Published online Aug 15, 2013. doi: 10.4251/wjgo.v5.i8.181
Revised: August 5, 2013
Accepted: August 11, 2013
Published online: August 15, 2013
Processing time: 77 Days and 15.8 Hours
A 77-year-old man with jaundice and a pancreatic head tumor was referred to our hospital in August 2006. The initial laboratory tests, computed tomography (CT) scan, magnetic resonance imaging (MRI), and endoscopic retrograde cholangiopancreatography suggested IgG4-related cholangitis and autoimmune pancreatitis. Oral prednisolone (PSL) was then administered. This treatment reduced the size of the pancreatic parenchyma, and the lower common bile duct (CBD) returned to its normal size. Thus, the oral PSL was gradually tapered to a maintenance dose. In February 2010, a CT scan and MRI showed segmental wall thickening and stenosis of the middle CBD, the progression of which led to extrahepatic obstructive jaundice. We suspected the emergence of a cholangiocarcinoma rather than the exacerbation of the IgG4-related sclerosing cholangitis because the stricture of the CBD was short and localized. Then, a percutaneous transhepatic biliary drainage was performed. The biopsy specimens obtained via the percutaneous transhepatic tract indicated an abnormal glandular formation, suggesting the presence of a moderate, well-differencated adenocarcinoma. The gross examination, microscopic examination and immunohistochemical analysis of the pancreaticoduodenectomy specimen suggested that a cholangiocarcinoma developed from the IgG4-related sclerosing cholangitis.
Core tip: Chronic biliary inflammation and cholestasis are risk factors for cholangiocarcinoma. However, an association of IgG4-related sclerosing cholangitis with cholangiocarcinoma has not been previously demonstrated. To date, only two report described have neoplasia in the bile duct in patients with IgG4-related sclerosing cholangitis. Risk factors for cholangiocarcinoma include chronic biliary inflammation and cholestasis, both of which can contribute to the malignant transformation of cholangiocytes in patients with sclerosing cholangitis. Because of a long duration of overt IgG4-related sclerosing cholangitis and the affected location of the patient’s cholangiocarcinoma, we presumed a cause-and-effect relationship between the sclerosing cholangitis and bile duct cancer.
- Citation: Douhara A, Mitoro A, Otani E, Furukawa M, Kaji K, Uejima M, Sawai M, Yoshida M, Yoshiji H, Yamao J, Fukui H. Cholangiocarcinoma developed in a patient with IgG4-related disease. World J Gastrointest Oncol 2013; 5(8): 181-185
- URL: https://www.wjgnet.com/1948-5204/full/v5/i8/181.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v5.i8.181
Autoimmune pancreatitis (AIP) is a type of chronic pancreatitis characterized by an inflammatory process that presents with unique clinicopathologic features, including enlargement of the pancreas and pancreatic ductal narrowing, elevated levels of IgG or IgG4, dense lymphoplasmacytic infiltration, storiform fibrosis, obliterative venulitis, and a favorable response to steroid therapy[1,2]. Most patients (65%-75%) develop painless jaundice[3,4] , resulting from stenosis in the intrapancreatic portion of the common bile duct (CBD) induced by both extrinsic compression of the inflamed pancreatic head and inflammatory changes of the CBD itself[4,5]. Distal CBD strictures and irregular narrowing of the main pancreatic duct usually improve after steroid therapy[3] .
An association of primary sclerosing cholangitis (PSC) with malignancy, i.e., cholangiocarcinoma, is well-established with an incidence rate of 1.5% per year[6]. However, an association of IgG4-related sclerosing cholangitis with invasive carcinoma of the biliary duct has not been demonstrated. Recently, a case of biliary intraepithelial neoplasia in a background of sclerosing cholangitis and autoimmune pancreatitis[7] and a case of intrahepatic cholangiocarcinoma in a background of sclerosing cholangitis[8] have been described. Chronic biliary inflammation and cholestasis are risk factors for cholangiocarcinoma; however, cholangiocarcinoma in a background of IgG4-related sclerosing cholangitis has rarely been reported. The department of radiology at our university reported this case in terms of the interesting computed tomography (CT) scan and magnetic resonance imaging (MRI), findings in a study by Rinsho-hoshasen[9]. Here, we present a case of cholangiocarcinoma that developed 4 years after the diagnosis of IgG4-related sclerosing cholangitis in regards to its intriguing clinical course from the viewpoint of the attending physician.
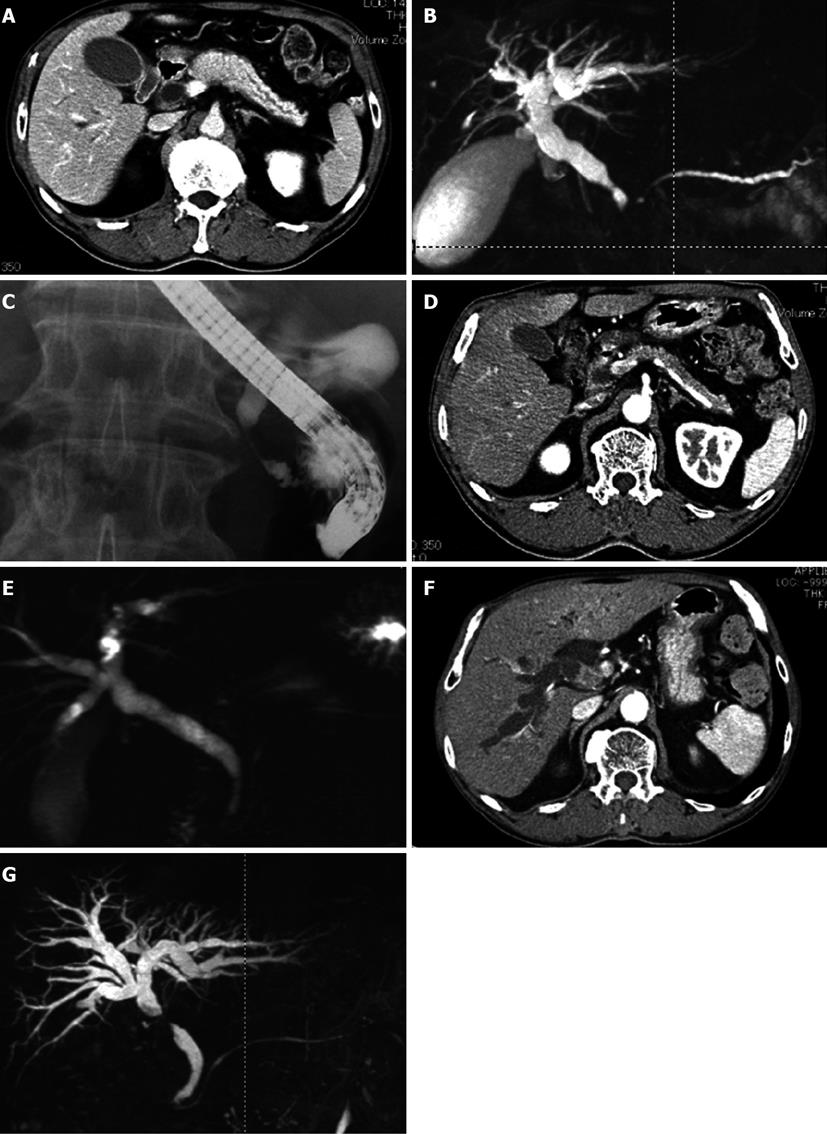
The patient was a 77-year-old man with jaundice and a pancreatic head tumor. He was referred to our hospital in August 2006. The physical examination revealed icteric sclerae without abdominal pain. The initial laboratory tests showed 2.3 mg/dL total bilirubin (normal level: 0.2-1.5 mg/dL), 742 IU/L aspartate aminotransferase (normal level: 12-32 IU/L), 545 IU/L alanine aminotransferase (normal level: 5-36 IU/L), 1104 IU/L gamma-glutamyltransferase (normal level: 11-69 IU/L), 1350 IU/L alkaline phosphatase (normal level: 80-260 IU/L), 1663.4 mg/dL IgG (normal level: 860-1800 mg/dL), 299 mg/dL IgG4 (normal level: 4.8-105 mg/dL), and an ANA of 160 (normal level: < 40). The CT scan showed a diffuse enlargement of the pancreas with a capsule-like rim and segmental wall thickening and an enlargement of the lower CBD (Figure 1A). The MRI revealed a narrowed main pancreatic duct and narrowed lower CBD (Figure 1B). The endoscopic retrograde cholangiopancreatography (ERCP) also confirmed the narrowed lower CBD (Figure 1C). The laboratory and radiologic findings suggested IgG4-related cholangitis and autoimmune pancreatitis. Oral PSL (30 mg, 0.6 mg/kg per day) was then administered for 2 wk. The treatment reduced the size of the pancreatic parenchyma, and the lower CBD returned to its normal size. The normal enhancement pattern in the pancreatic parenchyma was recovered (Figure 1D, E), and oral PSL was gradually tapered to a maintenance dose of 2.5 mg/d over a period of 2 mo.
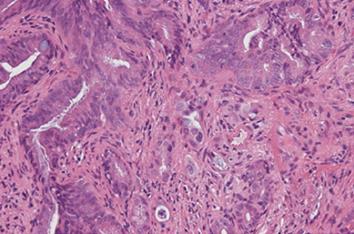
In February 2010, a CT scan showed segmental wall thickening with enhancement of the middle CBD (Figure 1F). An MRI revealed stenosis of the middle CBD (Figure 1G), and its progression led to extrahepatic obstructive jaundice. We suspected the emergence of a cholangiocarcinoma rather than the exacerbation of the IgG4-related sclerosing cholangitis because the stricture of the CBD was short and localized. We failed in our attempt at ERCP-guided tissue diagnosis of the biliary stricture. The biopsy guided by percutaneous transhepatic cholangiography was difficult because the intrahepatic bile duct was not sufficiently dilated. Because informed consent was obtained, we increased the PSL dose from 2.5 to 15 mg/d for a differential diagnosis. A month later, the CBD stricture persisted and led to the dilation of the intrahepatic bile duct. Finally, a percutaneous transhepatic biliary drainage was performed. The biopsy specimens obtained via the percutaneous transhepatic tract showed an abnormal glandular formation, suggesting a well-moderate differentiated adenocarcinoma (Figure 2).
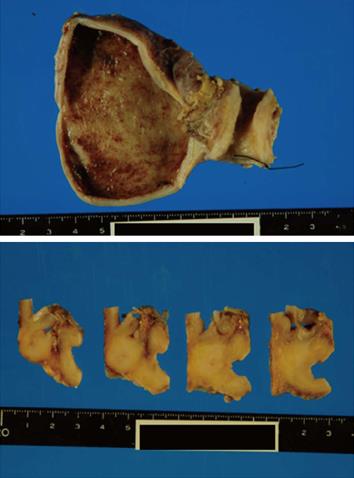
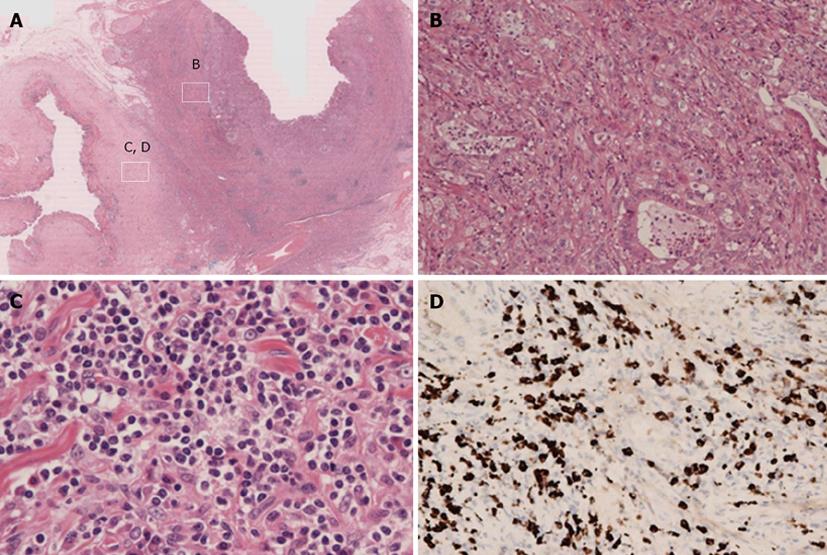
A pancreaticoduodenectomy was performed. At gross examination of the pancreaticoduodenectomy specimen, the wall of the middle CBD was markedly thickened without cancer invasion of the serosa (Figure 3). At microscopic examination, there was abnormal glandular formation, suggesting a moderate, well-differentiated adenocarcinoma from the mucosa to the subserosa in the middle CBD (Figure 4B). Lymphoplasmacellular infiltrate with lymph follicles and obliterative phlebitis were present around the CBD cancer (Figure 4C). Immunohistochemistry was performed to identify IgG4-positive plasma cells. More than 10 IgG4-positive plasma cells/high power fields were detected in the CBD (Figure 4D). Thus, we arrived at the diagnosis of cholangiocarcinoma developed from IgG4-related sclerosing cholangitis.
IgG4-related sclerosing disease is a systemic disease characterized by a dense lymphoplasmacytic infiltrate rich in IgG4-positive plasma cells and the storiform fibrosis of various organs. Major clinical manifestations of this disease are apparent in the pancreas, bile duct, gall bladder, salivary glands, retroperitoneum, periorbital tissues, kidneys, lungs, lymph nodes, meninges, aorta, breasts, prostate, thyroid, pericardium, and skin. While IgG4-related sclerosing cholangitis and AIP can both appear as a tumor mass mimicking cholangiocarcinoma or pancreatic cancer, these diseases have not been demonstrated to be associated with an increased risk of developing cancers of the bile duct or pancreas.
To date, only a few cases of synchronous autoimmune pancreatitis and pancreatic ductal adenocarcinoma have been reported[10,11]. One report described an epithelial atypia in the common bile duct (suggestive of biliary intraepithelial neoplasia) concurrent with a bile duct affection caused by autoimmune pancreatocholangitis[8]. One report described an adenocarcinoma in the intrahepatic bile duct in a patient with IgG4-related cholangitis[7].
Risk factors for bile duct cancer include chronic biliary inflammation and cholestasis[12], both of which can contribute to the malignant transformation of cholangiocytes in patients with sclerosing cholangitis. In patients with PSC, the lymphocytic infiltration is dominant near the lumen of the extrahepatic bile duct and is followed by erosion and damage of the surface epithelium. Furthermore, a multistep carcinogenesis from biliary intraepithelial neoplasia (BilIN: equivalent to biliary dysplasia) has been suggested[13]. However, for patients with IgG4-related sclerosing cholangitis, the inflammation and fibrosis are observed in the whole layer of the extrahepatic bile duct and the surface epithelium is less damaged compared to patients with PSC. A recent study showed that a significant K-ras mutation occurs most frequently in the pancreatobiliary regions of patients with AIP[14], although no relationship of this mutation with carcinogenesis is known. The mechanism of carcinogenesis from IgG4-related sclerosing cholangitis still needs to be investigated. Because our patient had a long duration of overt IgG4-related sclerosing cholangitis and the location of the patient’s cholangiocarcinoma was in the affected bile duct, we presumed a cause-and-effect relationship between the IgG4-related sclerosing cholangitis and the bile duct cancer in this case. Until now, reports of cholangiocarcinoma with a histological background of IgG4-related sclerosing cholangitis have been rare, but it appears that these reports will increase as the concept of IgG4-related sclerosing cholangitis becomes well known.
In conclusion, if unspecific morphological changes in the bile duct of a patient appear during the course of IgG4-related sclerosing cholangitis, proactive pathological examination is needed to detect potential cholangiocarcinomas at an early stage.
| 1. | Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 306] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 2. | Otsuki M. Autoimmune pancreatitis: a message from Japan. J Gastroenterol. 2007;42 Suppl 18:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Horiuchi A, Kawa S, Hamano H, Hayama M, Ota H, Kiyosawa K. ERCP features in 27 patients with autoimmune pancreatitis. Gastrointest Endosc. 2002;55:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 189] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Klöppel G, Lüttges J, Löhr M, Zamboni G, Longnecker D. Autoimmune pancreatitis: pathological, clinical, and immunological features. Pancreas. 2003;27:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 479] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | Straub BK, Esposito I, Gotthardt D, Radeleff B, Antolovic D, Flechtenmacher C, Schirmacher P. IgG4-associated cholangitis with cholangiocarcinoma. Virchows Arch. 2011;458:761-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Oh HC, Kim JG, Kim JW, Lee KS, Kim MK, Chi KC, Kim YS, Kim KH. Early bile duct cancer in a background of sclerosing cholangitis and autoimmune pancreatitis. Intern Med. 2008;47:2025-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Maeda S, Marugami N, Anai H, Takahama J, Sueyoshi S, Nishiofuku H, Kichikawa K, Shou M, Mitoro A, Enomoto Y. Bile duct cancer from IgG4-related sclerosing cholangitis: CT and MR findings. Rinsho-hoshasen. 2013;58:In press. |
| 10. | Inoue H, Miyatani H, Sawada Y, Yoshida Y. A case of pancreas cancer with autoimmune pancreatitis. Pancreas. 2006;33:208-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Witkiewicz AK, Kennedy EP, Kennyon L, Yeo CJ, Hruban RH. Synchronous autoimmune pancreatitis and infiltrating pancreatic ductal adenocarcinoma: case report and review of the literature. Hum Pathol. 2008;39:1548-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 345] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 13. | Zen Y, Adsay NV, Bardadin K, Colombari R, Ferrell L, Haga H, Hong SM, Hytiroglou P, Klöppel G, Lauwers GY. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Kamisawa T, Tsuruta K, Okamoto A, Horiguchi S, Hayashi Y, Yun X, Yamaguchi T, Sasaki T. Frequent and significant K-ras mutation in the pancreas, the bile duct, and the gallbladder in autoimmune pancreatitis. Pancreas. 2009;38:890-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
P- Reviewers Pinlaor S, Ramia JM S- Editor Wen LL L- Editor A E- Editor Lu YJ