THE BURDEN
Despite its incidence decreasing, gastric cancer remains the fourth most prevalent tumor and second most frequent cause of cancer-related mortality in the world[1]. As a primary prevention, some behavior modifications have been suggested, including reduction of salt intake with the diet, increase of vitamin C consumption and abolition of smoking[2]. Moreover, Helicobacter pylori (H. pylori) eradication is recommended as it is able to reduce gastric cancer incidence up to 35%[3]. As a secondary prevention, a radiological or endoscopic-based screening program is performed in a few Asian countries, including Japan, Korea and Matsu Island in Taiwan, where the gastric cancer incidence remains extremely high[4]. Such a population-based screening is not feasible in other countries due to a distinctly lower gastric cancer incidence. Follow-up of precancerous lesions is the other possible procedure intended to either reduce gastric cancer onset, i.e., by removing dysplasia areas at endoscopy or to diagnose neoplasia in an early stage, so that patient survival is distinctly improved[5]. It is well known that intestinal metaplasia (IM) and gastric dysplasia are the main precancerous lesions of the stomach; IM also being the most frequently encountered[6]. Few studies indicated that endoscopic-histological follow-up in patients with IM is able to detect gastric cancer in an early stage with a considerable mortality reduction[7,8]. However, no guidelines on the endoscopic follow-up of patients with IM are available. Consequently, physicians remain uncertain on the surveillance strategy to be clinically adopted in patients with IM. We therefore analyzed the available data, aiming to clarify the potential pros and cons of endoscopic follow-up of IM for gastric cancer prevention.
EPIDEMIOLOGY OF IM
Since IM is not associated with specific symptoms, it is impossible to assess its prevalence in the general population without performing an upper endoscopy with histological evaluation. Therefore, data on asymptomatic patients are scanty. A Chinese trial with 1630 consecutive healthy persons (mean age: 42.2 years) with H. pylori infection recruited in a screening program for gastric cancer found an IM prevalence of 29.3%[9]. On the other hand, a lower IM prevalence rate was observed in Hong Kong, with values of 13.9% in H. pylori-infected and 9% in controls, in both males and females[10]. A study performed in the Netherlands found IM in 25.3% out of 533 consecutive dyspeptic patients who underwent upper endoscopy[11], with a prevalence that varied according to age, H. pylori status and gastric disease. In detail, IM prevalence was significantly higher in patients aged > 50 years (31.9%) compared to those younger (10.4%) and was also detected in 46.6% of > 80 years and in only 5.2% of those < 40 years. IM prevalence was significantly increased in patients with H. pylori (33.9%) compared to those uninfected (15.2%) and the presence of infection also significantly lowered the mean age of IM onset (64 years vs 72 years). Moreover, IM was present in 29.5% of patients with non-ulcer dyspepsia, in 55.2% of those with gastric ulcer, and in 100% of those with intestinal type gastric cancer. Similar data were observed in Germany, where IM was observed in 25.7% of 1446 H. pylori-infected patients, with a prevalence varying from 16.9% in duodenal ulcer patients to 23.6% in non-ulcer dyspepsia, and to 47.4% in those with gastric ulcer[12]. Moreover, mean age was significantly higher in IM patients than in controls (58.3 years vs 50.7 years). A multicenter European study found an IM prevalence of 31.4% in 401 H. pylori-infected patients and the prevalence rate was higher in antral than in the gastric body mucosa[13]. In a cross-sectional study performed in a high-risk gastric cancer region of Colombia, IM was detected in 25.7% of 1670 patients, with a similar distribution between males and females and a significant increase with age (11% in those aged < 34 years; 35.6% aged 25-54 years and 45.2% in those > 55 years)[14]. In different Italian studies with consecutive dyspeptic patients with H. pylori infection, IM was detected in 12.7% of 300 cases with a mean age of 49 years[15], in 15.5% of 375 patients with median age of 48 years[16], in 19.3% of 273 patients with a mean age of 54 years[17] and in 32.4% of 179 patients with a mean age of 69.5 years[18].
In a Japanese study with consecutive patients[19], IM was detected in as many as 37% of 1426 H. pylori positive patients (mean age: 52.3 years) compared to only of 2% of 280 uninfected patients (mean age: 52.7 years). In a province of north-western Iran where both H. pylori prevalence and gastric cancer incidence are high, IM was detected in 13% of antral mucosa and 8.3% of gastric body mucosa of 1011 patients (mean age: 53.3 years)[20]. On the other hand, in Malaysia where both H. pylori infection rate (4.8%) and gastric cancer incidence (4.3/100 000) are low in the general population, IM was detected in only 7.7% of 234 patients (mean age: 53.4 years) who underwent upper endoscopy, suggesting a close association with the infection[21].
All these data suggest that the IM is the result of a chronic, inflammatory injury of the gastric mucosa. The long-lasting active gastritis associated with H. pylori infection would appear to be the main etiological factor, increasing IM risk in the stomach by 4.5-9-fold[22-24]. A study found that infection with cagA-positive H. pylori strains is associated with a significant increased IM prevalence compared to those strains without it[25]. Moreover, in H. pylori infected patients, a current smoker of over 20 cigarettes daily and a high butter consumption are associated with a further increase risk of IM of 4.75-fold (95% CI: 1.33-16.99) and 2.17-fold (95% CI: 1.14-4.11), respectively[26].
The IM prevalence rate has been shown to be higher in first-degree relatives of gastric cancer patients compared to controls. Indeed, the IM prevalence rate in first-degree relatives compared to matched controls was 28.4% vs 12.2% in Germany[27], 26.1% vs 12.9% in Korea[28] and 19% vs 11.7% in UK[29], but not in Brazil[30]. Indeed, a recent meta-analysis calculated an odds ratio of 1.982 (95% CI: 1.363-2.881) for IM on 1500 first-degree subjects compared with 2638 controls[31]. A recent Iranian study on 808 first-degree relatives found a similar IM prevalence between those subjects with 1 and those with > 1 cases in the family, with no difference when the index case was male or female[32].
Regarding a possible interaction between H. pylori and a family history, we observed an overall IM prevalence of 35.8% in 39 consecutive first-degree relatives, with a prevalence rate as high as 52.6% in those with H. pylori infection compared to 20% in those uninfected[33]. Therefore, a possible synergistic effect between H. pylori infection and a family history in IM development may be hypothesized. Indeed, a possible genetic predisposition towards IM development in the stomach has been highlighted and the available data have been comprehensively analyzed in two recent reviews[34,35].
In summary: (1) IM is detected in nearly 1 of every 4 patients undergoing upper endoscopy; (2) H. pylori infection significantly raises IM prevalence; (3) IM prevalence rate increases with patient age; (4) IM prevalence is higher in first-degree relatives of gastric cancer patients; and (5) smoking (> 20 cigarettes/daily) further increases IM prevalence.
IS IM REVERSIBLE?
H. pylori infection, through a chronic inflammatory process on the gastric mucosa, is recognized as the main factor leading to IM development in the stomach[36]. Consequently, there have been several attempts aimed at inducing IM regression with bacterial eradication. However, a meta-analysis of 7 studies found that, different from atrophy, no significant regression of IM following H. pylori eradication occurred, either in antral mucosa (OR: 0.795, 95% CI: 0.587-1.078) or in the gastric body mucosa (OR: 0.891, 95% CI: 0.633-1.253)[37]. These findings were confirmed by a more recent meta-analysis of 12 studies, including data of 2582 patients with IM in the antrum and 2460 in the gastric body mucosa[38]. In detail, only 1 study showed that IM in the antrum was reversed after H. pylori eradication and no study showed that IM in the corpus was improved following bacterial eradication.
Of note, in a trial performed in Hong Kong on 435 patients[39], H. pylori eradication significantly prevented IM progression with an odds ratio of 0.48 (95% CI: 0.32-0.74), suggesting that bacterial eradication may play a role in slowing down IM progression rather than in inducing its regression. Moreover, some evidence would suggest that a high dietary consumption of ascorbic acid tends to reduce the risk of IM development in patients with H. pylori infection[22,40]. In addition, we observed that a 6 mo ascorbic acid supplementation following H. pylori eradication significantly helped to reduce IM in the stomach[41]. A COX-2 expression was found in the gastric mucosa of patients with H. pylori infection and IM and successful eradication promoted a down-regulation of COX-2 expression but failed to reverse IM at 1 year[42]. Of note, a recent pilot study in Taiwan found IM regression in 24.2% of 33 patients following 8 wk of treatment with celecoxib 200 mg/d after H. pylori eradication[43]. However, a randomized study performed in China on 136 patients with precancerous gastric lesions cured for H. pylori infection, a therapy with either 200 mg celecoxib twice daily or placebo for 3 mo, found a similar IM regression rate[44]. The high rate of drop-out cases in both due to side-effects (10 patients) and lost to follow-up (66 patients) deeply undermines the results of this trial. Therefore, further studies are urged.
In summary: (1) IM does not appear to regress following H. pylori eradication; (2) H. pylori eradication may slow IM progression; and (3) a potential chemoprevention with ascorbic acid supplementation and the potential role of celecoxib deserve further investigations.
GASTRIC CANCER RISK IN PATIENTS WITH IM
The role of IM in gastric carcinogenesis is unanimously recognized. Most likely, in the Correa’s sequence[45], IM is the “breaking point” of carcinogenesis between chronic active gastritis, i.e., the benign, completely reversible step of the sequence, and dysplasia, i.e., the non-invasive neoplasia, according to the Padova classification[46]. Some data would support such an assumption. Indeed, a prospective, randomized, 7-year follow-up Chinese trial found that H. pylori eradication failed to significantly prevent gastric cancer development in patients harboring IM at entry, whilst cancer did not develop in the eradicated patients without IM[9].
Gastric cancer incidence in IM patients was shown to vary from 0% to 10% in a recent systematic review[46]. However, such a wide range of gastric cancer risk could depend on the huge differences among the included studies in terms of either sample size (from 14 to 2628 patients) or follow-up period (from 2 to 23 years)[6].
In a Japanese trial of 1246 patients with both H. pylori infection and IM followed for a mean follow-up of 7.8 years, gastric cancer developed in 36 patients, with a relative risk for presence of IM of 6.4 (2.6-16.1)[19]. In a recent prospective, Korean study of 541 gastric cancer patients, the presence of moderate-severe IM in the antrum and lesser curvature of body mucosa was associated with a 7.52 (95% CI: 3.06-18.5) and 9.25 (95% CI: 2.39-35.8) increased risk of gastric cancer, respectively[47].
A recent nation-wide, histological-based Dutch study assessed the role of IM in gastric cancer development[48]. In a cohort of 61 707 patients with IM, gastric cancer developed in 874 cases, corresponding to a cumulative 10-year incidence of 1.8%, with an estimated yearly incidence of 0.18%. However, this analysis has been criticized; as many as 0.7% of cancer was diagnosed within 10.8 mo of follow-up[49]. Indeed, by excluding these prevalent cases, a most likely 10-year incidence of 1.1% (0.11% yearly) of gastric cancer in IM patients has been calculated[49].
In summary: (1) IM most likely represents the “breaking point” of gastric carcinogenesis; (2) a 6-fold increased risk of gastric cancer is present in IM patients; and (3) the actual incidence of gastric cancer in IM patients still needs to be defined because of the wide interval reported by the available studies.
ARE ALL PATIENTS WITH IM AT EQUAL RISK OF GASTRIC CANCER?
IM consists of the replacement of normal gastric epithelium with intestinal type epithelium as a result of a chronic injury[50]. IM can be either complete or incomplete. Complete IM (type I) is characterized by goblet cells scattered among columnar absorptive cells and incomplete with goblet cells interspersed among mucin-secreting columnar cells partly resembling gastric foveolar or colorectal cells[8,36]. Incomplete IM includes type II (sialomucin-secreting cells; presence of Paneth cells) and type III (sulphomucin-secreting columnar cells; absence of Paneth cells), identified through high-iron diamine stain. Patients harboring incomplete IM seem to be at higher risk of gastric cancer compared to those with complete IM. Indeed, in a recent study performed in Spain, gastric carcinoma developed in 16 (18.2%) out of 88 patients with incomplete IM and in only 1 (0.96%) out of 104 patients with complete IM after a mean follow-up of 12.8 years; incomplete IM also showed the highest risk of developing a gastric cancer at multivariate analysis (HR 11.3, 95% CI: 3.8-33.9)[51]. In a follow-up study performed in Portugal, 31% and 6.9% of 58 with incomplete IM developed low- and high-grade dysplasia, respectively, compared to only 8% of 62 patients with complete IM who developed low-grade dysplasia[52]. In a retrospective study in Slovenia on cancer registry, the cumulative incidences of gastric cancer in those patients previously diagnosed with IM were 1.3% in complete IM-type I, 2.8% in incomplete IM-type II and 9.8% in incomplete IM-type III patients[53].
A pattern of IM presence in the stomach has been also shown to play a role in gastric cancer risk. In a study in Colombia, compared to focal or antral-predominant IM distribution (arbitrarily assigned to be 1), its extension through the entire lesser curve increased gastric cancer risk by 5.7-fold (95% CI: 1.3-26), whilst the diffuse pattern (antral plus gastric body) showed a 12.2-fold (95% CI: 2.0-72.9) increased risk[54]. Of note, this study also showed that incomplete IM presents as diffuse more frequently than a focal pattern. The association between IM extension in the stomach and gastric cancer risk was confirmed in Italy[8]. In this study, the rate of gastric cancer appeared to increase with increasing IM extension. In particular, a > 20% IM extension at first examination was suggested to identify patients at increased risk for cancer.
In summary: (1) the presence of incomplete-type IM is associated with a higher gastric cancer risk compared to complete-type IM; (2) gastric cancer risk is associated with IM distribution in the stomach; and (3) an extension of IM over 20% seems to be a valuable cut-off.
IS FOLLOW-UP OF IM PATIENTS COST-EFFECTIVE?
The awareness that IM represents a definite precancerous lesion for gastric cancer, coupled with the dismal prognosis of such a neoplasia when diagnosed in an advanced stage, poses ethical concerns about leaving these patients without a scheduled follow-up. However, an appropriate use of endoscopic procedures is essential to the rational use of finite resources. Indeed, to dissipate economic resources in performing serial endoscopic controls in all IM patients, most of which would never develop gastric cancer, would also be unethical.
To address this issue, some parameters may be regarded as critical: (1) the annual incidence of gastric cancer in IM in order to estimate the loss of life-expectancy; (2) the stage of gastric cancer at diagnosis to estimate the increase of patient survival rate; (3) the role of endoscopic removal of dysplasia lesions by mucosectomy/dissection to estimate the real reduction of gastric cancer incidence; and (4) the interval between two endoscopies in order to estimate the cost of endoscopic follow up.
We recently constructed a decision analysis model to compare a strategy of performing an upper endoscopy every year for a 10-year period (surveillance strategy) following a new diagnosis of IM to a policy of no surveillance in a simulated cohort of 10 000 60 years old American patients[55]. The considered 10-year cancer risk in patients with IM was 1.8% (0.18% yearly)[48], whilst the downstaging of gastric cancer achieved in the surveillance was estimated to be 58% from regional to local and 84% from distant to regional[7]. The cost of upper endoscopy was $358 (299-416) and $39 080 (27 000-63 000) for gastric cancer treatment. An incremental cost-effectiveness ratio (ICER) of $100 000 per life years gained was used as a general threshold to differentiate a potentially efficient procedure from an inefficient procedure. The strategy of endoscopic surveillance was associated with the discounted saving of 0.041 year per person and with a discounted increase in cost of $2969 per person. The incremental cost-effectiveness of endoscopic surveillance was $72 519, so this strategy appeared to be a cost-effective option compared to no surveillance[55]. Obviously, cost-effectiveness of such a surveillance strategy strictly depends on the parameters used. Indeed, if the 1.8% (0.18% yearly) cumulative 10-year gastric cancer incidence in IM patients was reduced to 1.1% (0.11% yearly)[49], surveillance strategy would not be cost-effective. Conversely, the surveillance strategy could be even more advantageous in a different scenario, including those areas where either there is an increased incidence of gastric cancer in IM patients or the cost of upper endoscopy is less than $358. In an Italian study, 26 gastric cancers occurred in 471 patients with IM who were followed for a median of 52 mo, with a gastric cancer incidence (events per 100 persons/year) ranging from 0.24 (95% CI: 0.03-1.72) in those patients with IM extension < 20% to 3.85 (95% CI: 2.28-6.49) when IM extension was > 75%[8]. These data would render endoscopic surveillance highly cost-effective in Italy, as well as in those areas with similar conditions. A cause for concern in surveillance of IM patients is the timing of endoscopy follow-up. Unfortunately, there are no randomized studies comparing different strategies, with endoscopic control performed yearly, every 2 years or less frequently. However, in a UK study with a yearly endoscopic control[7], 36% of detected gastric cancers were stage I disease, a rate which would appear similar to the 38% achieved in Italy with a 2-year endoscopic control[8].
Cost-effectiveness of IM surveillance, with eventually developed dysplastic and cancerous lesions removed by endoscopic mucosal resection, was evaluated in another study modeling the strategy on a cohort of 50 years old men[5]. According to this simulation, IM surveillance with 5-10 years upper endoscopy was not cost-effective, the incremental cost-effectiveness ratio being > $500 000 per life-year saved. The apparent discrepancy between this model and the previous one[55] is likely to be the different estimate of cancer risk in IM patients. Indeed, in the latter study[5], the life-time risk of gastric cancer in IM patients was only 1%, much lower than that observed in clinical studies[8,49]. When the authors simulated a higher gastric cancer risk (i.e., in immigrants from a high-risk region of China), a 5-year endoscopic surveillance strategy was found to be potentially attractive (ICER = $80 600 per QALY)[55].
In summary: (1) scheduled endoscopic control could be cost-effective in IM patients; and (2) yearly and 2 yearly controls seem to be equally effective but specific studies are needed in this setting.
A PRAGMATIC BEHAVIOR
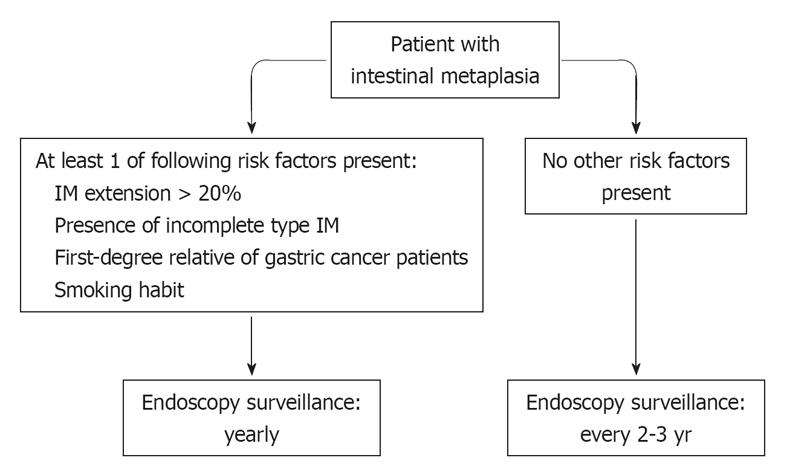
Based on the high prevalence of IM in endoscopic series, each endoscopist faces a case of IM virtually every day and is required to suggest a follow-up or not. Unfortunately, he/she cannot base this choice on the results of large prospective, randomized studies. Therefore, a pragmatic behavior could be suggested for IM patient surveillance in primary care. To date, the impact of upper endoscopy for gastric cancer prevention would not appear as impressive as that of colonoscopy for colorectal cancer prevention, especially when performed outside a screening program. There is evidence that we are performing a plethora (> 20%) of inappropriate upper endoscopies[56] while leaving several patients with a definite precancerous lesion, i.e., IM, without a scheduled control. While waiting for international guidelines on IM management[57], a yearly endoscopic control would appear justified in all IM patients with at least one of these conditions: (1) IM extension > 20%; (2) presence of incomplete type IM; (3) first-degree relative of gastric cancer patients; and (4) smokers. In the remaining IM patients, a less intensive (2-3 years) could be proposed (Figure 1). Such stratification requires a meticulous endoscopic and pathological approach. Endoscopic procedures should be carefully performed, especially in Western countries where missed diagnosis of gastric cancer would appear to be higher than in Asian series and the detection rate of early gastric lower[58]. At least 2 biopsies need to be taken on the antral region, 1 on the incisura angularis and 2 on the gastric body mucosa according to the current gastritis classification system[59], although the IM detection rate increases from 90% to 97% when performing 9 instead of 5 biopsies[60]. The pathologist is expected to report both IM type (complete/incomplete) and its extension (< 20% or > 20%)[8]. Despite the fact that H. pylori eradication seems to be unable to promote IM regression, curing the infection would appear a reasonable approach in all IM patients.
Figure 1 A pragmatic endoscopic surveillance for intestinal metaplasia patients.
IM: Intestinal metaplasia.
CONCLUSION
The overall 5-year survival in gastric cancer remains disappointingly low, the neoplasia frequently being detected when either endoscopic or surgical therapeutic approaches are less effective[61]. Therefore, to reduce gastric cancer mortality, the neoplasia needs to be diagnosed in an early stage. IM is a definite precancerous lesion for gastric adenocarcinoma. It is frequently detected in endoscopic series, especially in H. pylori infected patients and in first-degree relatives of gastric cancer patients, the prevalence increasing with age. Patients with incomplete type IM harbor a higher risk of gastric cancer compared to those with complete type IM. However, incomplete type IM is much less frequent than complete type (Type III: 21.5% of 1281 IM cases)[60], so both IM types similarly account for the overall gastric cancer development. Besides IM type, its distribution in the stomach plays an important role; involvement of both antral and gastric body mucosa represents a higher risk. A surveillance strategy could be cost-effective, at least in those patients with adjunctive risk factors for gastric cancer. A large prospective, randomized, multicenter study is needed to give definite information on when, how and why we should care for our IM patients.
Peer reviewers: Jian-Kun Hu, MD, PhD, Associate Professor, Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu 610041, Sichuan Province, China; John Griniatsos, MD, Assistant Professor, Department of Surgery, University of Athens, Medical School, 1st LAIKO Hospital, 17 Agiou Thoma str, GR 115-27, Athens, Greece
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM