Published online Apr 15, 2012. doi: 10.4251/wjgo.v4.i4.76
Revised: March 1, 2012
Accepted: March 10, 2012
Published online: April 15, 2012
Digestive endoscopy is currently the main diagnostic procedure for investigation of the digestive tract when a digestive disease is suspected. The use of computers and electronic medical records for the management of endoscopic data are an important key to improving endoscopy unit efficiency and productivity. This technology supports optimal program operation, monitoring and evaluation colorectal cancer screening. This article is a comprehensive survey of endoscopic electronic medical records and information systems. Computerized clinical records have the capability of identifying patients due for screening and to calculate baseline rates of colorectal cancer screening by patient characteristics and by primary care physician and practice group. This paper describes data flow in the endoscopy unit, the minimum data set of colorectal cancer and key features of endoscopic electronic medical record. In addition, the researchers state standards in different aspects, especially terminology standards and interoperability standards for image and text.
- Citation: Maserat E, Safdari R, Maserat E, Zali MR. Endoscopic electronic record: A new approach for improving management of colorectal cancer prevention. World J Gastrointest Oncol 2012; 4(4): 76-81
- URL: https://www.wjgnet.com/1948-5204/full/v4/i4/76.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v4.i4.76
The concept of the computerized endoscopic medical record (CEMR) or endoscopic electronic medical record systems (EEMR) has existed since the development of the endoscope[1,2] and a substantial amount of work has been done for more than a decade in the design and development of endoscopic databases and application software[3-13]. Electronic medical records have been developed to modernize procedural information management in the endoscopy unit[14]. The advantage of the CEMR is that it is possible to search any database created, perform statistical analysis and avoid the need for hand-written or typed reports[11]. There is a growing recognition of the need to base cancer control policies on accurate, detailed and timely information on cancer management and outcomes. With the development of the National Cancer Control program, it is obvious that an integrated cancer information system, incorporating a national cancer dataset, is needed to provide detailed timely and consistent information across the country. This would ensure that the care received by cancer patients is consistent and conforms to national guidelines, that information on trends in incidence, survival and mortality is readily available for planning and evaluation and that inequality in the delivery or outcome of services is quickly identified. EEMR not only have great potential to contribute to advantages such as better quality and safety in endoscopy and increased productivity due to automated data entry and report generation, but also aid in clinical research and education by recording complete and accurate data. It has repeatedly been reported in studies that structured reports are superior to free-text reports in endoscopy as they offer a built-in quality control into the report by specifying the terms to be used together unambiguously with their attributes and values[13-19].
In the last decade, the introduction of electronic endoscopes in the daily practice of digestive endoscopy has dramatically increased the possibilities of documenting endoscopic procedures with high quality pictures. Combined with computers, the electronic endoscopes constitute actual “endoscopic workstations”[11]. Available features of CEMR include: (1) patient scheduling: multi-user configurable; (2) patient monitoring: vital signs, pulse oximetry; (3) procedural coding: pre-procedural diagnosis, current procedure terminology (CPT) and ICD; (4) report generation: endoscopic record with images; (5) pathology interface and tracking; (6) discharge planning; (7) correspondence and networkable; (8) billing: automated billing for insurance; (9) quality assurance; (10) instrument tracking, usage and maintenance; (11) inventory control for pharmaceutical and supplies; (12) practice management; (13) clinical investigations; (14) risk management: completeness of documentation; (15) image management; (16) video clip management; (17) remote access internet; (18) patient education material; (19) searchable fields; (20) nursing note module; and (21) office note module[1,14].
Patient scheduling systems normally allow the user to enter essential information. The user may customize lists of frequently used descriptors (e.g., procedure types, referring physicians and performing physicians).
Patient monitoring may be entered into the endoscopic record manually or in an automated process. The ability to generate a natural language report diminishes with increasing complexity of the report. All report generators are capable of generating standardized negative examinations. Procedure related medications may be entered using a menu, “default” or free text.
Procedural findings are usually taken from a customizable list. Free text entries are usually allowed but weaken the utility of the database.
Some systems use a graphical display of the GI tract to input and/or report the findings. CEMR systems are capable of generating discharge instructions, physician recommendations and correspondence that may be printed or distributed electronically (e.g., fax and e-mail).
Most CEMR systems can report CPT codes. However, certain systems may be unable to adjust the CPT code if the actual procedure performed differs from the planned procedure. Diagnostic ICD code may be generated automatically or manually selected.
Quality assurance can be performed using CEMR by identifying immediate complications and sentinel events (e.g., oxygen desaturation, use of supplemental oxygen or use of reversal agents). However, data regarding delayed complications and procedural outcomes may be limited by the lack of follow-up information. CEMR can monitor instrument usage and endoscopy unit inventory[14]. Other features, such as automated follow-up and endoscopy unit statistics, may streamline practice management[20].
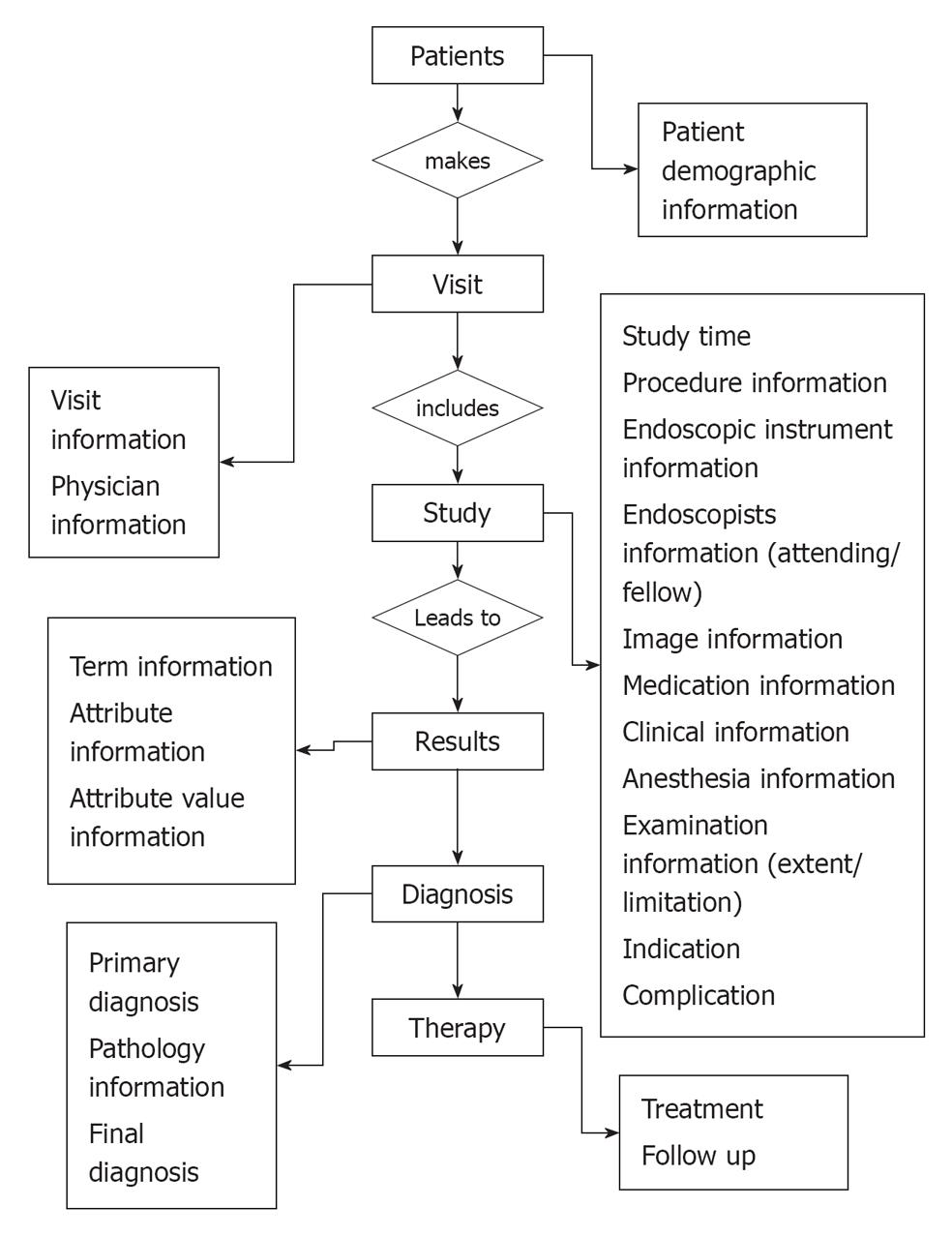
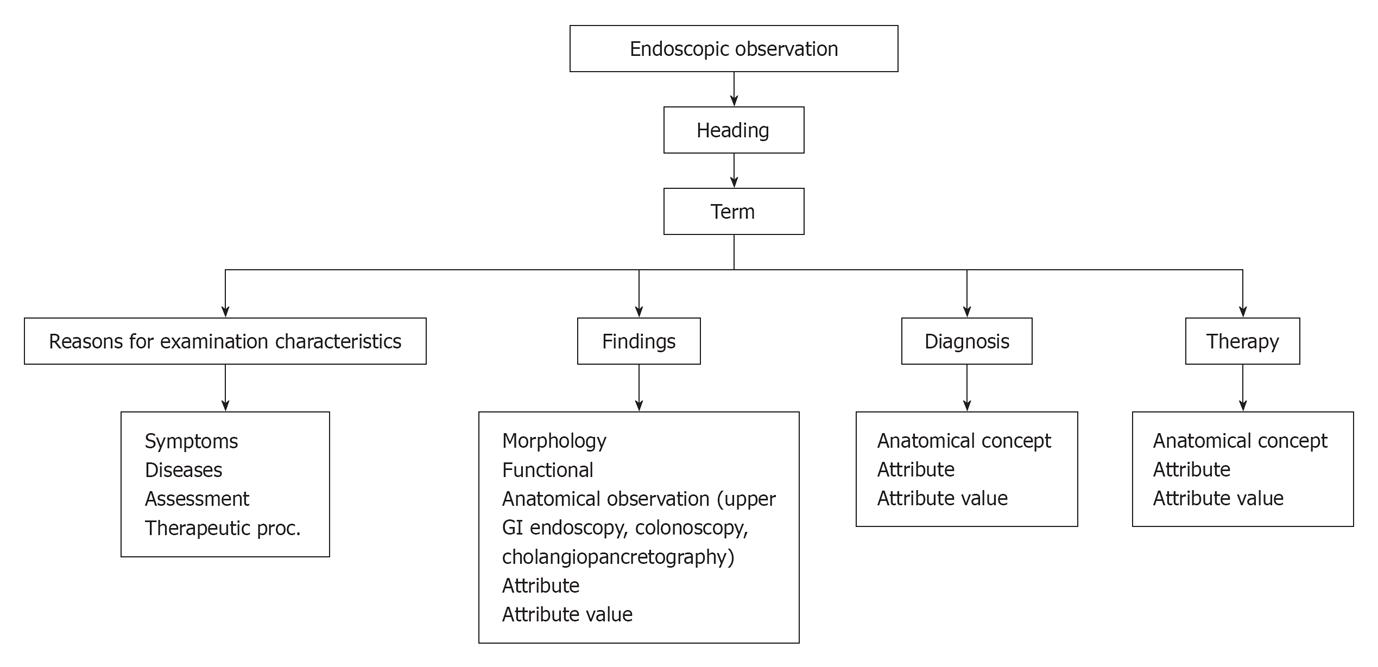
Although modern computing and communication technology holds great promise, its role in medicine has been limited by the absence of lexical and data exchange standards[18]. Furthermore, endoscopy reports have suffered from a lack of uniform terminology and content. The CEMR has evolved an increasing need for documentation of gastrointestinal procedures[14]. Standard endoscopy record flow is illustrated in Figure 1. The Minimal standard terminology (MST) is the result of a global effort to establish a common structure and vocabulary for electronic endoscopic reports[18]. Hierarchy of minimum standard terminology and examples of general data element in endoscopy are illustrated in Figure 2 and Table 1. This flow data contain elements to describe: (1) reasons for performing an endoscopy, although a list of “Indications” is available in many countries and is intended as a means of assessing the relevance and necessity for an endoscopic examination. This list was devised on the basis of the appropriateness of an individual examination. While appreciating the reasons behind this decision, the committee felt that it was more important to record why a particular examination had been undertaken rather than instruct users when an examination was acceptable. “Reasons for” have, therefore, been divided into: (a) symptoms: to allow a user to record the symptoms for which an endoscopic examination is required. This is particularly important when a disease is difficult to define; (b) diseases: this lists the common diseases for which an endoscopic examination may be required. These can be qualified by “Suspected ¡”, “For exclusion of ¡”, “For follow-up of ¡” or “For therapy of ¡”; (c) assessment of: this category was introduced in the “Reasons for” list in order to allow the recording of examinations performed to evaluate the status of a part of the GI tract before or after a surgical procedure; and (d) diagnostic sampling: this was included as a “Reason for” as it was recognized that some examinations may be performed only to collect a sample; (2) endoscopic findings; and (3) endoscopic diagnosis: at the end of the list of terms for each examination, a diagnostic list appears. This indicates a diagnosis that the endoscopist feels is most likely on the basis of macroscopic findings. This is not necessarily the final diagnosis, which takes into account the findings of any additional procedures performed, such as biopsy/cytology. The diagnostic list has been split into two parts: (a) main diagnoses ordered by expected prevalence; and (b) other (rarer) diagnoses listed alphabetically. The decision as to which list a particular diagnosis appears is based on the expected frequency of this finding in a European context. This “diagnosis” could be used to implement a “conclusion” field within any report generated based on a synthesis of all of the findings recorded. This is particularly true when a number of different lesions are described, such as in inflammatory bowel diseases at colonoscopy. It is also recommended that it should be possible to record a “negative conclusion” as well as a positive one. It is often just as important to record when a feature is not present as it is to describe it, e.g., failure to find any sign of bleeding when a patient presents with an apparent gastrointestinal bleed. It is suggested that it should be possible to qualify a diagnosis by “certain”, “suspected”, “probably not present” and “definitely excluded”.
| Elements | Example |
| Headings: Type of observation | Excavated Lesion |
| Term: Observation | Ulcer |
| Attribute: Characteristics of the term that expands the observation | Size |
| Attribute value: Defined characteristics | Size in mm |
| Anatomical concept: region + site + epicenter + locus | Regions (e.g., stomach, colon), sites (e.g., antrum, fundus), epicenters (e.g., extrinsic, intralumenal, wall), and loci (e.g., lumen, contents, mucosa) |
| Findings | Normal: Should be used if the organ has been examined entirely and everything is normal in it |
| Lumen: Contains all terms regarding an abnormality of the size of the organ, any deformity, compression and the evidence of previous surgery | |
| Contents: Terms describing the presence of various materials within the organ | |
| Mucosa: Terms describing patterns of the mucosa that are mainly diffuse and which may involve all the mucosa of one limited area. These terms are not applicable to individual lesions | |
| Flat lesions: Terms to be used for individual lesions which remain in the plane of the mucosa | |
| Protruding lesions: Terms to be applied to lesions growing above the plane of the mucosa | |
| Excavated lesions: Terms to be applied to lesions where the surface is beneath the plane of mucosa | |
| Therapy: intervention related to observation (coding from SNOMED or Clinical LOINC or ICD databases) | Biopsy |
Using these standards can overcome a lack of interoperability between colorectal cancer databanks at national and international level. Standard data elements can be used in their databases. Core datasets for colorectal cancer are: (1) macroscopic; (2) site of tumor; (3) maximum tumor diameter; (4) distance to the near nearer end resection margin; (5) tumor perforation; (6) relationship of rectal tumors to the potential reflection; (7) microscopic; (8) histological type; (9) histological differentiation; (10) maximum extent of local invasion (pT stage); (11) lymph node status; (12) extramural venous invasion; (13) evidence of regression following therapy; (14) histologically confirmed distant metastases; (15) background abnormalities; (16) other; (17) TNM stage; (18) Dukes stage; (19) completeness; and (20) SNOMED (Systematized nomenclature of medicine clinical terms) codes[21].
The widespread use of gastrointestinal endoscopy for diagnosis and treatment requires effective, standardized report systems[22]. Standardization of the endoscopic report is a key issue for future research in the field of digestive endoscopy[11]. Report generators should provide essential information, including patient identifier, physician identifier, date of procedure, relevant medical history, procedure type, medications, indication for procedure, extent of procedure, limitations of examination, findings, tissue acquired, adverse events, final diagnosis, results of therapeutic interventions, notation if images were acquired, complications and disposition[14]. The central role of the EEMR continues to be generation of the endoscopy procedure report[1]. Standard endoscopy report data element is illustrated in Table 2.
| Patient name |
| Address |
| Date of birth |
| Sex |
| SSN |
| Patient ID (internal) |
| Telephone No. (home) |
| Telephone No. (work) |
| Study date (date of procedure) |
| Study time |
| Study type (type of procedure) |
| Referring physician |
| Endoscopist (procedure MD) |
| Endoscopic instrument |
| Anesthesia status |
| Medication |
| Reason for examination |
| Indication |
| Anatomic extent of examination |
| Limitation of examination |
| Complication |
| Finding |
| Site |
| Term |
| Attribute |
| Attribute value |
| Therapeutic procedure |
| Diagnostic impression |
| Diagnostic impression IC9 code |
| Pathologic result |
| Final diagnosis |
| Final diagnosis ICD9 code |
| Recommendation |
Nomenclature standard: Vague and insignificant forms of speech and abuse of language have passed for mysteries of science for so long, and hard and misapplied words with little or no meaning have, by prescription, been be taken for deep learning and height of speculation, that it will not be easy to persuade either those who speak them or those who hear them, that they are but the covers of ignorance and hindrance of true knowledge. The importance of precise language in medicine cannot be overestimated. All medical activity arises from the ability to observe and communicate intelligibly. Endoscopists view the GI tract and create text and images that reflect their observations and transmit this information to others who are also involved in the care of the patient. The increasing fragmentation of care, pressure for increased productivity and lack of rapid access to the patients’ clinical reports make effective automation crucial to the future of medicine[18]. SNOMED is a system of standardized medical terminology. SNOMED Clinical Terms® or SNOMED CT is a comprehensive computerized clinical terminology covering clinical data for diseases, clinical findings and procedures. It is a “comprehensive and precise clinical reference terminology that provides unsurpassed clinical content and expressivity for clinical documentation and reporting”. It allows a consistent way to index, store, retrieve and aggregate clinical data across specialties and sites of care. It also helps structure and computerizes the medical record, reducing the variability in the way data is captured, encoded and used for clinical care of patients and research. SNOMED created a common clinical language that is a necessary element of a health care information infrastructure[23]. The goal of SNOMED is to create a comprehensive nomenclature for indexing the entire medical record, including signs, symptoms, diagnosis and procedures. SNOMED contains 156 602 unique concepts that, when linked to the MST, would permit endoscopic records to be automatically cross-indexed to other parts of the medical record[18].
Coding standard (ICD, CPT, logical observation identifier names and codes): In the course of creating an endoscopic report and submitting a claim for reimbursement, practitioners are required to classify the endoscopy according to coding systems: CPT and ICD. At the end of each procedure, the endoscopist must select a CPT code that indicates what was done and an ICD code that defines the indication for the procedure and what was found. Automation of these processes would improve the accuracy of the codes[18]. Different fields that need a specific code in endoscopic information systems are illustrated in Table 3.
| Reason for endoscopy |
| Medication use |
| Sedation and medication during endoscopy |
| Preparation |
| Procedure for investigation |
| Endoscopic diagnosis/findings |
| Therapeutic and diagnostic interventions |
| Histology results |
| Therapy started |
| Advice to referring doctor |
| Complications |
Logical observation identifier names and codes (LOINC) is one of therapy coding in EEMR[24]. The LOINC database provides a set of universal names and ID codes for identifying laboratory and clinical observations[25]. They are mainly intended to identify the test results. LOINC was developed to facilitate the electronic transmission of laboratory results to hospital, physician, third party payers and other users of laboratory data. Each record in the LOINC database identifies a clinical observation and contains a formal six-part name and identifying code with a check digit, synonyms and other useful information[26].
Standard of interface of data and image (health level 7, Digital Imaging and Communications in Medicine): EEMRs can also be configured to interface promptly with a hospital electronic medical record systems (EMR), usually via standard technical compatibilities, such as health level 7 (HL7)[1]. HL7 is one of several American National Standards Institute-accredited Standards Developing Organizations (SDOs) operating in the health care arena. Most SDOs produce standards (sometimes called specifications or protocols) for a particular health care domain such as pharmacy, medical devices, imaging or insurance (claims processing) transactions. The HL7 domain is clinical and administrative data. HL7 develops specifications; the most widely used is a messaging standard that enables disparate health care applications to exchange key sets of clinical and administrative data (California Office of HIPPA implementation, 2008).
The advent of the video endoscope has revolutionized the practice of gastrointestinal endoscopy[27]. Images are critical components of the clinical record. Since the 1970s, when digital images first became widely used in clinical practice (with routine use of computerized tomography), there has been an ever-increasing need for a generic image-file format and exchange protocol to enable interchange of diagnostic images and related information in electronic form. The Digital Imaging and Communications in Medicine (DICOM) standard was developed by the American College of Radiology and the National Electrical Manufacturers Association to meet this need. DICOM is a set of engineering specifications for a generic image file format, a network image-interchange protocol and an explicit semantic data model for images and related information. The DICOM standard has been very favorably received by industry and professional organizations. Since publication of DICOM in 1993, digital image management systems enabled by DICOM interfaces have been widely implemented in radiology. Images from a variety of sources (video, fluoroscopy and US) should be DICOM compatible and can often be stored in the EEMR with easy export to other sources[28].
Network connectivity (e.g., LAN, WAN) is available with many of these endoscopic medical record systems, allowing sharing of information with other health care systems. The ability to table interface with other clinical systems may enhance exchange of endoscopic information[14]. Newer functions include interfaces with hospital-wide EMR and pathology databases, improved communication with referring physicians through automated faxes or e-mail, and internet access to allow clinicians secure remote connections[20]. During the procedure, some systems allow automatic transfer of data from the patient’s vital sign monitor to the EEMR[14].
Colorectal cancer is over 90% preventable. Screening of this disease is key for detecting and preventing colorectal cancer. New technologies enhance colorectal cancer screening. Electronic technology can effectively reduce mortality and increase successful treatment by evidence - based screening. Applications of this technology were developed to handle data entry, reporting, telecommunications and data sharing. Furthermore, health informatics is cost efficient for patient management and facilitates data access in any time and any place[29].
EEMR has the key role to greatly increase the efficiency of both the endoscopist and the entire endoscopy unit. It decreases duplication of procedures and increases the utility of databases for clinical research and education purposes. Current features of EEMR can improve patient care and reduce the cost of procedures. Capabilities of this innovation in information management of preprocedure, intraprocedure and postprocedure data can reduce duplication of documentation and reduce total patient time in the endoscopy center. Standard EEMR has the capability of sharing and integrating information among the many stakeholders involved in EEMR, such as participant, family physician, specialist, hospitals, laboratories and pharmacist. Application of endoscopic standard in this technology can be used to improve the quality of endoscopic reporting by integrating images and text, creating large image bases and facilitating clinical research by use of a common lexicon. The minimum datasets for reporting tumors are used in the system of standard setting, data collection, audit and feedback for those involving in caring for these patients. This technology provides minimum datasets for reporting colorectal cancer status and other gastrointestinal cancers. This tool facilitates data access and applications of this technology are cost efficient for patient management, health care organization management, documentation management and material management in the field of colorectal cancer. Electronic technology decreases errors of reporting and duplication in endoscopy activities and health care provider access to comprehensive information for decision making.
| 1. | Conway JD, Adler DG, Diehl DL, Farraye FA, Kantsevoy SV, Kwon R, Mamula P, Rodriguez B, Shah RJ, Song LM. Endoscopic electronic medical record systems. Gastrointest Endosc. 2008;67:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Nelson DB, Block KP, Bosco JJ, Burdick JS, Curtis WD, Faigel DO, Greenwald DA, Kelsey PB, Rajan E, Slivka A. Technology status evaluation report: computerized endoscopic medical record systems: November 1999. Gastrointest Endosc. 2000;51:793-796. [PubMed] |
| 3. | Atalağ K, Bilgen S, Gür G, Boyacioğlu S. Evaluation of the Turkish translation of the Minimal Standard Terminology for Digestive Endoscopy by development of an endoscopic information system. Turk J Gastroenterol. 2007;18:157-164. [PubMed] |
| 4. | Gouveia-Oliveira A, Raposo VD, Azevedo AP, Salgado NC, Almeida I, Silva AM, de Melo FG, Correia JP. SISCOPE: a multiuser information system for gastrointestinal endoscopy. Endoscopy. 1991;23:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Gouveia-Oliveira A, Raposo VD, Salgado NC, Azevedo AP, Almeida I, de Melo FG, Correira JP. Modification of the OMED nomenclature: a system approach based on the SISCOPE data model. Endoscopy. 1992;24 Suppl 2:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Vicary R. System design: which requirements should be met. Endoscopy. 1992;24 Suppl 2:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 7. | Schapiro M. Computerization of endoscopic reports--an ASGE proposal. American Society for Gastrointestinal Endoscopy. Endoscopy. 1992;24 Suppl 2:478-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | de Dombal FT. Organization of data input--the importance of rapid/high quality data collection. Endoscopy. 1992;24 Suppl 2:490-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 9. | Venables CW. Clinical experience with computerised endoscopic record systems in the UK. Endoscopy. 1992;24 Suppl 2:481-486. [PubMed] |
| 10. | Kuhn K. Knowledge-based user guidance for endoscopic database systems. Endoscopy. 1992;24 Suppl 2:499-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 11. | Delvaux M, Korman LY, Armengol-Miro JR, Crespi M, Cass O, Hagenmüller F, Zwiebel FM. The minimal standard terminology for digestive endoscopy: introduction to structured reporting. Int J Med Inform. 1998;48:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Delvaux M. Image management: the viewpoint of the clinician. Gastroenterologist. 1996;4:3-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Crespi M, Delvaux M, Schapiro M, Venables C, Zwiebel F. Minimal standards for a computerized endoscopic database. Ad hoc Task Force of the Committee for Minimal Standards of Digestive Endoscopy of the European Society of Gastrointestinal Endoscopy (ESGE). Am J Gastroenterol. 1994;89:S144-S153. [PubMed] |
| 14. | Axon AT, Sobala GM. Computers in endoscopy--scientific aspects and research. Endoscopy. 1992;24 Suppl 2:532-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Zwiebel FM, Sauerbruch T. Quality assurance by computerized endoscopy record systems. Endoscopy. 1992;24 Suppl 2:527-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | O'Mahony S, Naylor G, Axon A. Quality assurance in gastrointestinal endoscopy. Endoscopy. 2000;32:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Robertson DJ, Lawrence LB, Shaheen NJ, Baron JA, Paskett E, Petrelli NJ, Sandler RS. Quality of colonoscopy reporting: a process of care study. Am J Gastroenterol. 2002;97:2651-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Korman LY, Delvaux M, Crespi M. The minimal standard terminology in digestive endoscopy: perspective on a standard endoscopic vocabulary. Gastrointest Endosc. 2001;53:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Naylor G, Gatta L, Butler A, Duffet S, Wilcox M, Axon AT, O'Mahony S. Setting up a quality assurance program in endoscopy. Endoscopy. 2003;35:701-707. [PubMed] |
| 20. | Enns RA, Barkun AN, Gerdes H. Electronic endoscopic information systems: what is out there. Gastrointest Endosc Clin N Am. 2004;14:745-754, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Royal college of pathologists. Minimum dataset for gastric cancer, 2000. Available from: http://www.wales.nhs.uk/sites3/Documents/456/Appendix%209%20%2D%20Pathology%20Guidelines.pdf. |
| 22. | Groenen MJ, Hirs W, Becker H, Kuipers EJ, Van Berge Henegouwen GP, Fockens P, Ouwendijk RJ. Gastrointestinal endoscopic terminology coding (GET-C): a WHO-approved extension of the ICD-10. Dig Dis Sci. 2007;52:1004-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Available from: http://www.ihtsdo.org. |
| 24. | Korman LY, Bidgood WD. Representation of the Gastrointestinal Endoscopy Minimal Standard Terminology in the SNOMED DICOM microglossary. Proc AMIA Annu Fall Symp. 1997;434-438. [PubMed] |
| 25. | Degoult , Patrice and Fieschi, Marius . Introduction to clinical informatics. Switzerland: WHO 1988; 74. |
| 26. | Wager KA. Managing Health Information Systems. USA: Jossey-bass 2005; 238-240. |
| 27. | Groenen MJ, Kuipers EJ, van Berge Henegouwen GP, Fockens P, Ouwendijk RJ. Computerisation of endoscopy reports using standard reports and text blocks. Neth J Med. 2006;64:78-83. [PubMed] |
| 28. | Electronic data exchange standards. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1998;48:683-684. [PubMed] |
| 29. | Elham M, Nasraran Z, Reza ZM. Health informatics and information system: an integrated evidence-base tool for colorectal cancer screening. Asian Pac J Cancer Prev. 2008;9:537-540. [PubMed] |
Peer reviewers: Angelo Zullo, MD, Department of Gastroenterology and Digestive Endoscopy, “Nuovo Regina Margherita” Hospital, Via E. Morosini 30, Rome 00153, Italy; Antonio Russo, MD, PhD, Associate Professor, Genetic and Molecular Oncology Unit, Interdepartmental Center of Research in Clinical Oncology, School of Medicine, University of Palermo, Via del Vespro 127-90127 Palermo, Italy
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM