version 19.0 (SPSS Inc., Chicago, IL, United States) and Stata, version 12.0 (StataCorp LP, College Station, Texas, United States). The statistical methods of this study were reviewed by Elferink MA, from the Netherlands Comprehensive Cancer Organization.
Patient characteristics were compared using Pearson χ2 tests and one-way ANOVA. Interobserver reproducibility for the absolute and categorized CPs was analyzed by using Cohen’s Kappa (κ) coefficient. A κ-value of 0.0 or less was considered to represent poor agreement, 0.01-0.20 slight agreement, 0.21-0.40 fair, 0.41-0.60 moderate, 0.61-0.80 sufficient to good, and 0.81-1.00 near-perfect agreement[11]. Survival analyses based on categorized CPs included comparison of OS, disease free survival (DFS), and disease specific survival (DSS) by Kaplan-Meier survival analysis and log-rank statistics. Follow-up time in OS analyses was defined as the period between the date of primary surgery and the date of death from any cause, or the date of last follow-up. The DSS was restricted to death from rectal cancer only. Follow-up time in DFS analyses was defined as the time from the date of primary surgery until the date of a local recurrence or distant metastasis (irrespective of site). In DFS analyses, cases were censored in case of a second primary tumor (colorectal or other types) or death. The date of last follow-up was used as endpoint to calculate follow-up time, if none of these events occurred.
The relation between categorized TSR and survival (OS, DFS, and DSS) was analyzed, and adjusted for confounders (age, gender, grading, pathological T- and N-stage, and adjuvant treatment), using Cox proportional hazards model. Probability values < 0.05 (2-sided) were considered statistically significant.
A total of 154 patients met the inclusion criteria for this study. Three types of resections were used: Abdominoperineal resection in 67 (43.5%), low anterior resection in 63 (40.9%), and Hartmann resection, a modulated low anterior resection without construction of an anastomosis, in 24 patients (15.6%). The median follow-up of all patients was 5.3 years. Out of the analyzed samples, 36 (23.4%) were scored as TSR-low, 70 (45.4%) as TSR-intermediate, and 48 (31.2%) as TSR-high. There were more lymph node metastasis positive patients with a low TSR in comparison with patients with a higher TSR (P = 0.029), who consequently received adjuvant treatment. Radiotherapy was the most common form of adjuvant therapy. Detailed patient characteristics are shown by categorized TSR in Table 1.
| TSR-low (n = 36) | TSR-intermediate (n = 70) | TSR-high (n = 48) | P-value1 | ||||
| n | % | n | % | n | % | ||
| Gender | NS | ||||||
| Male | 24 | 66.7 | 44 | 62.9 | 32 | 66.7 | |
| Female | 12 | 33.3 | 26 | 37.1 | 16 | 33.3 | |
| Age (yr) | M 68.0 | SD 8.0 | M 67.3 | SD 10.3 | M 65.7 | SD 10.3 | NS2 |
| (range 49.0-82.0 ) | (range 40.0-87.0) | (range 43.0-91.0) | |||||
| Treatment | NS | ||||||
| APR | 19 | 52.6 | 31 | 44.3 | 17 | 35.4 | |
| LAR | 11 | 30.6 | 28 | 40.0 | 24 | 50.0 | |
| Hartmann | 6 | 16.7 | 11 | 15.7 | 7 | 14.6 | |
| T-status | NS | ||||||
| pT1 | 1 | 2.8 | 1 | 1.4 | 4 | 8.3 | |
| pT2 | 9 | 25.0 | 23 | 32.9 | 18 | 37.5 | |
| pT3 | 24 | 66.7 | 43 | 61.4 | 25 | 52.1 | |
| pT4 | 2 | 5.6 | 3 | 4.3 | 1 | 2.1 | |
| N-status | 0.029 | ||||||
| pN0 | 16 | 44.4 | 44 | 62.9 | 34 | 70.8 | |
| N1 | 15 | 41.7 | 13 | 18.6 | 11 | 22.9 | |
| N2 | 5 | 13.9 | 13 | 18.6 | 3 | 6.3 | |
| Stage | NS | ||||||
| I | 7 | 19.4 | 21 | 30.0 | 17 | 35.4 | |
| II | 9 | 25.0 | 23 | 32.9 | 17 | 35.4 | |
| III | 20 | 55.6 | 26 | 37.1 | 14 | 29.2 | |
| Grading | NS | ||||||
| Well | 0 | 0 | 1 | 1.4 | 3 | 6.3 | |
| Moderate | 31 | 86.1 | 58 | 82.9 | 40 | 83.3 | |
| Poor | 5 | 13.9 | 11 | 15.7 | 5 | 10.4 | |
| Adjuvant treatment | 19 | 52.8 | 21 | 30.0 | 11 | 22.9 | 0.0123 |
| Radiotherapy | 17 | 18 | 9 | ||||
| Chemoradiotherapy | 1 | 3 | 2 | ||||
| Chemotherapy | 1 | - | - | ||||
A third opinion about a final TSR in case of interobserver disagreement about CPs with > 10% difference in determined CPs leading to different TSR categories was needed in 12 patients (7.8%). Mainly strong heterogeneity of the tumor complicated the determination of the CP for the total section. CPs were scored in a range between 10 and 90 percent. Lower CPs were found in mucinous adenocarcinomas.
Cohen’s Kappa (κ) coefficient for interobserver agreement of the absolute CP showed a moderate agreement (κ = 0.522, concordance 59.1%). By categorizing the CP into three categories TSR (TSR-low, TSR-intermediate, and TSR-high) the κ-value improved and showed a good agreement (κ = 0.724, concordance 82.5%).
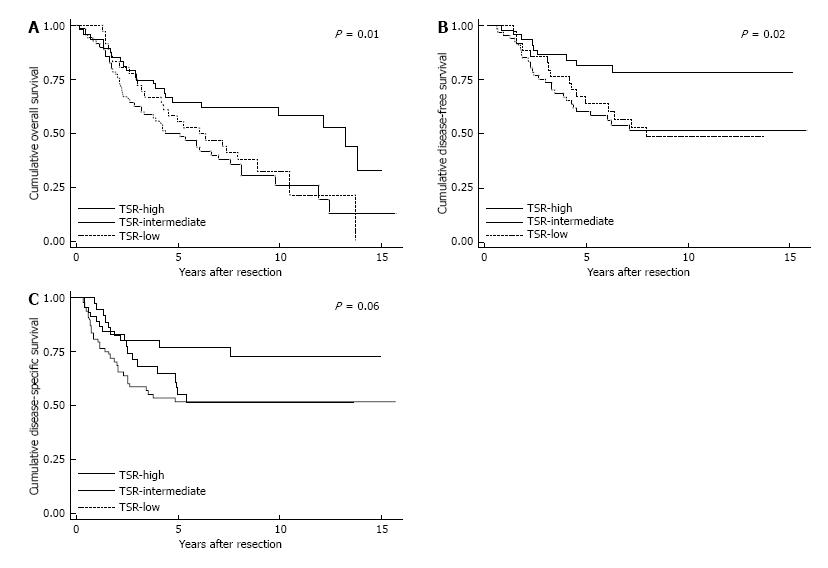
The 5-year survival rate for OS was 64.6% in the TSR-high population, vs 50.0% and 55.6% in the TSR-intermediate and TSR-low population, respectively. For the DFS, the 5-year survival rates for TSR-high, TSR-intermediate, and TSR-low were 77.2%, 51.8%, and 55.2%, respectively. The OS and DFS were significant different between the three TSR categories (P = 0.01 and P = 0.02, respectively). The 5-year survival rates for DSS were 81.6% for TSR-high, 60.3% for TSR-intermediate, and 63.9% for TSR-low. Although a higher DSS for the TSR-high population was thereby seen, the differences between the three TSR categories were just not significant (P = 0.06). The Kaplan-Meier survival curves are shown in Figure 3.
After adjusting for known prognostic factors (age, grading, and the use of adjuvant therapy), an intermediate TSR in lymph node metastasis negative patients showed a trend to a lower OS rate (HR = 2.04, 95%CI: 0.99-4.21) in comparison with a high TSR. There were no statistical differences between the TSR categories in OS among lymph node metastasis positive patients (Table 2). A statistically significant worse DFS was seen among the lymph node metastasis negative patients with an intermediate TSR (HR = 6.41, 95%CI: 1.84-22.28) compared to patients with a high TSR. Among lymph node metastasis positive patients, no statistically significant differences were seen between TSR categories for DFS (Table 3). Lymph node metastasis negative patients with an intermediate TSR had a higher risk of dying from rectal cancer (HR 5.27, 95%CI: 1.54-18.10) in comparison with patients with a high TSR. These differences were not seen in lymph node metastasis positive patients (Table 4).
| N0 | N+ | |||
| HR | 95%CI | HR | 95%CI | |
| Age | ||||
| < 70 yr | 1 | Ref. | 1 | Ref. |
| > 70 yr | 3.32a | 1.75-6.28 | 2.26a | 1.10-4.65 |
| Grading | ||||
| Poor | 1 | Ref. | 1 | Ref. |
| Moderate | 1.03 | 0.43-2.49 | 0.52 | 0.25-1.10 |
| Well | 0.36 | 0.04-2.99 | 0.56 | 0.06-5.31 |
| Adjuvant treatment | ||||
| No | 1 | Ref. | 1 | Ref. |
| Yes | 0.47 | 0.06-3.50 | 0.66 | 0.27-1.60 |
| TSR | ||||
| TSR-high | 1 | Ref. | 1 | Ref. |
| TSR-intermediate | 2.04 | 0.99-4.21 | 1.19 | 0.50-2.84 |
| TSR-low | 1.43 | 0.57-3.60 | 1.04 | 0.40-2.69 |
| N0 | N+ | |||
| HR | 95%CI | HR | 95%CI | |
| Age | ||||
| < 70 yr | 1.00 | Ref. | 1.00 | Ref. |
| > 70 yr | 0.36 | 0.13-1.01 | 0.89 | 0.36-2.22 |
| pT-status | ||||
| T1 | 1.00 | Ref. | 1.00 | Ref. |
| T2 | 0.11 | 0.01-1.21 | 0.66 | 0.10-4.44 |
| T3 | 0.85 | 0.09-7.58 | 1.51 | 0.31-7.30 |
| T4 | 1.61 | 0.07-39.27 | 1 | |
| Grading | ||||
| Poor | 1.00 | Ref. | 1.00 | Ref. |
| Moderate | 1.43 | 0.29-6.99 | 0.58 | 0.25-1.35 |
| Well | 1 | 1 | ||
| Adjuvant treatment | ||||
| No | 1.00 | Ref. | 1.00 | Ref. |
| Yes | 0.2 | 0.01-2.57 | 1.04 | 0.32-3.41 |
| TSR | ||||
| TSR-high | 1.00 | Ref. | 1.00 | Ref. |
| TSR-intermediate | 6.41a | 1.84-22.28 | 1.31 | 0.49-3.51 |
| TSR-low | 3.7 | 0.84-16.42 | 0.93 | 0.31-2.74 |
| N0 | N+ | |||
| HR | 95%CI | HR | 95%CI | |
| Age | ||||
| < 70 yr | 1.00 | Ref. | 1.00 | Ref. |
| > 70 yr | 0.47 | 0.16-1.40 | 1.19 | 0.53-2.67 |
| pT-status | ||||
| T1 | 1.00 | Ref. | 1.00 | Ref. |
| T2 | 0.12 | 0.01-1.29 | 0.48 | 0.06-3.50 |
| T3 | 0.69 | 0.08-6.25 | 1.29 | 0.27-6.07 |
| T4 | 0.46 | 0.02-8.70 | 1 | |
| Grading | ||||
| Poor | 1.00 | Ref. | 1.00 | Ref. |
| Moderate | 1.03 | 0.20-5.30 | 0.46 | 0.21-1.04 |
| Well | 1 | 1 | ||
| Adjuvant treatment | ||||
| No | 1.00 | Ref. | 1.00 | Ref. |
| Yes | 0.2 | 0.01-2.57 | 1.04 | 0.32-3.41 |
| TSR | ||||
| TSR-high | 1.00 | Ref. | 1.00 | Ref. |
| TSR-intermediate | 5.27a | 1.54-18.1 | 1.60 | 0.54-4.70 |
| TSR-low | 3.48 | 0.78-15.55 | 1.22 | 0.41-3.66 |
This study, analyzing data of 154 patients with rectal adenocarcinoma diagnosed in the period 1996-2006, showed that the TSR is a prognostic factor for patients without lymph node metastasis. In such cases, a high TSR had a longer local recurrence and distant metastasis free period, and a lower risk of death from rectal adenocarcinoma. Besides, a high TSR was associated with a lower risk of death from any cause. The determination of the TSR may therefore contribute to stratify patients for prognosis. Determination of the TSR turned out to be feasible and reproducible among observers on routinely made sections of rectal cancers. The TSR therefore has the potential to contribute to decision making regarding the individual treatment policy in rectal cancer.
The relation between the prognosis and the TSR may be explained pathophysiologically. A dual effect of the tumor stroma in the tumor-host interaction has been described. The tumor stroma is able to exert inhibitory effects on the malignant cells at first. With ongoing tumor growth, the tumor can exploit its stroma, for example by changing its composition (e.g., vasculature), to promote tumor growth and metastasis. A process called stromagenesis, which occurs parallel with tumor progression. Stromagenesis is characterized by bidirectional communication between the tumor and its stroma. The interactional pathways are multiple and complex[12-14]. Despite this complexity it is justifiable to conclude that the stromal tissue is not a passive component surrounding the tumor. A sufficient amount of stroma contributes to a more aggressive phenotype of tumor, as is shown in this study as well.
Indeed, the poor prognosis for lymph node negative patients with an intermediate TSR is remarkable. The survival rates for death from all causes, death from rectal cancer, and the occurrence of local recurrences and distant metastasis are the lowest for this group of patients. This may be explained by a favorable balance between the tumor and its stroma. In this way, the tumor may be able to exploit the surrounding tumor stroma very efficiently. The concept of a balance between pro- and antitumor factors had been hypothesized earlier. For example, there is a relation between the degree of the peritumoral inflammatory reaction and its ability to destroy invading colorectal malignant cells[15]. Lymph node metastasis can be seen as an expression of a developed tumor that has exploited its environment successfully. When lymph node metastasis has occurred, the effect of the tumor micro-environment may be negligible. This statement may explain why we did not find differences in survival in lymph node metastasis positive patients survival based on the TSR.
The effect of a high TSR on survival demonstrated in this study is in line with other studies on the prognostic impact of the TSR in colorectal carcinomas (CRCs) and other malignancies[6-10,16]. The present study is however the first that has identified a subgroup of patients with rectal cancer, namely lymph node metastasis negative patients with an intermediate TSR, whereby the TSR is a strong prognostic factor.
The interobserver agreement for absolute scores was moderate. The correlation coefficient improved to good, when grouping as TSR-low, TSR-intermediate, and TSR-high. The categorization into these categories was performed with the aim of generating enhanced prognostic information based on the TSR, which had been executed earlier in a previous study on the TSR in oesophageal adenocarcinomas[17]. Other studies concerning the TSR used an arbitrary cut-off value of 50%. No differences in the given survival rates were found at this and other cut-off values in our population (Appendix 1). The rate of agreement of the present study is slightly lower compared to these studies[7,8], which may be attributed to the determination of absolute CPs before the categorization and the addition of an extra CP-category.
This study has some shortcomings to be noted. Neo-adjuvant radiotherapy for rectal malignancies was applied more frequently at our hospital relatively late in the study period and consequentially patients received adjuvant therapy frequently. Neoadjuvant treated patients were excluded, while in most cases a neoadjuvant (chemo)radiotherapy regimen is given nowadays. Though, it remains valuable to investigate tissue based prognostic factors in non-pretreated patients. There is a tendency to treat elderly, for whom there is an increasing incidence of rectal cancer, without neoadjuvant radiotherapy due to postoperative wound complications in The Netherlands. According to the Dutch Surgical Colorectal Audit, no neoadjuvant therapy was used in 26% of cT2 patients aged > 75 years with rectal carcinoma in comparison with 14% in younger patients[18]. Furthermore, there is still a debate about adjuvant chemotherapy for rectal cancer. Future research about the balance between the oncological benefit, i.e., relative risk reduction of 50% in local recurrences and the side effects, i.e., relative risk increase of 50% in acute treatment-relate toxicity, and long-term anorectal and sexual dysfunction[19-21] of neoadjuvant radiotherapy will help to determine the position of pretreatment dependent tissue-based markers like the TSR in predicting an individual prognosis.
It would be of interest to analyze the TSR and its prognostic value in biopsy specimens of well described areas of a rectal tumor. Prognostic information could then be provided before the use of neoadjuvant therapy. The visual estimation of the TSR could be made more accurate by the use of tumor or stroma specific stainings. Besides, it would be desirable to develop a more objective instrument to determine the TSR than the visual estimation in this study. This could be provided by the use of tumor specific staining and the development of computer software in the growing field of digital pathology.
Determination of the TSR has the potential to identify patients without lymph node metastasis with a good and a poor clinical outcome and can thereby help in decision making on (neo)adjuvant treatment policy in individual cases. Determination of the TSR in routine sections is feasible and can be done with a good concordance by different observers.
Colorectal cancer is one of the most common form of cancer in both men and women. The TNM staging system, the most common system to stage colorectal tumors, is used to discriminate between patients with a better and a poor prognosis, but it seems insufficiently able to predict the prognosis of the individual patient. Additional prognostic factors are desirable, because a part of the patients is overtreated and consequently exposed to a higher risk on therapy related complications. The tumor-stroma ratio (TSR), the proportion of carcinoma relative to the proportion of tumor stroma in the histopathological section through the tumor, has proven to be of prognostic value in several malignancies.
A previous study, on a small number of rectal cancer patients, showed that a high TSR is possibly related to both a longer disease free and overall survival. In this respect, it is meaningful to explore the relevance of the TSR in a larger cohort of patients with rectal adenocarcinoma, as the authors did.
This paper showed that the TSR has potential as a prognostic factor for survival in surgically treated rectal cancer patients, especially in lymph node negative cases. The effect of a high TSR on survival demonstrated in this study is in line with other studies on the prognostic impact of the TSR in colorectal carcinomas and other malignancies. The present study is however the first that has identified a subgroup of patients with rectal cancer, namely lymph node metastasis negative patients with an intermediate TSR, whereby the TSR is a strong prognostic factor.
The determination of the TSR may contribute to stratify patients for prognosis and has the potential to contribute to decision making regarding the individual treatment policy in rectal cancer.
The TSR is the proportion of carcinoma relative to the proportion of tumor stroma in the histopathological section through the tumor. The carcinoma percentage (CP) is a derivative of the TSR and is complementary to the percentage of stroma and other components, like mucus. TSR-low including the CP-values ≤ 30%, TSR-intermediate including the CP-values 40%, 50% and 60%, and TSR-high including the CP-values ≥ 70%.
The study is well designed and clearly presented and the topic of high interest for oncologists that should decide after surgery what patients will benefit more from an adjuvant treatment.
| 1. | Utrecht: IKNL. Nederlandse Kankerregistratie, beheerd door IKNL. 2011-2016. Accessed April 10. 2017; Available from: http://www.cijfersoverkanker.nl. |
| 2. | Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008;61:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev. 2008;CD005390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Witz IP, Levy-Nissenbaum O. The tumor microenvironment in the post-PAGET era. Cancer Lett. 2006;242:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Mesker WE, Junggeburt JM, Szuhai K, de Heer P, Morreau H, Tanke HJ, Tollenaar RA. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29:387-398. [PubMed] |
| 7. | de Kruijf EM, van Nes JG, van de Velde CJ, Putter H, Smit VT, Liefers GJ, Kuppen PJ, Tollenaar RA, Mesker WE. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat. 2011;125:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Marsh D, Suchak K, Moutasim KA, Vallath S, Hopper C, Jerjes W, Upile T, Kalavrezos N, Violette SM, Weinreb PH. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223:470-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 9. | Yanagisawa N, Li R, Rowley D, Liu H, Kadmon D, Miles BJ, Wheeler TM, Ayala GE. Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum Pathol. 2007;38:1611-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | West NP, Dattani M, McShane P, Hutchins G, Grabsch J, Mueller W, Treanor D, Quirke P, Grabsch H. The proportion of tumour cells is an independent predictor for survival in colorectal cancer patients. Br J Cancer. 2010;102:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Koch GG, Landis JR, Freeman JL, Freeman DH Jr, Lehnen RC. A general methodology for the analysis of experiments with repeated measurement of categorical data. Biometrics. 1977;33:133-158. [PubMed] |
| 12. | Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2949] [Cited by in RCA: 2810] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 13. | Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 1540] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 14. | Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol. 2005;15:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2007;212:260-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Courrech Staal EF, Wouters MW, van Sandick JW, Takkenberg MM, Smit VT, Junggeburt JM, Spitzer-Naaykens JM, Karsten T, Hartgrink HH, Mesker WE. The stromal part of adenocarcinomas of the oesophagus: does it conceal targets for therapy? Eur J Cancer. 2010;46:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Courrech Staal EF, Smit VT, van Velthuysen ML, Spitzer-Naaykens JM, Wouters MW, Mesker WE, Tollenaar RA, van Sandick JW. Reproducibility and validation of tumour stroma ratio scoring on oesophageal adenocarcinoma biopsies. Eur J Cancer. 2011;47:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Dutch Surgical Colorectal Audit. Jaarrapportage 2011. Accessed April 10. 2017; Available from: http://www.dsca.nl/images/documenten/2012/jaarrapportage%202011.pdf. |
| 19. | Marijnen CA, van de Velde CJ, Putter H, van den Brink M, Maas CP, Martijn H, Rutten HJ, Wiggers T, Kranenbarg EK, Leer JW. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 482] [Article Influence: 23.0] [Reference Citation Analysis (4)] |
| 20. | Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 892] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 21. | Fleming FJ, Påhlman L, Monson JR. Neoadjuvant therapy in rectal cancer. Dis Colon Rectum. 2011;54:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cecchin E, Fogli L, Velenik V S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ