Published online Feb 15, 2012. doi: 10.4251/wjgo.v4.i2.22
Revised: December 16, 2011
Accepted: December 23, 2011
Published online: February 15, 2012
Some authors have suggested that intraductal papillary mucinous neoplasms of the bile duct (IPMN-B) could be the the biliary counterpart of IPMN of the pancreas (IPMN-P) since they share several clinical-pathological features. These include prominent intraductal papillary proliferation pattern, a gastrointestinal phenotype, frequent mucin hyper-secretion and progression to mucinous carcinoma. To date there are just four reported cases of patients with synchronous IPMN-B and IPMN-P all of which were treated surgically. We hereby report the case of a 76-year-old woman who was incidentally diagnosed with both an asymptomatic 3 cm bulky fluid lesion obstructing the bile duct lumen, diagnosed as a malignant IPMN-B, and synchronous multiple pancreatic cystic lesions (10-13 mm) communicating with an irregular Wirsung, diagnosed as branch duct IPMN-P. Since surgery was ruled-out because of the patient’s age and preferences, she underwent a conservative management regimen comprising both chemotherapy and radiotherapy. This was effective in decreasing the mass size and in resolving subsequent jaundice. This is also the first reported case of IPMN-B successfully treated with chemoradiotherapy. Clinicians should consider medical treatment as an option in this clinical scenario, in patients who may be unfit for surgery.
- Citation: Valente R, Capurso G, Pierantognetti P, Iannicelli E, Piciucchi M, Romiti A, Mercantini P, Larghi A, Federici GF, Barucca V, Osti MF, Di Giulio E, Ziparo V, Delle Fave G. Simultaneous intraductal papillary neoplasms of the bile duct and pancreas treated with chemoradiotherapy. World J Gastrointest Oncol 2012; 4(2): 22-25
- URL: https://www.wjgnet.com/1948-5204/full/v4/i2/22.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v4.i2.22
Intraductal papillary mucinous neoplasms of the pancreas (IPMN-P) are lesions generally characterized by proliferation of the mucinous epithelium with vary degrees of ductal and cystic dilatation[1,2]. By definition, these tumours reside within the main pancreatic duct or its side branches and frequently manifest with grossly visible papillary growths producing mucin.
Depending on their location within the pancreatic ductal system, they are subclassified as branch duct IPMN, main duct IPMN, or combined-IPMNs, the latter being characterized by the involvement of both the main pancreatic duct and the branch-ducts. The reported 5-year risk of developing high grade dysplasia or invasive carcinoma is directly related to this localization with a risk exceeding 70% for tumours arising from the main duct, and substantially lower (25%) risk for lesions of the branch ducts[3,4].
Accordingly, surgery has been indicated for patients with main duct IPMN-P, for branch duct lesions of size > 30 mm or when particular features, such as nodules or thick walls, as present[5].
The clinical behaviour of IPMN-P is less aggressive than pancreatic adenocarcinoma, even when the lesion shows malignant features. The overall 5-year survival rate is indeed higher in patients with malignant invasive IPMN than in those with pancreatic ductal carcinoma (36% vs 21%)[6].
In the past few years a rare subtype of cholangiocarcinoma, which shows a mainly papillary proliferation into the bile duct lumen, has been identified and classified as papillary cholangiocarcinoma. The term intraductal papillary mucinous neoplasms of the bile duct (IPMN-B) has also been employed for this tumour type, as it shares some histopathologic and clinical aspects with IPMN-P. Indeed, IPMN-B seem to have a better clinical prognosis than the other major types of cholangiocarcinoma[7], with a reported 5-year survival rate for IPMN-B after surgical resection close to 38%[8]. However, most reported cases or series of IPMN-B have been treated surgically, and the possible role of medical treatments in patients with IPMN-B has not been investigated.
Interestingly, a few cases of IPMN-B and IPMN-P occurring simultaneously have been reported[9-12], possibly suggesting a common carcinogenesis pathway.
As all reported cases of simultaneous IPMN-B and IPMN-P were also treated surgically, the possible outcome of such patients when managed conservatively is unknown. We hereby report a case in which IPMN-B and IPMN-P were diagnosed, simultaneously and incidentally, in a patient who received chemoradiotherapy, with excellent results in long-term follow-up.
A 76-year-old woman, with a history of hypertension, hypercolesterolemia and type II diabetes was incidentally diagnosed with an asymptomatic mass in the liver during routine abdominal ultrasonography (US) performed for evaluation of haematuria. She subsequently underwent abdominal computer tomography (CT) scan and magnetic resonance imaging, resulting in the identification a 25 mm × 18 mm intrahepatic cholangiocarcinoma. Moreover, both imaging modalities disclosed the presence of multiple pancreatic cystic lesions (ranging 10-13 mm) located in the uncinate process and in the tail of the pancreas.
The patient was referred to our Centre 3 mo after the initial US, and a magnetic resonance cholangiopancreatography (MRCP) was performed. This demonstratied a 30-mm bulky fluid lesion, with a 25-mm endoluminal solid component, occupying the bile duct lumen in the right liver, as well as multiple pancreatic cystic lesions (ranging 10-13 mm) in the uncinate process and in the tail of pancreas and communicating with an irregular Wirsung, consistent with the diagnosis of diffuse branch duct pancreatic IPMN. Endoscopic ultrasound (EUS) confirmed the presence of an hepatic irregular fluid lesion originating from the bile duct (major diameter 23 mm) with an internal solid component, and multiple dilatations in both the uncinate process and of in the tail of the pancreas, suggestive for branch duct IPMN-P. However, an EUS-guided fine needle aspiration of the biliary lesion was inconclusive for cancer cells. At that time the patient was asymptomatic, the carbohydrate antigen 19-9 levels were normal and there was no biliary tract dilatation or biochemical abnormalities. The possibility of surgery was discussed with the patient, who refused this option and a follow-up was programmed.
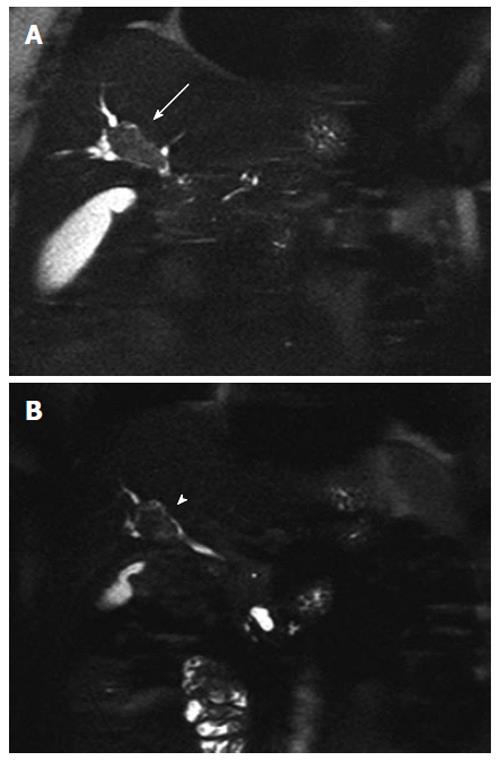
Seven months later a follow-up MRCP demonstrated disease progression (Figure 1A) with an increased size of the bile duct lesion (40 mm), which now extended to the biliary confluence. Its content was described as a fluid proteic component consistent with mucin. The pancreatic lesions were stable. Blood tests showed initial signs of cholestasis [aspartate transaminase 331 U/L (n.v 15-46 U/L), alanine transaminase 273 U/L (n.v 11-66 U/L), γ glutamyl transferase 476 U/L (n.v 8-78 U/L), alkaline phosphatase 161 U/L (n.v 35-128 U/L)]. An endoscopic retrograde cholangiopancreatography (ERCP) was performed and a biliary plastic stent (10 FR) was inserted. Multiple endoscopic bioptic and brushing assays on the biliary lesion indicated biliary duct cells with a papillary growth, showing severe dysplasia and cancer foci, within lakes of mucin. The findings were considered suggestive for an invasive IPMN-B.
Given the patient’s preferences, age and co-morbidities, a conservative management was chosen and the patient underwent concomitant chemotherapy (5-fluoruracine, 5 d infusion for 6 wk 225 mg/m2 per die) and radiotherapy (total 50.4 Gy; 1.8 Gy/28 fractions, for 6 wk).
Three months after the end of the treatment (18 mo after the diagnosis) she underwent a re-staging CT-scan and a MRCP which showed a decrease in the size of the biliary lesion (30 mm) and a prevalence of the endoluminal component (Figure 1B), and an absence of bile duct dilation. The pancreatic lesions were again stable in size. As the patient was asymptomatic and blood tests in the normal range, a new ERCP was performed and the plastic biliary plastic was removed.
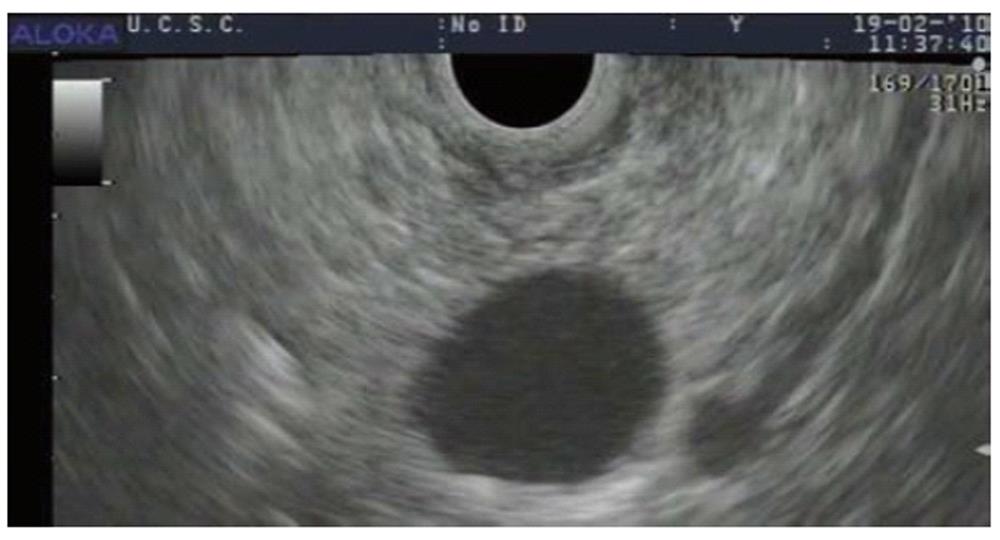
Five months later (23 mo after the diagnosis) a new MRCP again showed signs of disease progression, with an increase in the size of both the biliary (45 mm) and of the largest pancreatic lesions (31 mm). This increase in size of > 1 cm in few months, with a lesion size exceeding 3 cm is considered to be suggestive of a potentially malignant behaviour[2,13]. A second EUS confirmed the presence of the biliary lesion extending from the median third of the main biliary duct to the hepatic hilum, and of multiple pancreatic lesions communicating with a dilated Wirsung’s duct. EUS-FNA of the largest cystic pancreatic lesion (Figure 2) in the uncinate process was performed and fluid collected for further analyses. The cystic fluid level of Carcinoembryonic Antigen (CEA) was 27 U/L and amylase was 23.8842 U/L. Cytological examination showed dysplastic epithelium within lakes of mucin.
The patient then underwent second-line chemotherapy treatment (Gemcitabine 1000 mg/m2, 1,8,15 q28, 3/4 wk).
A restaging CT-scan 28 mo after diagnosis showed a new stabilization, of both the biliary (45 mm) and of the pancreatic lesions. This was further confirmed by another MRCP conducted 3 mo later (31 mo after the diagnosis), which demonstrated a significant decrease in size of the major pancreatic lesion (20 mm).
Currently (36 mo after the diagnosis) the patient is asymptomatic and in good clinical conditions, the disease is stable, and she is undergoing her 26th administration of chemotherapy.
We have described a case of concomitant biliary and pancreatic IPMNs that were diagnosed incidentally and were managed conservatively by endoscopic stent placement and chemo-radiotherapy with a good clinical outcome in a 3-year follow-up.
The term biliary IPMN refers to a recently classified subtype of cholangiocarcinoma, which is considered the biliary counterpart of pancreatic IPMN, as they share several clinico-pathological features. These include a prominent intraductal papillary proliferation pattern, a gastrointestinal phenotype, frequent mucin hyper-secretion and progression to mucinous carcinoma[8,14].
Such intraductally-growing tumours account for only a small fraction of cholangiocarcinomas, and represent an equivocally-classified entity that has been described in the literature with several different names (mucin-producing intrahepatic cholangiocarcinoma, biliary papillary tumor, mucin producing bile duct tumor, intrahepatic biliary intraductal papillary mucinous neoplasia, IPMN-B, biliary IPMN)[15].
IPMN-Bs present with striking similarities to pancreatic IPMNs, including intraductal papillary growth, the production of mucin and the relatively indolent clinical behaviour. As the biliary tract and the pancreas have a common embryologic origin from the ventral foregut, it seems plausible that some common genetic events may lead to the development of IPMN-P and IPMN-B.
Interestingly, it has recently been proposed that only IPMN-Bs with proper mucin production, such as the one diagnosed in the present report, share histopathological and molecular similarities with IPMN-P[16], and that although the clinicopathological features of IPMN-B resemble those of IPMN-P, the biliary lesions have a higher malignancy rate[17].
To the best of our knowledge, there have been only four reported cases of patients with synchronous IPMN-B and IPMN-P, all of which weresurgically treated[9-12]. We have made the first presentation of the clinical course of a case treated conservatively with a good clinical outcome in a long-term follow-up. This suggests that even in aggressive cases, with clear signs of malignant transformation, concomitant IPMN-B and IPMN-P may be conservatively managed with chemoradiotherapy in patients with a relatively high surgical risk.
Intraductal papillary mucinous neoplasms of the pancreas are being increasingly diagnosed due to improved imaging techniques and greater awareness[18]. Therefore, their incidental diagnosis often occur in elderly patients undergoing imaging procedures for other disorders[19,20]. In such cases, surgery may be ruled-out, even in presence of factors associated with a higher risk of malignancy.
However, there have been no reported studies specifically investigating the possible efficacy of chemotherapy and/or radiotherapy in this setting. Our finding of a radiologic response of both biliary and pancreatic intraductal papillary mucinous neoplasms to medical treatments may therefore prove interesting for similar cases, in the absence of evidence from clinical trials.
On the other hand, whether the coincidence of these two neoplasms is merely accidental, or part of a defined phenotype requires further detailed clinical and molecular studies on larger series.
Our observation seems to confirm that IPMN-B and IPMN-P, even when occurring simultaneously and with signs suggesting a malignant behaviour, have a relatively good prognosis and require a specific multidisciplinary therapeutic approach.
| 1. | Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. World Health Organization International Histological Typing of Tumors of the Exocrine Pancreas. Berlin: Springer–Verlag 1996; 1-61. |
| 2. | Sahani DV, Lin DJ, Venkatesan AM, Sainani N, Mino-Kenudson M, Brugge WR, Fernandez-Del-Castillo C. Multidisciplinary approach to diagnosis and management of intraductal papillary mucinous neoplasms of the pancreas. Clin Gastroenterol Hepatol. 2009;7:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Salvia R, Crippa S, Partelli S, Armatura G, Malleo G, Paini M, Pea A, Bassi C. Differences between main-duct and branch-duct intraductal papillary mucinous neoplasms of the pancreas. World J Gastrointest Surg. 2010;2:342-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Lévy P, Jouannaud V, O'Toole D, Couvelard A, Vullierme MP, Palazzo L, Aubert A, Ponsot P, Sauvanet A, Maire F. Natural history of intraductal papillary mucinous tumors of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1458] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 6. | Maire F, Hammel P, Terris B, Paye F, Scoazec JY, Cellier C, Barthet M, O'Toole D, Rufat P, Partensky C. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717-722. [PubMed] |
| 7. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 8. | Barton JG, Barrett DA, Maricevich MA, Schnelldorfer T, Wood CM, Smyrk TC, Baron TH, Sarr MG, Donohue JH, Farnell MB. Intraductal papillary mucinous neoplasm of the biliary tract: a real disease? HPB (Oxford). 2009;11:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Yamaguchi Y, Abe N, Imase K, Mizuno H, Chinen K, Mori H, Sugiyama M, Atomi Y, Ishida H, Takahashi S. A case of mucin hypersecreting intraductal papillary carcinomas occurring simultaneously in liver and pancreas. Gastrointest Endosc. 2005;61:330-334. [PubMed] |
| 10. | Joo YH, Kim MH, Lee SK, Seo DW, Yoo KS, Min YI, Chang JJ, Yu E. A case of mucin-hypersecreting intrahepatic bile duct tumor associated with pancreatic intraductal papillary mucinous tumor. Gastrointest Endosc. 2000;52:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Ishida M, Seki K, Honda K, Kimura T, Katayama K, Hirose K, Dojo M, Azuma T, Imamura Y, Hutchins RR. Intraductal mucinous tumors occurring simultaneously in the liver and pancreas. J Gastroenterol. 2002;37:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Zalinski S, Paradis V, Valla D, Belghiti J. Intraductal papillary mucinous tumors of both biliary and pancreatic ducts. J Hepatol. 2007;46:978-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Bae SY, Lee KT, Lee JH, Lee JK, Lee KH, Rhee JC. Proper management and follow-up strategy of branch duct intraductal papillary mucinous neoplasms of the pancreas. Dig Liver Dis. 2012;44:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 14. | Kim HJ, Kim MH, Lee SK, Yoo KS, Park ET, Lim BC, Park HJ, Myung SJ, Seo DW, Min YI. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000;32:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Paik KY, Heo JS, Choi SH, Choi DW. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008;97:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, Furukawa K, Takeuchi D, Takayashiki T, Suda K. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol. 2011;35:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Kloek JJ, van der Gaag NA, Erdogan D, Rauws EA, Busch OR, Gouma DJ, ten Kate FJ, van Gulik TM. A comparative study of intraductal papillary neoplasia of the biliary tract and pancreas. Hum Pathol. 2011;42:824-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 447] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 19. | Girometti R, Intini SG, Cereser L, Bazzocchi M, Como G, Del Pin M, Baccarani U, Toniutto P, Zuiani C. Incidental pancreatic cysts: a frequent finding in liver-transplanted patients as assessed by 3D T2-weighted turbo spin echo magnetic resonance cholangiopancreatography. JOP. 2009;10:507-514. [PubMed] |
Peer reviewer: Yo-ichi Yamashita, MD, PhD, Department of Surgery, Hiroshima Red Cross Hospital and Atomic Bomb Survivors Hospital, Senda-machi 1-9-6, Naka-ku, Hiroshima 730-8619, Japan
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM