INTRODUCTION
Colorectal cancer (CRC) remains a major contributor to cancer cancer worldwide and accounts for about 9% of overall cancer incidence[1]. There is, however, a quite remarkable country-to-country variation and CRC appears to be primarily a disease of Western life-style[1]. In India the incidence of CRC is about one seventh that of the United States[2]. Moreover, immigrants from low-risk countries who move to high-risk countries generally assume the high-risk profile of the new host country[1]. These data suggest that environmental factors could be important in the etiology of sporadic CRC and provide some hope that chemopreventive/lifestyle strategies could have a significant impact on reducing CRC incidence. The primary purpose of this review is to evaluate the potential role that pro-oxidant and antioxidant factors play in the development of CRC. The roles of these factors are of major importance to the advice that oncologists provide to cancer patients and that nutritionists provide to the general population. The food industry also has a vested interest in these issues since many foods are labeled as being “high in antioxidants” with the implicit promise of promoting health.
PRO-OXIDANTS AND ANTIOXIDANTS IN CRC
The role of both antioxidants and pro-oxidants in colon cancer has become a topic of intense interest and controversy[3-6]. A great body of evidence supports the view that in vivo oxidative stress and the accompanying reactive oxygen species (ROS) are genotoxic and contribute to the development of colon cancer and cancers in general (Figure 1)[7]. ROS are thought to be a major source of endogenous DNA damage and at least one hundred oxidative modifications to DNA have been identified[8,9]. It is plausible to assert, therefore, that antioxidants could be beneficial by minimizing the genotoxic insults caused by ROS and thereby reduce the incidence of cancers. In this capacity, antioxidants in food or in dietary supplements would be acting as long-term chemopreventive agents. Moreover, it is now well recognized that many cancer cells exhibit an enhanced level of intrinsic oxidative stress that plays a causative role in the expression of many oncogenic phenotypes[10,11]. The ROS giving rise to the enhanced level of intrinsic oxidative stress in cancer cells are thought to promote oncogenic phenotypes by virtue of their roles in modulating redox sensitive signal transduction mechanisms[12]. It follows, that in vivo antioxidant agents (chemical and enzymatic) that lower ROS could potentially inhibit the expression of aggressive cancer phenotypes. Antioxidants could, therefore, be chemopreventive by reducing both genotoxicity and by slowing cancer progression.
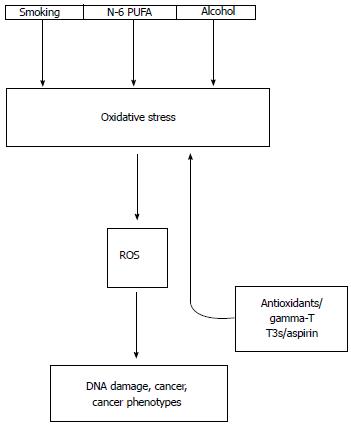
Figure 1 Connections between known risk factors of colorectal cancer and oxidative stress.
Smoking, dietary n-6 polyunsaturated fatty acids (n-6 PUFA) and heavy alcohol consumption contribute to in vivo oxidative stress with an accompanying overproduction of genotoxic reactive oxygen species (ROS) that give rise to mutations, cancer and also promote cancer phenotypes. Antioxidants such as gamma-tocopherol (gamma-T), tocotrienols (T3s) and aspirin reduce oxidative stress and ROS overproduction (up arrow).
DO ANTIOXIDANTS INTERFERE WITH CHEMOTHERAPY?
On the other hand, many effective pro-oxidant chemotherapeutic agents rely on inducing additional oxidative stress in cancer cells, thereby driving them into apoptotic cell death (Figure 2). This process is thought to have some degree of selectivity since cells with a “normal” level of oxidative stress would not be sufficiently stressed by pro-oxidant chemotherapeutic agents to reach the threshold at which apoptosis would be triggered. A key concern, however, is that cancer patients with a high level of dietary/supplement antioxidant intake could be resistant to pro-oxidant chemotherapeutic agents[3,6]. It is likewise plausible that a high level of antioxidant intake could shield normal tissues from the cytotoxic effects of pro-oxidant chemotherapeutic agents, thereby reducing many of the severe side effects associated with these agents. These issues were expertly reviewed in 2008 with the conclusion that supplemental antioxidants should be avoided during chemotherapy and radiation therapy based on their potential for protecting tumors and reducing the effectiveness of the pro-oxidant therapies. Little has changed from 2008 and this assessment remains valid. Nevertheless, this remains an area where more evidence-based medicine is needed. As is often the case in clinical research, a more nuanced approach is required that is cancer specific, dose and time controlled and focused on specific antioxidants.
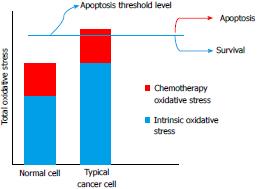
Figure 2 Conceptual framework for the selective action of chemotherapeutic agents that induce oxidative stress.
Many cancer cells show a high level of intrinsic oxidative stress (“blue” component of total oxidative stress) compared to normal cells. Chemotherapeutic agents often act by inducing an additional level of oxidative stress (“red” component of total oxidative stress) that is sufficient to reach an apoptotic threshold (blue line) in a typical cancer cell but not a normal cell.
A SYSTEMS BIOLOGY APPROACH TO REDOX ISSUES IN CRC
The complex set of interconnected events that give rise to CRC are unlikely to be fully replicated (or even well elucidated) in cell-based studies, animal models, observational studies or even short term clinical intervention trials. A systems biology approach in which an organism is considered a dynamic set of interacting organs, tissues, cells and molecular level components is more realistic, especially since time-dependency and interconnecting environmental factors are also key to this approach[13].
OXIDATIVE STRESS AND THE INTESTINAL MICROBIOME
The utility of a systems biology approach to oxidative stress and CRC is firmly illustrated when considering the role of the intestinal microbiome. The intestinal microbiome is the community of commensal, symbiotic, and pathogenic microorganisms sharing the space within the intestinal lumen. CRC, in most cases, arises from the epithelial cells of the large intestine, which have suffered DNA damage and a subsequent loss of epithelial differentiation towards a phenotypic expression closer to that of mesenchymal cells (the epithelial-to-mesenchymal transition or EMT)[14]. The EMT of DNA-damaged colonic epithelial cells is thought to promote metastasis to other essential organs. The small intestine, despite its name, has an epithelial surface area some 30 times larger than the large intestine. This large difference is due to the fact that the small intestine, unlike the large intestine, is very convoluted and has villi. Nevertheless, cancers of the small intestine are rare, amounting to only 2.3% of cancers of digestive system in the United States[15]. A complete understanding of this differential rate of cancer incidence along the digestive track is lacking but accumulating data suggest that oxidative stress could be an important factor.
A major difference between the small intestine and large intestine is the number of bacteria present. The duodenum and jejunum (66% of the small intestine) contain low numbers of bacteria but this number markedly increases by about four orders of magnitude in the distal ileum and in the large intestine[16]. Owen et al[17] have found that the human fecal matrix is capable of generating abundant ROS. In marked contrast, many isolated and cultured aerobic or anaerobic fecal bacteria do not generate abundant ROS[17]. Babbs et al[18] also found that fecal matrix generates a high flux of ROS being the equivalent of 10000 rads of gamma irradiation per day. This high level of ROS production stopped after autoclaving the feces suggesting the direct involvement of fecal bacteria. The in vitro experiments of both Owen et al[17] and Babbs et al[18] must be interpreted with some caution and future work in this area is needed given the importance of understanding the role of digestive system microflora in cancer development. Nevertheless, these studies suggest that the interaction between fecal bacteria and the fecal matrix generates oxidative stress.
IS COLONIC OXIDATIVE STRESS SUFFICIENT TO CAUSE GENOTOXIC DAMAGE?
Whether or not colonic oxidative stress arising from colonic bacteria is capable of causing significant in vivo genotoxicity in humans is not yet known. The in vitro results of Wang and Huycke[19] are, however, very relevant in this regard. These investigators found that Enterococcus faecalis (E. faecalis), a prevalent fecal bacteria that uniquely generates extracellular superoxide, is quite effective at promoting chromosomal instability (CIN) in mammalian cells at a level equal to that 2 gray (or 200 rad) of gamma-irradiation. E. faecalisis thought to generate superoxide by virtue of a rudimentary respiratory chain in which an electron is transferred to oxygen by membrane-associated demethylmenaquinone[19].
Under normal circumstances, fecal bacteria do not come into contact with the epithelial cells of the large intestine, which is covered by layers of dense mucin (10-90 microns thick). With this fact in mind, Wang and Huyche[19] reasoned that M cells (or microfold cells) in the colon could potentially transport E. faecalis across the intestinal lumen to macrophage cells (antigen presenting cells) across the epithelial barrier (into the subepithelial dome) for immunological processing (i.e., the innate immune system). This is schematically illustrated in Figure 3. Accordingly, these investigators tested the hypothesis (in vitro) that macrophages that have phagocytized E. faecalis could generate diffusible oxidation products that could induce CIN in surrounding hybrid hamster cells containing human chromosome 11. Their results were consistent with this hypothesis: a 2.5 fold increase in CIN was found in hamster cells co-incubated macrophages that phagocytized E. faecalis compared to hamster cells co-incubated with macrophages not having phagocytized E. faecalis. Control experiments using Escherichia coli, which generates only low levels of superoxide, elicited only a modest degree of mammalian cell CIN in their model.
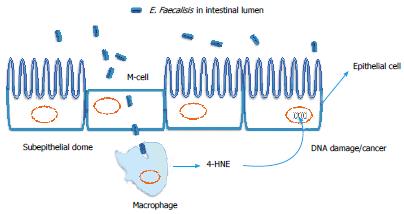
Figure 3 Role of pro-oxidant intestinal bacteria in colorectal cancer.
Enterococcus faecalis (E. faecalis) an intestinal bacteria with the unique ability to generate superoxide radicals. Intestinal microfold cells (M-cells) may transport E. faecalis from the intestinal lumen to macrophages in the subepithelial dome where macrophage cyclooxygenase-2 (COX-2) and lipid peroxidation can generate 4-hydroxynonenal (4-HNE), which promotes DNA damage/chromosomal instability to nearby epithelial cells[20].
GAMMA-TOCOPHEROL BUT NOT ALPHA-TOCOPHEROL INHIBITS CHROMOSOMAL INSTABILITY IN MAMMALIAN CELLS INDUCED BY ENTEROCOCCUS FAECALIS
Quite interestingly, Wang and Huyche[19] found that 200 μmol gamma-tocopherol, but not alpha-tocopherol, was able to completely inhibit the CIN inflicted on hamster cells when co-incubated with macrophages having phagocytized E. faecalis. Moreover, cyclooxygenase-2 (COX-2) overexpression was found in the macrophages having phagocytized E faecalis and COX-2 inhibitors (as well as superoxide dismutase) blocked the induced CIN in hamster cells.
In a logical follow-up to this in vitro work, these investigators provided convincing evidence that the diffusible product of oxidative stress induced by E. faecalis was 4-hydroxy-2-nonenal (4-HNE), which is a well-known aldehyde by-product arising from the lipid peroxidation of omega-6 polyunsaturated fatty acids (e.g., arachidonic acid)[20] (Figure 4). As mentioned below, arachidonic acid is a proinflammatory dietary fatty acid and dietary arachidonic acid is a risk factor for CRC. Moreover, silencing glutathione-S-transferase alpha4 (GST-alpha4) in colonic epithelial cells increased their susceptibility to 4-HNE CIN. GST-alpha4 detoxifies 4-HNE by covalently complexing it with GSH. Similarly, silencing COX-2 decreased 4-HNE production by E. faecalis infected macrophages and depleting GSH (the primary intracellular antioxidant) increased 4-HNE production[21].
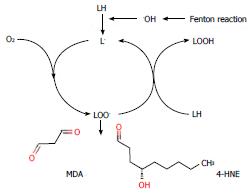
Figure 4 Lipid peroxidation and mutagens.
Lipids containing PUFA (LH) are oxidized to form damaging lipid peroxy radicals (LOO.). These radicals can generate mutagenic aldehydes such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) from n-6 PUFAs. Lipid peroxidation can be initiated by the formation of highly reactive hydroxyl radicals (.OH) arising from the Fenton reaction.
In an outstanding pre-clinical in vivo experiment, these investigators also found that interleukin-10 knockout mice (IL-10-/-) colonized with superoxide producing E. faecalis developed both inflammation and CRC[20]. IL-10-/- mice develop colitis and are a model for human inflammatory bowel disease. In a parallel experiment with a strain of E. faecalis that does not produce superoxide (delta-men) the IL-10-/- mice showed inflammation but not CRC[20]. Unfortunately, the investigators did not determine if supplementation with gamma-tocopherol prevented CRC in the animal model using the superoxide producing E. faecalis. The overall model proposed by these investigators is provided in Figure 3. Collectively, this work provides a very compelling hypothesis for the etiological factors promoting CRC, i.e., M-cells transport pro-oxidant intestinal bacteria to macrophages in the subepithelial dome and through the action of macrophage COX-2 and lipid peroxidation on pro-inflammatory n-6 n-6 polyunsaturated fatty acid (PUFA) generate 4-HNE which diffuses to nearby epithelial cells inducing genotoxicity eventually resulting in CRC. This is a powerful model and does much to explain the interconnections between the known risk factors for CRC and their relationship to the oxidative stress (Figure 1).
DYSFUNCTION OF THE INTESTINAL MICROBIOME, INFLAMMATORY BOWL DISEASE AND OXIDATIVE STRESS
It is becoming increasingly clear that dysfunction of the intestinal microbiome is very much related to inflammatory bowl disease(s) such as Crohn’s disease and ulcerative colitis. Inflammatory bowel diseases (IBDs) are known to increase the risk of CRC and inflammation (in general) is also accompanied by increased oxidative stress[20]. With rapid technical advances it will soon be routinely possible for individuals to have their exomes sequenced in an effort to identify markers for cancer susceptibility. For most types of cancer, exome sequencing alone should be sufficient but CRC might be an exception. The collective genomes of the intestinal microbiome is estimated to be about 100 times that of the human genome and is of direct significance to CRC. Although beyond the scope of this review, it is now clear that that there are complex interactions between the intestinal microbiome, IBD and metabolic processes in the large intestine[22]. Of particular interest, however, is the model proposed by Morgan et al[22] which suggests that IBD could be accompanied by a shift in the normal microbiome to microbes utilizing mucin as a primary energy source and thereby compromise the barrier function of mucin in protecting colonic epithelial cells from contact with microbes. Mucin is rich in the sulfur-containing amino acids needed to synthesize glutathione (GSH), which is a key intracellular antioxidant. The loss (or thinning) of the mucin barrier would, in turn, result in increased inflammation and oxidative stress that could then select for those microbes efficient at using GSH to survive in an oxidatively stressed environment.
THE GENETIC CHARACTERISTICS OF CRC AND OXIDATIVE STRESS
The types of mutations that occur in CRC are of interest since this information is relevant to etiological factors and their origin. The lung, the esophagus and the colon, unlike most other internal organs, are directly exposed to a wide variety of environmental mutagens and, not unexpectedly, have a large number of non-synonymous mutations per tumor compared with other adult solid tumors[23]. Nonsynonymous mutations are those that alter the encoded sequence of a protein. Lung cancer is thought to be largely (about 90%) due to mutagens in cigarette smoke. Cigarette smoking is a major oxidative stressor and it is reasonable to suggest that smoke derived carcinogens and oxidants in tobacco tar could make their way to the colon.
OXIDATIVE STRESS CAN INACTIVATE THE DNA MISMATCH REPAIR MECHANISM SYSTEM IN CRC
CRC mutations fall into two general categories: (1) those with mutations accompanied by microsatellite instability (MSI) in which there is a defective DNA mismatch repair (MMR) mechanism during DNA replication (about 15% of all CRC); (2) those with mutations accompanied by microsatellite stability (MSS) but with chromosomal instability[23]. Microsatellites are repeating sequences of 2-6 base pairs of DNA.
MSI CRC is associated with a very large number of non-synonymous mutations yet has a better prognosis than MSS CRC[23,24]. A high frequency form of MSI (MSI-H), where over 40% of the assayed microsatellites are mutated, is associated with germline mutations in the protein complexes forming the MMR system or with epigenetic silencing of MMR protein expression by DNA hypermethylation[25]. A second, low frequency form of MSI (MSI-L) also occurs in which less than 20% of the assayed microsatellites are mutated[25]. Quite curiously, 10%-20% of sporadic CRC of the MSI-L variety show no evidence of mutations in MMR proteins and are only rarely associated with epigenetic silencing of MMR protein expression by DNA hypermethylation[25]. MSL-L is, however, found in CRC associated with ulcerative colitis, a long lasting inflammatory bowel disease with well-documented evidence of oxidative stress[25,26]. Chang et al[25] have shown that oxidative stress can inactivate the MMR system leading to the suggestion that this mechanism could be responsible for the MSI-L seen in CRC associated with chronic inflammation such as ulcerative colitis and/or smoking and/or high alcohol consumption which are all oxidative stressor factors (see below).
WHAT DO OBSERVATIONAL STUDIES TELL US ABOUT THE INCIDENCE OF CRC AND ENVIRONMENTAL ANTIOXIDANTS AND PRO-OXIDANT FACTORS?
Observational studies are fraught with limitations and do not directly speak to causality. Nevertheless, such studies are often all that is available and have the advantage that very large subject populations can be studied over very long time periods at a reasonable cost. Observational studies are very useful for hypothesis building, particularly when combined with pre-clinical data from cell and animal models. The genetic data, summarized above, suggests that environmental mutagens contribute to CRC. As detailed below, a strong case can be made that many of the environmental factors known to contribute to CRC incidence are also sources of oxidative stress and genotoxicity.
CIGARETTE SMOKE AS AN ENVIRONMENTAL SOURCE OF OXIDATIVE STRESS AND GAMMA-TOCOPHEROL AS A DIETARY ANTIOXIDANT
Cigarette smoking and is thought to contribute to about 12% CRC incidence[1]. For reasons that are not clear, the increased risk of CRC due to smoking appears to be greater in women than in men[27]. Smoking is a major source of free radicals in both the gaseous and tar phases[28,29] and is a major contributor to in vivo oxidative stress. Overwhelming evidence shows that smoking increases many systemic biomarkers for oxidative stress such as breath pentane[30], plasma protein carbonyls[31], F2 isoprostanes[32] and also causes an increased vitamin E consumption through an oxidative stress pathway[33-35].Cigarette smoke contains a significant amount of reactive nitrogen species (RNS) as well as ROS[36]. Gamma-tocopherol (the main dietary form of vitamin E) has a unique ability to react with RNS to form 5-nitro-gamma-tocopherol (NGT) and levels of NGT are about two fold higher in smokers compared to nonsmokers[36]. We do not yet know if supplementation with gamma-tocopherol would reduce the genotoxic effects of RNS cigarette smoke and thereby reduce CRC or cancer in general.
CIGARETTE SMOKE, MUTAGENIC POLYCYCLIC AROMATIC HYDROCARBONS AND LIPID PEROXIDATION
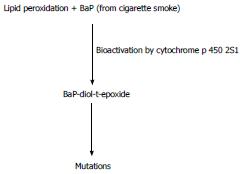
Cigarette smoke (and the high temperature cooking of meat) is also a source of polycyclic aromatic hydrocarbons (PAHs), which are subsequently activated to potent mutagens. A recent case-cohort study suggests that a 57% increase in CCR is associated with a doubling of the level of aromatic DNA adducts[37]. Benzo[a]pyrene (BaP) is a major and potent PAH carcinogen found in cooked meats and tobacco smoke. It has long been known that the activation of BaP to its ultimate carcinogen can be promoted by the process of lipid peroxidation[38]. DNA adducts of carcinogenic BaP metabolites have been found in human colon mucosa[39].
Recent work now suggests (Figure 5) that lipid hydroperoxides support the cytochrome P450 mediated activation of benzo[a]pyrene-trans-7,8-dihydrodiol (BaP-7,8-diol) into the highly mutagenic the highly mutagenic and carcinogenic benzo[a]pyrene-r-7,t-8-dihydrodiol-t-9,10-epoxide (BaP-diol-t-epoxide)[40]. Cytochrome P450s (CYPs) are a superfamily of enzymes that catalyze the oxidation of xenobiotic organic substances such PAHs as well as a variety of endogenous compounds. CYP2S1 is the particular cytochrome P450 that effectively activates BaP-7,8-diol[40]. It is very relevant, therefore, that the P450 profile of CRC has been determined and CRC tissues show a higher level of CYP2S1 expression compared to normal CR tissue[41]. Moreover, a higher CRC expression of CYP2S1 was associated with poor prognosis[41]. Collectively, the above suggests that smoking induced oxidative stress, and increased exposure to BaP, could result in lipid peroxidation driven production of carcinogenic BaP-diol-t-epoxide with increased CCR incidence and poor prognosis.
Figure 5 Lipid peroxidation, smoking and the activation of benzo[a]pyrene.
Lipid peroxidation and cytochrome P450 2S1 (CYP2S1) can activate benzo[a]pyrene (BaP) to the potent mutagen, benzo[a]pyrene-r-7,t-8-dihydrodiol-t-9,10-epoxide (BaP-diol-t-epoxide). A high expression of CYP2S1 is associated with colorectal cancer and a poor prognosis (see text).
DIETARY ARACHIDONIC ACID INTAKE AS A PRO-OXIDANT STRESSOR
Some observational studies suggest that the dietary consumption of arachidonic acid, a proinflammatory and pro-oxidant (as detailed below) PUFA is associated with an increased risk of CRC[42,43]. In contrast n-3 PUFAs (primarily from marine sources) are generally considered to be anti-inflammatory and have anti-neoplastic properties, at least in animal and human cell models[44]. A recent large-scale study of over 73000 Chinese women suggests that the ratio of dietary total n-6 to n-3 PUFA ratio is strongly associated with the incidence of CRC: compared to women in the lowest quintile, the relative risk of CRC was 1.95 for women in the highest total n-6 to n-3 PUFA quintile[44].
COX-2 OVEREXPRESSION, OXIDATIVE STRESS AND CRC
The cyclooxygenase enzymes catalyze the rate-limiting step of prostaglandin formation from arachidonic acid. There are two known isoforms of cyclooxygenase: cyclooxygenase-1 (COX-1) and COX-2. COX-1 has a constitutive promoter and is expressed in many tissues to maintain normal physiological functions such as the maintenance of renal blood flow, gastric mucosa, and platelet aggregation. COX-2 has an inducible promoter that contains a number of active regulatory elements including: FOXM1, cyclic AMP response element binding protein (CREB), NFkappaB, AP-1, p53, and PPAR gamma[45,46]. COX-2 overexpression is strongly associated with a number of cancers[47]. A large amount of evidence supports the view that a constitutive expression of the COX-2 enzyme is a contributing factor in the promotion of colon carcinogenesis as well as other cancers[48,49]. COX-2 overexpression is an unfavorable prognostic factor for numerous cancers including CRC[47-49] while silencing COX-2 reduces the tumorigenesis of CRC as well as the metastatic potential of CRC and other cancers[50,51]. COX-2 activation can occur through numerous signals, which contribute to the fact that the mechanism behind COX-2 activation has not been fully elucidated. Inflammation, viral and bacterial infections, phorbol esters, lipopolysaccharides, transforming growth factor beta, UVB exposure, gamma-irradiation, and mechanical shear stress can all be responsible for the activation of COX-2[52,53].
In HT-29 human colon cancer cells nontoxic doses of hydrogen peroxide (10 μmol) results in cancer cell proliferation, but toxic doses of 1000 μmol induce apoptosis[54]. The stimulation of cell proliferation was accompanied by an increase in COX-2 and apoptosis from the high-dose hydrogen peroxide was negatively correlated with COX-2 expression[54]. These data suggests that the balance of proliferation and apoptosis of cancer cells is dependent upon the concentration of ROS and can be correlated with COX-2 expression[54].
However, the roles COX-2 activation and suppression have on pro-oxidants, ROS, and antioxidants in carcinogenesis are not clear. In some studies COX-2 expression has led to increased oxidative stress, while in other studies increased oxidative stress has occurred through the inhibition of COX-2. For example, COX-2 mediated arachidonic acid metabolism was identified as a potential source of ROS in human intestinal epithelial cells (FHs 74 Int) exposed to monohaloacetic acids[55]. Viral induction of COX-2 has led to increased oxidative stress[56]. In a study examining the effects of nitric oxide-releasing non-steroidal antin flammatory drugs (NO-NSAIDs), treatment of human colon cancer cells lines with NO-NSAIDs produced a cytotoxic effect in all cell lines tested and an increased COX-2 activity was observed with concomitant oxidative stress[57].
There are examples of chemotherapy agents that enhance the expression of COX-2. For example, oral mucosal staining following cytotoxic chemotherapy (with various chemotherapeutic regimens including: doxorubicin/docetaxel/cyclophosphamide/methotrexate/5-fluorouracil; cyclophosphamide/methotrexate/5-fluorouracil; docetaxel alone; 5FU/folinic acid; and 5FU/leucovorin) demonstrate an increase COX-2 expression[58]. Colorectal tissues from patients treated with preoperative radiotherapy demonstrated increased expression of COX-2[59]. While the above data suggests that expression of COX-2 induces oxidative stress, there is a balanced amount of evidence showing that inhibition of COX-2 induces oxidative stress. For example, inhibition of aldose reductase (AR), an enzyme that catalyzes the reduction of lipid aldehydes and their glutathione conjugates, results in a growth reduction of human colon cancer cell through the inhibition of TNF-alpha induced activation of PKC and NFkappaB, which results in the abrogation of COX-2 mRNA and protein expression[60]. AR inhibition results in suppression of oxidative stress in inflammatory disorders[61]. Further, inhibition of phorbol ester-mediated induction of COX-2 in colon carcinoma cells by 15-deoxy-delta(12,14)-prostaglandin J(2) (15d-PGJ(2)) results in intracellular oxidative stress through the inhibition of AP-1 activation[62].
The chemical treatment of animals with azoxymethane (AOM) is a commonly accepted model for carcinogenesis, which results in the formation of aberrant crypt foci (ACF), precursor lesions to colon cancer. Pterostilbene (PS) had been reported to prevent chemical-induced colon carcinogenesis by anti-inflammatory and pro-apoptotic properties[63]. In a study examining the effects of PS on AOM-induced colon tumorigenesis, it was discovered that PS reduced AOM-induced tumor formation, ACF, as well as lymphoid nodules. In addition, PS treatment resulted in reducing the expression of oxidative inflammatory markers NFkappaB, inducible nitric oxide synthase, COX-2 and AR, while enhancing the expression of antioxidant enzymes such as hemeoxygenase-1 and glutathione reductase via NF-E2 related factor 2 signaling[63]. While it is not clear what conditions lead to oxidative stress through COX-2 signaling, these data suggest that the role of COX-2 in carcinogenesis is correlated with antioxidant signaling/pro-oxidant signaling and that more investigation is needed to understand these mechanisms in CRC.
HEAVY ALCOHOL CONSUMPTION AS A PRO-OXIDANT STRESSOR
Heavy alcohol consumption has been linked to an increase CRC risk as well well as an increased incidence of tumors in the distal colon[1]. Individuals with a family history of CRC are particularly at risk, with a relative risk of 2.8 compared to nondrinking individuals with no family history of CRC[64]. Similarly, patients with chronic alcohol dependence also show an increased level of oxidative stress biomarkers such as plasma protein carbonyl levels[65]. Alcohol is, however, likely to be procarcinogenic by multiple mechanisms. Alcohol is metabolized to acetaldehyde, which is a highly toxic mutagen causing point mutations[66,67]. Alcohol metabolized by the cytochrome P450 system of the endoplasmic reticulum leads to the production of both acetaldehyde and ROS[67]. ROS can directly cause DNA damage and can also lead to increased lipid peroxidation with the generation of genotoxic aldehydes, e.g., MDA and 4-HNE.
ANTIOXIDANT ENVIRONMENTAL FACTORS
Fruit and vegetables
Fruit and vegetables have a relatively high content of antioxidant compounds and many observational studies have shown that their frequent consumption is associated with a decreased risk of CRC. Nevertheless, a very large scale and well-conducted study in 2000 found “high consumption of fruit and vegetables did not appear to be protective against cancers of the colon and rectum in our large United States cohorts”[68]. Recent results from the Shanghai Men’s Health Study showed that the consumption of fruits but not vegetables was associated with a reduced risk of CRC in middle-aged and older Chinese men[69]. In the United States, folate added to many common foods items and present in most multivitamins may be preventing colon cancer and negating the need to get this vitamin from fruits and vegetables[68].
The Iowa Women’s health study (35000 women) found that a high intake of vitamin E was associated with a reduced risk of CRC[70]. Most of the vitamin E intake in this study was from multivitamin supplements and the form of vitamin E (see below) was not specified. Moreover, during the time period (1986-1990) for this study, most multivitamin supplements had very high levels of iron which is a quite potent pro-oxidant that can promote lipid peroxidation[71].
ASPIRIN, AN ANTIOXIDANT CHEMOPREVENTIVE FACTOR
It has long been known that the daily consumption of aspirin is associated with a significant decrease in CRC risk. Aspirin is a direct quencher of the genotoxic hydroxyl radical and the formation of hydroxylated aspirin derivatives (2,3- and 2,5-dihydroxybenzoic acid) has long been utilized as very sensitive in vivo biomarker for oxidative stress[72]. Most relevant to this review is the well-documented ability of aspirin to inhibit COX-1 and COX-2[73]. Data from two large studies now suggests that aspirin’s protective effect can be modified by the BRAF gene[74] which codes for a protein called serine/threonine-protein kinase B-Raf (a member of the Raf kinase family). Constitutive activation of the Ras-mitogen-activated protein kinase (MAPK) kinase pathway is of major importance in CRC and this can occur by oncogenic mutations in BRAF that upregulate the Ras-MAPK kinase pathway resulting (among many other important cancer related events) in an overexpression of COX-2[75]. Nishihara et al[74] found that aspirin use reduced the risk of CRC in individuals with wild type BRAF but not in individuals with oncogenic mutated BRAF. As also mentioned above, these data emphasize the potential interconnections between COX-2, CRC and oxidative stress.
LARGE SCALE CLINICAL TRIALS OF VITAMIN E AND CANCER PREVENTION-THE MANY DETAILS AND THE MANY DEVILS
The Selenium and Vitamin E Cancer Prevention Trial (SELECT) was a $130 million trial that concluded that “vitamin E” did not prevent prostate cancer, colon cancer, or any other cancer[76]. In a more ominous note, a follow up to the SELECT trial concluded that “Dietary supplementation with vitamin E significantly increased the risk of prostate cancer among healthy men”[77]. Vitamin E is the major in vivo lipid soluble antioxidant and it quenches the lipid peroxyl radicals that propagate during lipid peroxidation (c.f.[78]). As is often the case in biomedical research, there are many “devils” in the details of the SELECT study that are worthy of notice. An often-overlooked detail lies in the particular form of vitamin E used in the study. For the SELECT trial this was all-racemic-alpha-tocopheryl acetate at a dose of 400 IU/d taken over a period of about 5.5 years in men who were 50-55 or older at the start of the study. As it happens, “vitamin E” is not a single-organic compound and there are at least eight natural forms, i.e., alpha-, beta-, gamma- and delta-tocopherols as well as the corresponding four tocotrienols (c.f.[79,80]). To make matters more complicated, vitamin E isoforms have asymmetric carbons where each such carbon is attached to four different groups of other atoms. Tocopherols, for example, have three asymmetric carbons, each of which could have an R- or S-stereo-configuration.
All naturally occurring forms of tocopherols have the R-stereo-configuration. The form of vitamin E used in the SELECT study was “synthetic” where the configuration at each of the asymmetric carbons is an equimolar mixture of both the R- and S-stereoisomers (at each of the three asymmetric carbons): this is the form of vitamin E found in most commercial supplements although it is sometimes mistakenly labeled dl-alpha-tocopheryl acetate. All-racemic alpha-tocopheryl acetate is an equimolar mixture of eight stereoisomers with only one eighth of which is the naturally occurring RRR-alpha-tocopherol. The other seven stereoisomers are essentially xenobiotics whose detailed biochemical properties (and potential modulation of signal transduction pathways) are largely unstudied. Moreover, the bioavailability of all-racemic-alpha-tocopheryl acetate is about half that of RRR-alpha-tocopheryl acetate. Nevertheless, the ability of R- or S-vitamin E isomers to quench free radicals (lipid peroxyl radicals) and prevent lipid peroxidation is very similar.
ARE DIETARY ANTIOXIDANTS USELESS AS CHEMOPREVENTIVE AGENTS?
The SELECT study certainly suggests, that in healthy middle aged men, taking a potent lipid soluble antioxidant for half a decade or more did nothing to prevent prostate cancer, colon cancer or any other cancer diagnosed in this study. Does this mean that dietary antioxidants are useless as chemoprevention agents? Using a strictly evidenced based approach the answer is “we cannot be sure.” CRC is very much a disease of aging with the incidence markedly increasing after the age of 50. This suggests that many of the driver mutations responsible for CRC have already accumulated by mid-age. For any antioxidant to be effective as a chemopreventive agent that blocks free radical mediate genotoxicity it is reasonable to suggest that it must be consumed at an effective level starting at an early age. By mid-life too many oncogenic driver mutations may already be in play and a five-year period may also be too short. It may also be that the particular chemical form of the antioxidant is of critical importance since this is likely to play a role: (1) in its bioavailability; (2) what ROS/RNS are being modulated; (3) what organs/tissues is the antioxidant being delivered to; (4) the cellular and subcellular distribution of the antioxidant; and (5) does the antioxidant have any other relevant anticancer properties unrelated to its ability to function as an antioxidant (e.g., modulate carcinogenic signal transduction pathways).
WAS THE RIGHT FORM OF VITAMIN E USED IN THE SELECT TRAIL?
Neither tissue culture experiments nor animal models support of a strong anti-cancer role for all-racemic-alpha tocopherol or RRR-alpha-tocopherol. In contrast, both gamma-tocopherol[81,82] and tocotrienols[83] have anticancer effects that are now well documented. Gamma-tocopherol is the primary form of vitamin E in the American diet. It is quite interesting that the anticancer effects of tocotrienols are attenuated by supplementation with alpha-tocopherol[84]. Moreover, supplementation with alpha-tocopherol lowers plasma levels of gamma-tocopherol.
In addition to issues related to vitamin E stereochemistry, it should be noted that the form used in the SELECT study was a vitamin E ester (“yl”) rather than the free form (“ol”). Any vitamin E ester, such as alpha-tocopheryl acetate, is not an antioxidant since the functional phenol group is blocked by esterification. An esterase must act on the vitamin E before it is converted into an active “ol” antioxidant with the ability to quench lipid peroxyl radicals. In humans, vitamin E esters can have half the bioavailability compared to the corresponding free or unesterified form[85]. Much of the vitamin E in a high dose gel capsule (e.g., 200 IU) is not absorbed and is found in feces. It may well be that free tocopherol in the fecal matrix could reduce oxidative stress in the colon whereas tocopheryl esters would have no such effect. Quite surprisingly, some vitamin E esters (but not alpha-tocopheryl acetate) have anticancer effects that are not shared by the unesterified (free) alpha-tocopherol. Vitamin E succinate (or more precisely alpha tocopheryl succinate) for example has been found to be effective in reducing CRC in a mouse xenograft model[86,87].
The non-alpha-tocopherol forms of vitamin E have not yet been tested in large scale randomized, placebo-controlled studies. In any event, it is not biochemically justified to make clinical conclusions about all forms of vitamin E (or antioxidants in general) based on the results from a single form (such as all-rac-alpha-tocopheryl acetate) as was done in the SELECT study and in much of the “web” literature.
CONCLUSION
The evidence presented in this review presents a compelling case supporting the view that environmental oxidative stressors are causally related to the development of CRC. Moreover, the molecular and cellular details whereby oxidative environmental stress is translated into genotoxic damage to the epithelial cells of the large intestine are becoming increasingly clear as detailed above. The intestinal microbiome can be a source of oxidative environmental stress and can, via intestinal M-cells, transmit oxidative stress to macrophages in the subepithelial dome where subsequent genotoxic damage to colonic epithelial cells is likely.