Published online Sep 15, 2016. doi: 10.4251/wjgo.v8.i9.688
Peer-review started: March 27, 2016
First decision: May 13, 2016
Revised: June 9, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: September 15, 2016
Processing time: 169 Days and 13.7 Hours
To determine whether there is an increased risk of gastric adenocarcinoma associated with vitamin D deficiency (VDd).
A retrospective case control study was performed of all patients diagnosed with gastric adenocarcinoma between 2005 and 2015. After we excluded the patients without a documented vitamin D level, 49 patients were included in our study.
The average age of patients with gastric adenocarcinoma and documented vitamin D level was 64 years old (95%CI: 27-86) and average vitamin D level was 20.8 mg/dL (95%CI: 4-44). Compared to a matched control group, the prevalence of VDd/insufficiency in patients with gastric adenocarcinoma was significantly higher than normal vitamin D levels (83.7% vs 16.3%). Forty-one patients (83.7%) with adenocarcinoma showed VDd/insufficiency compared to 18 (37%) patients with normal vitamin D level without gastric cancer (OR: 8.8, 95%CI: 5-22, P value < 0.0001). The average age of males with gastric adenocarcinoma diagnosis was 60 years old vs 68 years old for females (P = 0.01). Stage II gastric adenocarcinoma was the most prevalent in our study (37%).
We reported a positive relationship between VDd and gastric adenocarcinoma, that is to say, patients with decreased VDd levels have an increased propensity for gastric adenocarcinoma.
Core tip: In recent years, vitamin D deficiency (VDd) has been associated with several gastrointestinal malignancies largely mediated by vitamin D receptors. It affects multiple cellular processes such as inhibiting differentiation, metastasis, proliferation and inducing apoptosis and cell cycle arrest. All these mechanisms support vitamin D’s anti-cancer role. VDd removes the tumorigenic activity that it elicits from regulating cell cycle, inhibiting cellular proliferation, angiogenesis and molecular signaling. Several studies have revealed that vitamin D3 substantially promotes apoptosis in undifferentiated gastric malignant cells, specifically HCG-27. A retrospective research was conducted to find an association between vitamin D serum levels and gastric cancer.
- Citation: Vyas N, Companioni RC, Tiba M, Alkhawam H, Catalano C, Sogomonian R, Baum J, Walfish A. Association between serum vitamin D levels and gastric cancer: A retrospective chart analysis. World J Gastrointest Oncol 2016; 8(9): 688-694
- URL: https://www.wjgnet.com/1948-5204/full/v8/i9/688.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i9.688
Vitamin D is a fat soluble secosteroid responsible for enhancing intestinal absorption of several key nutrients such as calcium, magnesium, iron and phosphate. It is known to affect intestinal, skeletal and biologic pathways such as immune cells and tumor microenvironment. Vitamin D exists in two forms - vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), which is obtained from foods such as fortified dairy products, eggs, fish and liver[1]. The development of vitamin D3 also stems from the conversion process of 7-hydrocholesterol in the dermis after exposure to sunlight (ultraviolet-B radiation)[2]. Vitamin D2 and D3, whether from diet or dermal synthesis, is considered biologically inactive. The activation process begins in the liver where hydroxylation occurs by enzyme 25-hydroxylase, which results in 25-hydroxy vitamin D [25(OH)D][1-3]. Next, 25(OH)D travels to the kidneys to become the active form of vitamin D; 1,25(OH)2D3 by 1 alpha hydroxylase[2,3]. Subsequently, vitamin D3 is released to serum where it acts on receptors of the intestine, bone and kidney to regulate calcium metabolism[2,4,5].
Measurement of 25(OH)D level is the highly indicative for overall vitamin D status. 25(OH)D accounts for sunlight exposure, dietary intake and conversion from adipose tissue[3,6,7]. Vitamin D deficiency (VDd) is defined as 25(OH)D < 15-20 mg/dL and sufficient range > 30 mg/dL.
In recent years, VDd has been associated with a multitude of gastrointestinal malignancies largely mediated by vitamin D receptors (VDRs). It affects multiple cellular processes such as inhibiting differentiation, metastasis, proliferation and inducing apoptosis and cell cycle arrest. All these mechanisms support vitamin D’s anti-cancer role. Numerous studies have associated colon cancer with low vitamin D, however limited data suggests an association with gastric cancer[8-11].
Gastric adenocarcinoma is considered the fourth most common cancer worldwide. A major risk factor for development of gastric adenocarcinoma is Helicobacter pylori (H. pylori) infection[2]. Other risk factors include smoking and alcohol use. However, recent studies show that VDd is associated with poor prognosis in gastric adenocarcinoma[3]. Recent cohort studies revealed that increased serum vitamin D levels are linked to a decreased risk of gastric cancer.
VDd removes the tumorigenic activity that it elicits from regulating cell cycle, inhibiting cellular proliferation, angiogenesis and molecular signaling[12]. Current research illustrates that vitamin D3 increases apoptosis in undifferentiated gastric malignant cells, specifically HCG-27 cell lines[3]. In order to find a correlation between serum levels of vitamin D and gastric adenocarcinoma a retrospective research was conducted.
A retrospective, single-centered study was performed of all subjects diagnosed with gastric adenocarcinoma between 2005 and 2015. This study was conducted at a tertiary medical center in one of the most diverse communities in the United States, which provided a significant cultural and epidemiological advantage. This study was approved by Icahn School of Medicine at Mount Sinai, Institutional Review Board.
Among 3200 patients with gastrointestinal malignancies diagnosed between 2005 and 2015, 304 patients had gastric adenocarcinoma. Patients who did not have a vitamin D level were excluded. Of the 304 patients with gastric adenocarcinoma, 49 patients had documented vitamin D levels, documented gastric pathology by pathologist report and were over the age of 18. Vitamin D levels were defined as following: 25(OH)D deficiency (< 20 ng/mL), insufficiency (20-29 ng/mL) and normal 25(OH)D level (≥ 30 ng/mL).
Demographic data such as age, gender and ethnicity as well as smoking, body mass index, H. pylori infection and stage of adenocarcinoma were assessed. Our cohort was compared to a matched control group of subjects who had no known history of cancers and with documented level of vitamin D (Table 1).
| Characteristics | Case (gastric adenocarcinoma) | Control |
| (n = 49) | (n = 49) | |
| Age, yr | 63.9% | 60.43% |
| Male | 24 (49%) | 24 (49%) |
| Female | 25 (51%) | 25 (51%) |
| Hispanic | 30 (61.2%) | 30 (61.2%) |
| Body mass index | 24 kg/m2 | 25 kg/m2 |
| SD (22-27) | SD (23-28) | |
| Vitamin D deficiency | 19 (38.8%) | 7 (14.3%) |
| Vitamin insufficiency | 22 (44%) | 24 (49.0%) |
| Combined vitamin D deficiency and insufficiency | 41 (83.68%) | 31 (63.27%) |
For all statistical analyses, the results were considered significant when two-tailed P-values were < 0.05. The distributions of demographic information and baseline comorbidity were compared between both groups.
The average age of patients with gastric adenocarcinoma and documented vitamin D level was 64 years old (95%CI: 27-86) and average vitamin D level was 20.8 (95%CI: 4-44). Compared to the matched controls, the prevalence of VDd and vitamin D insufficiency in patients who had gastric adenocarcinoma was significantly higher than in patients with normal vitamin D levels (83.7% vs 63.27%). There were 41 gastric adenocarcinoma patients with VDd/insufficiency (83.7%) compared to 18 (37%) patients with normal vitamin D level without gastric adenocarcinoma (OR: 8.8, 95%CI: 5-22, P value < 0.0001) (Figure 1).
Furthermore, patients with VDd had an OR that was 2.7 times higher for gastric adenocarcinoma compared to the normal vitamin D population (95%CI: 1.4-5, P value 0.002), while patients with VDd had a 1.4 OR. However, endpoints did not achieve statistical significance (P value 0.1) (Figure 2).
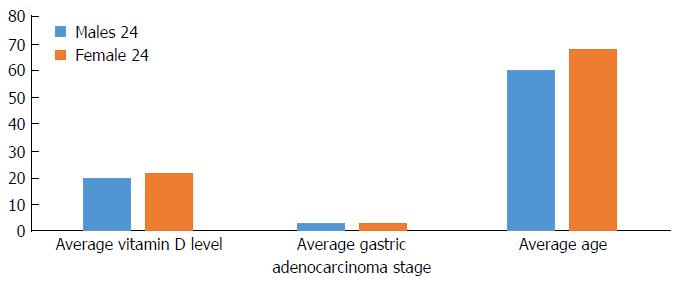
Among gastric adenocarcinoma groups, stage II gastric adenocarcinoma was most common (37%) followed by stages III (25%), I (20%) and IV (18%). The vitamin D level did not differ between different cancer stages. There were higher rates of Hispanics (61%) followed by the East Asian population (13%) (Table 2). The average age in males with a gastric adenocarcinoma diagnosis was 60 years old vs 68 years old for females (P = 0.01). There was no significant difference in average vitamin D level (20 ng/mL in males vs 22 ng/mL in females) or stage of cancer (Figure 3).
| Number of patients | Average vitamin D level | Average age | Average adenocarcinoma stage | |
| Hispanic | 30 (61%) | 20 | 63 | 3 |
| East Asian | 13 (27%) | 20 | 60 | 2 |
| Middle Eastern | 4 (8%) | 21 | 70 | 3 |
| Indian | 2 (4%) | 30 | 75 | 3 |
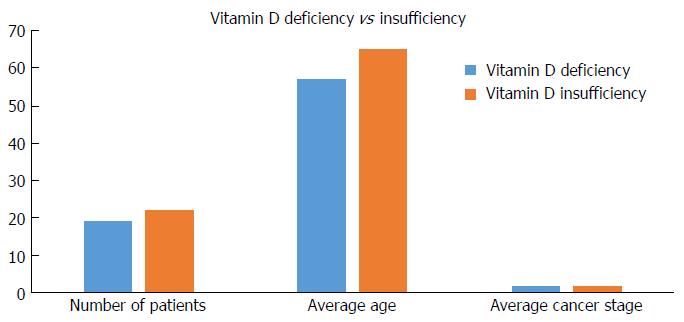
We sub grouped the patients with an abnormal vitamin D values into VDd and insufficiency; the average age of gastric adenocarcinoma in the deficiency group was 57.7 years old vs 65.5 years old in the insufficiency group (P = 0.07). There were no differences in gastric adenocarcinoma stage between the two groups (Figure 4).
In our study, our inclusion criteria were patients diagnosed with gastric adenocarcinoma with a vitamin D level. We had 49 patients as part of our cohorts. These cases were then matched to a cohort of similar age, gender and ethnicity with measured serum vitamin D level and no history of cancer. The mean age of the cases were 64 as opposed to the control group at 60. Gender ratio was similar - 49% male and 51% female. The predominant ethnicity was Hispanic, accounting for 61.2%. This can be attributed to the increased Hispanic patient population at Elmhurst Hospital Center. The diagnoses of gastric adenocarcinoma were by biopsy: 18% with stage IV, 25% with stage III, 37% with stage II and finally 20% with stage I. For our case group, the vitamin D value recorded was the one measured at the time of diagnoses and prior to repletion. We defined vitamin D levels in association with widely accepted thresholds. VDd is defined as < 20 mg/dL, insufficiency 20-29 mg/dL and sufficiency was ≥ 30 mg/dL.
The prevalence of VDd was compared between the gastric adenocarcinoma group and matched control group, using ORs and 95%CI. Our finding showed an increased prevalence of VDd in the case group as compared to the matched group - 39% vs 14% respectively. The primary end point suggests an association between VDd and gastric adenocarcinoma; however, the study also found no correlation between the degree of deficiency and the stage of gastric adenocarcinoma.
In general, there is now accumulating evidence of VDd associated with multiple malignancies due to its anticancer effects. There has been increasing data for the association of VDd with colon cancer[2,5,10]. More recently, there is some new research suggesting an association with esophageal, pancreatic and liver cancer as well[2]. However, there is limited data showing gastric adenocarcinoma. The anticancer biochemical role of vitamin D is largely mediated by the VDR. VDR is a steroid in the thyroid hormone receptor family. Free 1,25 D3 binds to the VDR causing phosphorylation of the receptor then the ligand activated VDR interacts with the retinoid X receptor (RXR) to form a heterodimer[8,9,11-14]. Subsequently, the heterodimer 1,25-VDR-RXR complex translocate to the nucleus and thus binds to vitamin D response elements (VDREs) in multiple regulatory regions located in the promoters of target genes[5,8,9,11-14]. The receptor signaling between RXR and VDR is a vital step for VDR transcriptional activity. VDREs are also utilized to initiate gene transcription. The RXR-VDR complex recruit specific coactivator molecules such as steroid receptor coactivators, histone acetyltransferases and its mediators. The significance of the RXR-VDR complex translocating to the nuclei and binding to VDREs is to allow for promotion or suppression of precise cellular events of tumorigenesis[2,4,5,15].
The most common malignancy of the stomach is adenocarcinomas arising from gastric epithelium. Gastric adenocarcinoma is the fourth most common malignancy worldwide. In the United States, gastric adenocarcinoma accounts for 1.5% of new cancer cases and 1.8% of cancer deaths. The 5-year relative survival rate for gastric cancer increased from 14.3% in 1975 to 29.3% from 2006-2011[16]. The 5-year observed survival rate for gastric adenocarcinoma patients after surgery ranges from 71% for stage IA to as low as 4% for stage IV[17].
Most gastric cancer patients are elderly when diagnosed with a median age of 69 in the United States. Less than 2% of gastric cancer cases arise in patients under 35 years of age. Asia and South America have higher rates of gastric cancer than in the United States. Gastric adenocarcinoma is marginally more prominent in males in the United States, whereas in Japan, it is the most common cancer diagnosed in men. However, worldwide, gastric cancer rates are twice as high in men compared to women. American Cancer Society’s estimates for stomach cancer in the United States for 2015 revealed that approximately 24590 cases will be diagnosed (15540 in men and 9050 in women) and that 10720 people will die from this type of cancer (6500 men and 4220 women).
Gastric adenocarcinoma is often asymptomatic, but some non-specific symptoms reported include indigestion, abdominal discomfort and appetite loss. Later in the disease phase there is bleeding which leads to anemia. Although causes of gastric cancer are multifactorial, H. pylori infections are an essential risk factor[17]. However, only 2% of people with H. pylori infections develop stomach cancer[18]. Duodenal ulcers are most likely due to inflammation of the pyloric antrum, whereas inflammation of the corpus causes gastric ulcers and gastric carcinoma[19]. There is a counter correlation between low socioeconomic status and this disease due to factors such as poor nutrition, poor sanitation and scarce treatment and conservation of food and water. Individuals who maintain diets high in fruits, green vegetables, beta-carotene and ascorbic acid are less at risk. There is also research that indicates decreased use of nitrites in prepared foods reduces the risk as well. It has also been well studied that dietary factors such as smoked foods and red meat[20] also increase the risk. With respect to vitamin D, there is now supporting evidence for the association of deficient levels and an increase risk for gastric adenocarcinoma. Several in vitro studies proved that 1,25-dihydroxyvitamin D3 has the anticarcinoma effects of anti-proliferation, promoting apoptosis[9,20-22]. More specifically, it has been demonstrated that vitamin D3 substantially stimulates apoptosis in the undifferentiated gastric cancer cell line HCG-27[3,23]. Moreover, specific to gastric adenocarcinoma, vitamin D3 inhibits gastric cancer cell growth and induces cell cycle arrest[9,20,23]. Bao et al[12] found that direct usage of 1,25 dihydroxy vitamin D3 induces cellular apoptosis in gastric cancer cells and also increases the expression of VDR, further supporting the antitumor role that vitamin D may activate in gastric adenocarcinoma[2]. One of the limitations of our study is the small population number as not every patient with gastric adenocarcinoma had documented vitamin D level. Another limitation that must be addressed is the role malnutrition in cancer patients. There are several pathogenesis of cancer associated malnutrition. The primary reason is decreased food intake due to systemic effects of the disease, local tumor effects, psychological effects or adverse effects of treatment. Other contributing factor is the malignancy releasing inflammatory markers systemically such as procytokines have been linked to malnutrition and cachexia[24]. However, this study is unable to assess the nutrition status of the patients included in this study but one must be aware malnutrition which includes low vitamin D could be a result of the malignancy itself.
The question becomes, can vitamin D supplementation decrease incidence of gastric adenocarcinoma? It is reasonable to suggest the role of supplementation of vitamin D in deficient patients because of indirect etiologies related to gastric adenocarcinoma. Patients who underwent gastrectomy subsequently altered vitamin D metabolism, especially amongst those who received total gastrectomy[3,25]. Supplementation is also recommended for patients who have malnutrition and malabsorption disorders, which is a consequence of malignancy or diseases such as celiac sprue. Lastly, there is new evidence that high doses of vitamin D plus calcium show significant reduction in cancer incidence in women[26]. With expanding evidence of vitamin D supplementation and its benefits as mentioned above, avoiding deficiency or adding vitamin D supplements might be an economical and safe way to reduce cancer incidence, or act as a supplement to chemotherapeutic agents[9,11,24,27]. Overall, VDd can be easily corrected by taking supplements, changing dietary habits or increasing sunlight exposure, which is why it is a practical reason for clinicians to assess vitamin D status. To further assess this association, additional prospective research should be done to clarify the benefit of vitamin D supplementation and possible prevention of gastric adenocarcinoma.
In summary, there is increasing research on the role of vitamin D and gastrointestinal cancers, but there is still limited data on the association of vitamin D and gastric adenocarcinoma. Our study showed VDd has an increased predisposition for gastric adenocarcinoma. There is however no correlation between the severity of VDd and stage of gastric adenocarcinoma.
Special thanks to Dr. Baum, Dr. Walfish and my fiance Amanda Thambounaris because without their help and guidance, this paper couldn’t have been completed.
In recent years, vitamin D deficiency (VDd) has been associated with a multitude of gastrointestinal malignancies. It affects multiple cellular processes such as inhibiting differentiation, metastasis, proliferation and inducing apoptosis and cell cycle arrest. All these mechanisms support vitamin D’s anti-cancer role. Numerous studies have associated colon cancer with low vitamin D, however limited data suggests an association with gastric cancer.
Vitamin D is a fat soluble secosteroid that is known to affect intestinal, skeletal and biologic pathways such as immune cells and tumor microenvironment. Vitamin D is related to many cancers and the current research hotspot is the potential use of vitamin D screening and supplementation to prevent or slow the progression of malignancy.
Understand the pathogenesis of vitamin D anticancer role. Several studies have found a link between low vitamin D levels and gastrointestinal malignancy. However, there is limited data suggesting a relationship between VDd/insufficiency in gastric adenocarcinoma.
This study showed VDd has an increased predisposition for gastric adenocarcinoma. Further studies are warranted to assess this association and the possible risk stratification of vitamin D supplementation in preventing or slowing the progression of gastric adenocarcinoma.
The anticancer biochemical role of vitamin D is largely mediated by the vitamin D receptor (VDR). VDR is a steroid in the thyroid hormone receptor family. Free 1,25 D3 binds to the VDR causing phosphorylation of the receptor then the ligand activated VDR interacts with the retinoid X receptor to form a heterodimer. This allows for the suppression or promotion of specific cellular events of tumorigenesis.
It is interesting that the VDd is associated with gastric adenocarcinoma. The article fits in perfectly with the global trend.
| 1. | Chen W, Dawsey SM, Qiao YL, Mark SD, Dong ZW, Taylor PR, Zhao P, Abnet CC. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97:123-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Hargrove L, Francis T, Francis H. Vitamin D and GI cancers: shedding some light on dark diseases. Ann Transl Med. 2014;2:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 3. | Ren C, Qiu MZ, Wang DS, Luo HY, Zhang DS, Wang ZQ, Wang FH, Li YH, Zhou ZW, Xu RH. Prognostic effects of 25-hydroxyvitamin D levels in gastric cancer. J Transl Med. 2012;10:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 802] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 5. | Picotto G, Liaudat AC, Bohl L, Tolosa de Talamoni N. Molecular aspects of vitamin D anticancer activity. Cancer Invest. 2012;30:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 560] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 8. | Wen Y, Da M, Zhang Y, Peng L, Yao J, Duan Y. Alterations in vitamin D signaling pathway in gastric cancer progression: a study of vitamin D receptor expression in human normal, premalignant, and malignant gastric tissue. Int J Clin Exp Pathol. 2015;8:13176-13184. [PubMed] |
| 9. | Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1030] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 10. | Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 971] [Article Influence: 80.9] [Reference Citation Analysis (2)] |
| 11. | Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 482] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 12. | Bao A, Li Y, Tong Y, Zheng H, Wu W, Wei C. Tumor-suppressive effects of 1, 25-dihydroxyvitamin D3 in gastric cancer cells. Hepatogastroenterology. 2013;60:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 13. | Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 523] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 14. | Pike JW, Meyer MB. Fundamentals of vitamin D hormone-regulated gene expression. J Steroid Biochem Mol Biol. 2014;144 Pt A:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: a review of molecular mechanisms. Biochem J. 2012;441:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 302] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 16. | González CA, Sala N, Rokkas T. Gastric cancer: epidemiologic aspects. Helicobacter. 2013;18 Suppl 1:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Ogata T, Araki K, Matsuura K, Kobayashi M, Inomata T, Yasuhiro O, Yoshida S. A 10-year experience of intraoperative radiotherapy for gastric carcinoma and a new surgical method of creating a wider irradiation field for cases of total gastrectomy patients. Int J Radiat Oncol Biol Phys. 1995;32:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Rust M, Schweinitzer T, Josenhans C. Helicobacter flagella, motility and chemotaxis. In: Helicobacter pylori. Molecular genetics and cellular biology. Caister Academic Press 2008; 61-86. |
| 19. | Jakszyn P, Gonzalez CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol. 2006;12:4296-4303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 262] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (6)] |
| 20. | Bao A, Li Y, Tong Y, Zheng H, Wu W, Wei C. 1,25-Dihydroxyvitamin D3 and cisplatin synergistically induce apoptosis and cell cycle arrest in gastric cancer cells. Int J Mol Med. 2014;33:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Wang W, Zhao CH, Zhang N, Wang J. Vitamin D analog EB1089 induces apoptosis in a subpopulation of SGC-7901 gastric cancer cells through a mitochondrial-dependent apoptotic pathway. Nutr Cancer. 2013;65:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Pan L, Matloob AF, Du J, Pan H, Dong Z, Zhao J, Feng Y, Zhong Y, Huang B, Lu J. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A /sodium butyrate-induced and 5-aza-2’-deoxycytidine-induced PTEN upregulation. FEBS J. 2010;277:989-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Park MR, Lee JH, Park MS, Hwang JE, Shim HJ, Cho SH, Chung IJ, Bae WK. Suppressive effect of 19-nor-1α-25-dihydroxyvitamin D2 on gastric cancer cells and peritoneal metastasis model. J Korean Med Sci. 2012;27:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9 Suppl 2:S51-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 405] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 25. | Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013;20:R31-R47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Pendás-Franco N, González-Sancho JM, Suárez Y, Aguilera O, Steinmeyer A, Gamallo C, Berciano MT, Lafarga M, Muñoz A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75:193-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586-1591. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Sakitani K, Song HS, Yang MH, Yuan AH, Zebrowska M S- Editor: Gong XM L- Editor: A E- Editor: Zhang FF