Published online Feb 15, 2013. doi: 10.4251/wjgo.v5.i2.38
Revised: January 3, 2013
Accepted: January 18, 2013
Published online: February 15, 2013
Processing time: 115 Days and 17.1 Hours
Hepatocellular carcinoma (HCC) is the fifth most common malignant disease worldwide, and curative treatment remains difficult because the majority of cases are diagnosed in the advanced stage. Sorafenib is the only known effective systemic treatment, but patients rarely achieve complete remission (CR). A 66-year-old man with a history of alcoholic liver cirrhosis with a diagnosis of advanced HCC, was initially treated with transarterial chemoembolization on four occasions. However, the disease progressed with portal vein thrombosis. Therefore, sorafenib was started, and 4 mo later, the patient achieved CR. The treatment was continued for 12 mo, and CR was maintained up to 4 mo after sorafenib discontinuation.
- Citation: Kim MS, Jin YJ, Lee JW, Lee JI, Kim YS, Lee SY, Chae MH. Complete remission of advanced hepatocellular carcinoma by sorafenib: A case report. World J Gastrointest Oncol 2013; 5(2): 38-42
- URL: https://www.wjgnet.com/1948-5204/full/v5/i2/38.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v5.i2.38
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor and ranks third on causes of death related to cancer[1]. However, only 30%-40% of HCC patients can expect to undergo curative treatment, as the majority of the patients are diagnosed in the advanced stage[2]. Therefore, only palliative treatment, such as, transarterial chemoembolization (TACE) or systemic treatment, is available for patients with advanced HCC[2].
At the molecular level, sorafenib inhibits several types of tyrosine protein kinases, such as, vascular endothelial growth factor (VEGF) receptors 1, 2, and 3, platelet-derived growth factor (PDGF) receptor, and Raf kinase[3]. The SHARP trial reported that sorafenib has significant survival benefit in advanced HCC, and that is the only effective systemic agent[4]. Treatment response to sorafenib in HCC manifests in different ways, but only a handful of reports of complete remission (CR) on sorafenib have been issued[5-8].
Here, we present a case of advanced HCC patient who have achieved CR on sorafenib despite disease progression after four sessions of TACE for multinodular intrahepatic HCC.
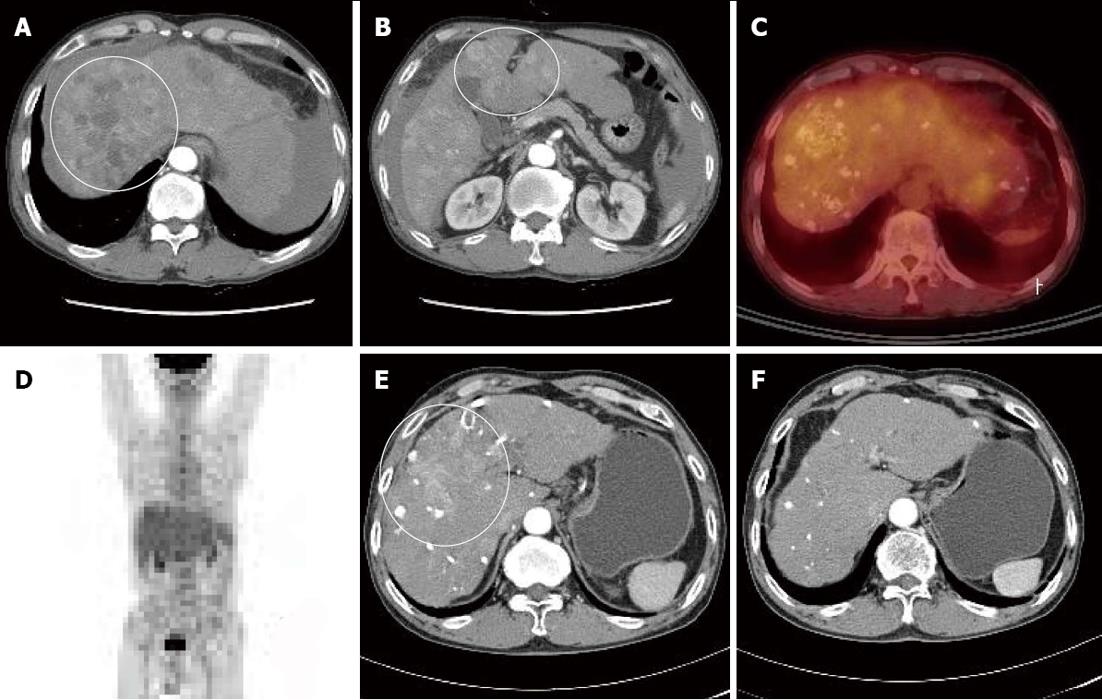
A 66-year-old male visited our hospital with abdominal pain. He had a 40-year history of 120 g/d of ethanol consumption, and had been diagnosed with alcoholic liver cirrhosis two years ago, for which he had not received any treatment. Laboratory test results at time of admission were as follows: aspartate aminotransferase 180 IU/L, alanine transaminase 75 IU/L, alpha-fetoprotein (AFP) 274 ng/mL, protein induced vitamin K absence > 2000 mAu/mL, total bilirubin 1.3 mg/dL, albumin 3.2 mg/dL, and prothrombin time international normalized ratio 1.25. He showed good reserve liver function with Child-Turcotte-Pugh class A, and did not have any ascites or findings of encephalopathy. Dynamic liver computed tomography (CT) findings depicted intrahepatic multinodular HCCs (Figure 1A and B). Tumor stage based on the Barcelona Clinical Liver Cancer (BCLC) staging system was BCLC stage B. Positron emission tomography (PET)-CT also showed increased multiple fluorine-18 2-fluoro-2-deoxy-D-glucose (PDG) uptake in liver without metastasis to any other organs (Figure 1C and D).
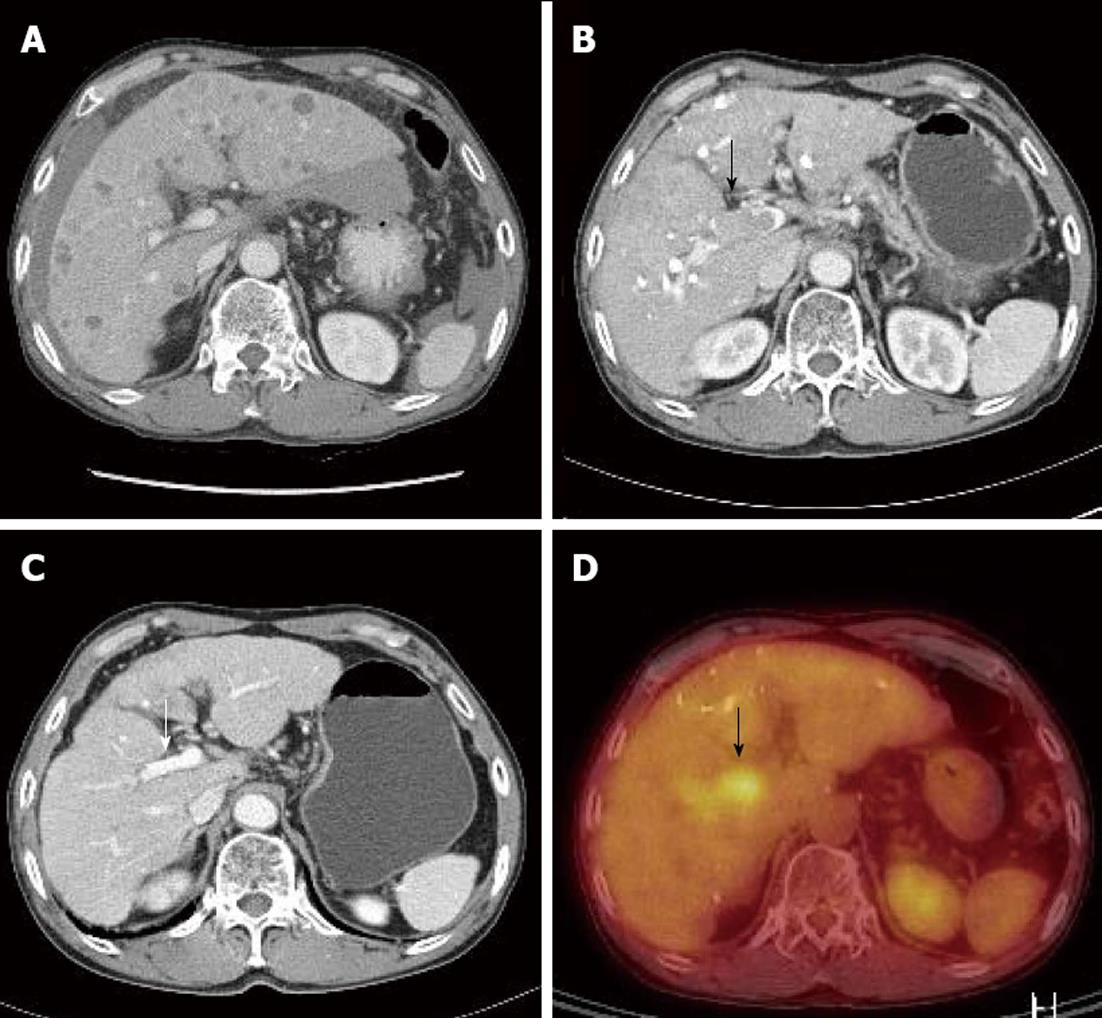
Because the tumor was not indicative for curative treatment, repeated TACE was performed with one or two month intervals. However, despite three sessions of TACE, follow-up dynamic liver CT revealed disease progression with viable intrahepatic HCCs (Figure 1E) and multiple paraaortic lymphadenopathy. At this time, serum AFP levels had increased to 2795 ng/mL, and thus, sorafenib was recommended. However, the patient refused for financial reasons, and TACE was performed once more. Followed-up CT scan taken after the fourth TACE session showed disease progression (Figure 2A) and portal vein tumor thrombosis (PVTT) that had not been previously identified was shown (Figure 2B and D).
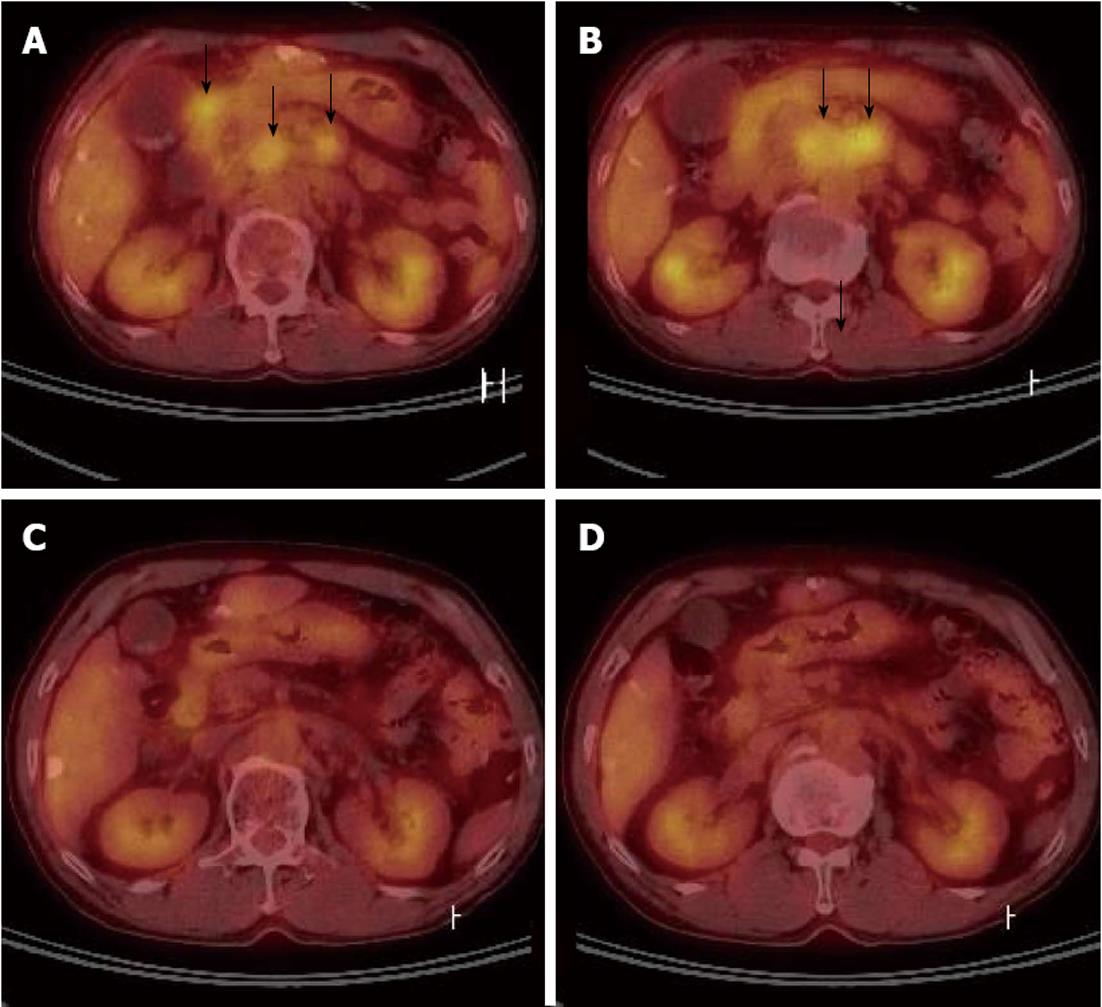
Due to the PVTT, transarterial chemoinfusion (TACI) was performed, and oral sorafenib (400 mg b.i.d) was initiated 4 d later. After 3 mo of sorafenib administration, serum AFP level returned to normal range (5.3 ng/mL), and dynamic liver CT visualized no remaining hypervascular intrahepatic mass, though it did show multiple lipiodol uptake. Furthermore, the PVTT and paraaortic lymphadenopathy had partially improved (Figure 2C). After 20 d of sorafenib administration, the patient developed a grade 2 hand-foot skin reaction and the dosage was reduced to 400 mg daily. After 6 mo of sorafenib treatment, serum AFP remained in normal range and no viable hypervascular mass was observed by dynamic liver CT (Figure 1F). The PVTT and the metastatic paraaortic lymph node had completely disappeared on dynamic liver CT, and no PDG uptake was observed by follow-up PET-CT (Figure 3). Therefore, he achieved clinical CR.
Sorafenib was maintained at 400 mg daily (200 mg, b.i.d) for 6 mo after the achievement of CR. However, sorafenib was discontinued after 12 mo of administration for financial reasons. The patient was lost to follow up at 2 mo after sorafenib discontinuation, at which time, he had maintained a CR status for 8 mo.
The majority of patients diagnosed with HCC have advanced stage disease, and for most, palliative treatments, such as, TACE or systemic treatment, are the only available therapeutic options. The SHARP trial report concluded that sorafenib was the only treatment regimen capable of providing a survival benefit for advanced HCC[3], and although several targeting agents and systemic cytotoxic agents have been developed, none has shown survival benefit in advanced HCC to date.
Sorafenib is a small molecule inhibitor of tyrosine protein kinases (e.g., VEGFR and PDGFR) and of Raf kinases. Sorafenib blocks VEGF and PDGF signaling, suppresses tumor angiogenesis, and interrupts Raf kinase signaling by suppressing tumor cell proliferation and inducing apoptosis. These actions of sorafenib are attributed to the inhibition of the serine/threonine kinases Raf-1 and B-Raf and to its inhibition of the receptor tyrosine kinase activities of VEGFRs 1, 2, and 3 and PDGFR-β. Furthermore, cellular signaling mediated by the Raf-1 and VEGF pathways has been implicated in the molecular pathogenesis of HCC, and this provides a rationale for the effect of sorafenib in HCC[9,10].
Two large phase III clinical trials, the SHARP trial and the Asia-Pacific trial, established consensus that sorafenib has survival benefit in advanced HCC. In the SHARP trial, 602 patients with advanced HCC and cirrhosis were enrolled. Of the patients treated with sorafenib, only seven (2%) achieved partial response and no complete response was recorded[3]. In the Asia-Pacific trial, no complete response to sorafenib was observed among the 226 patients enrolled, despite a partial response rate of 3.3%. Furthermore, median overall survival was 6.5 mo in the sorafenib group, and 4.2 mo in the placebo group[4]. Based on the findings of these studies, sorafenib is now used in HCC patients with BLCL stage C, but, increased survival can only be expected when it is used in indicative patients. Furthermore, the achievement of CR by sorafenib is rare and only a handful of cases have been reported[5-8]. In our case, PVT and intraabdominal lymphadenopathy were also improved by administrating sorafenib.
The treatment of advanced HCC with PVTT is much less effective than the treatment of HCC without PVTT, and median survival time of the former without treatment has been reported to be only 2.7-4 mo[11,12]. A variety of treatment modalities, such as, TACI, chemotherapy (5-fluorouracil, cisplatin, etc.), and radiotherapy have been attempted in cases with PVTT, but all have been found to be ineffective[13]. Interestingly, in the present case, PVTT improved after sorafenib treatment. PVTT is caused by portal vein invasion by cancer cells, and vascular specific growth factors are important for this process[14]. Sorafenib reacts by blocking VEGF and PDGF receptors, and is believed to promote PVT revascularization. Although PVTT revascularization after administering sorafenib has been reported in some cases, no case of complete response has been previously reported[15,16]. Furthermore, in the present case, sorafenib appeared to improve lymphadenopathy.
As in this case, the treatment for advanced HCC that has extrahepatic metastasis such as PVTT and abdominal lymphadenopathy is restricted[13]. The previous studies have reported the combined radiotherapy and TACE in advanced HCC with PVTT[17-19], and there are cases that report the effectiveness of radiation therapy in HCC that accompanies abdominal lymph node metastasis[20]. However, the standard treatment is not yet established. In this case, we started treatment with sorafenib according to BCLC guideline, and PVTT and abdominal lymphadenopathy responded to sorafenib.
The most common adverse effects of sorafenib are diarrhea (43%), rash (40%), fatigue (37%), hand-foot skin reaction (HFSR) (30%), and alopecia (27%); other adverse effects include nausea and pruritus, anorexia, hypertension[21]. HFSR is a common cause of dosage reduction, and affects quality of life, and usually occurs during the first 2-4 wk of administration. In our patient, grade 2 HFSR occurred after 20 d of administration and the dosage was halved to 400 mg daily, which was tolerable. Many patients treated with sorafenib complained about HFSR, and thus, require dosage adjustment or discontinuance. However, the dosage-response effects of sorafenib after dosage reduction have not been established. Well-designed studies are required to solve this issue in the future.
The pathogenesis of HFSR has not been elucidated. It has been reported that multi-targeting kinase inhibitors enter eccrine glands and directly affect toxicity to the skin[22]. However, HFSR is considered an indirect effect of sorafenib. Epidermal keratinocytes synthesize PDGF-α and PDGF-β, which activate dermal capillaries, fibroblasts, and PDGRF in eccrine glands[22]. Furthermore, eccrine glands present c-KIT and PDGFR, which are targeted by sorafenib. Therefore, because sorafenib suppresses VEGFR and PDGFR, HFSR is believed to be an indirect effect of the suppression of the angiogenic pathway[22].
We report a patient with advanced HCC patient with PVTT and abdominal lymphadenopathy, who achieved CR by sorafenib administration. Sorafenib is an important treatment option that has been shown to increase survival in HCC and to improve prognosis in selected cases. Therefore, we recommend active use of sorafenib to be considered in HCC patients capable of tolerating sorafenib but not indicative for curative treatment or TACE. Furthermore, our experience of this case recommands the use of sorafenib in HCC patients with PVTT, and suggests that sorafenib should be administered as aggressively as possible to such patients. In addition, given that many patients complain of HFSR during sorafenib administration, studies on the prevention and treatment of HFSR are required. Finally, studies are also required on the dosage/response characteristics of sorafenib with respect to ethnicity, age, gender, and disease status.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [PubMed] |
| 2. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3295] [Article Influence: 143.3] [Reference Citation Analysis (1)] |
| 3. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10537] [Article Influence: 585.4] [Reference Citation Analysis (9)] |
| 4. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4742] [Article Influence: 263.4] [Reference Citation Analysis (0)] |
| 5. | Inuzuka T, Nishikawa H, Sekikawa A, Takeda H, Henmi S, Sakamoto A, Saito S, Kita R, Kimura T, Osaki Y. Complete response of advanced hepatocellular carcinoma with multiple lung metastases treated with sorafenib: a case report. Oncology. 2011;81 Suppl 1:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Wang SX, Byrnes A, Verma S, Pancoast JR, Rixe O. Complete remission of unresectable hepatocellular carcinoma treated with reduced dose of sorafenib: a case report. Target Oncol. 2010;5:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Sacco R, Bargellini I, Gianluigi G, Bertini M, Bozzi E, Altomare E, Battaglia V, Romano A, Bertoni M, Capria A. Complete response for advanced liver cancer during sorafenib therapy: case report. BMC Gastroenterol. 2011;11:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (2)] |
| 8. | Chelis L, Ntinos N, Souftas V, Deftereos S, Xenidis N, Chamalidou E, Maltezos E, Kakolyris S. Complete response after sorafenib therapy for hepatocellular carcinoma in an HIV-HBV co infected patient: Possible synergy with HAART ? A case report. Med Oncol. 2011;28 Suppl 1:S165-S168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 286] [Article Influence: 13.0] [Reference Citation Analysis (3)] |
| 10. | Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851-11858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1236] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 921] [Article Influence: 34.1] [Reference Citation Analysis (2)] |
| 12. | Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, De Santis M, Manenti F. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 175] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561-7567. [PubMed] |
| 14. | Li Q, Xu B, Fu L, Hao XS. Correlation of four vascular specific growth factors with carcinogenesis and portal vein tumor thrombus formation in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2006;25:403-409. [PubMed] |
| 15. | Novi M, Lauritano EC, Piscaglia AC, Barbaro B, Zocco MA, Pompili M, Gasbarrini A. Portal vein tumor thrombosis revascularization during sorafenib treatment for hepatocellular carcinoma. Am J Gastroenterol. 2009;104:1852-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Yang MY, Jeong SW, Kim DK, Kim SG, Jang JY, Kim YS, Lee JS, Kim BS, Kim JH, Kim YJ. Treatment of hepatocellular carcinoma with portal vein thrombosis by sorafenib combined with hepatic arterial infusion chemotherapy. Gut Liver. 2010;4:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, Miyasaka Y, Nagayama K, Enomoto N, Sato C. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001;16:660-665. [PubMed] |
| 18. | Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, Nihei K, Ito Y, Maru Y, Ikeda H. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189-193. [PubMed] |
| 19. | Park MS, Kim SU, Park JY, Kim do Y, Ahn SH, Han KH, Chon CY, Seong J. Combination treatment of localized concurrent chemoradiation therapy and transarterial chemoembolization in locally advanced hepatocellular carcinoma with intrahepatic metastasis. Cancer Chemother Pharmacol. 2013;71:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Park YJ, Lim do H, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC, Nam HR, Oh DR, Park W. Radiation therapy for abdominal lymph node metastasis from hepatocellular carcinoma. J Gastroenterol. 2006;41:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Grünwald V, Heinzer H, Fiedler W. Managing side effects of angiogenesis inhibitors in renal cell carcinoma. Onkologie. 2007;30:519-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
P- Reviewer Capussotti L S- Editor Jiang L L- Editor A E- Editor Xiong L