INTRODUCTION
Peritoneal surface malignancies (PSM) are a loco-regional neoplastic dissemination that includes peritoneal carcinomatosis (PC), that is the progression of neoplastic diseases from abdominal, pelvic or extra abdominal organs[1-3], pseudomyxoma peritonei (PMP), an uncommon “borderline malignancy” generally arising from a perforated appendiceal epithelial tumor and, finally, primitive tumors of the peritoneum, such as diffuse malignant peritoneal mesothelioma (DMPM). The PC has long been considered a lethal clinical entity[4], with no curative options and a median survival rate of 3-6 mo[5], and it can be present at the moment of the diagnosis of primary cancer or, most frequently, after potentially curative surgery. The evolution of loco-regional treatments options occurred in the last two decades and has deeply changed the natural history of primitive and secondary PSM. In fact, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has become a promising treatment for patients with peritoneal malignancies thanks to the favorable results in terms of quality of life and outcome[6]. Several studies have proved the effectiveness of the combination of CRS plus HIPEC[7-12]. In fact, this type of approach has been established as the standard therapy for pseudomixoma peritonei with a mean survival rate of 156 mo[13] and a 5-year survival range from 62.5% to 100% for low grade and from 0 to 65% for high grade disease[14]. In PC from colorectal cancer, the mean survival rate varies considerably from 12 to 32 mo, with 1-year, 2-year, 3-year and, when reported, 5-year survival rates ranging from 65% to 90%, 25% to 60%, 18% to 47%, and 17% to 30% respectively[15]. For patients with a peritoneal diffusion of gastric cancer, the mean survival ranges from 8 to 11 mo and the 5-year survival from 6% to 16%[16,17]. In PC from epithelial ovarian cancer (EOC), the most lethal gynecological malignancy, a median overall and disease free survival of up to 64 mo and 57 mo, respectively[18] with a 5-year survival rate from 39% to 60.7%[9-19] has been reported. Finally, for peritoneal mesothelioma, after aggressive surgery combined with HIPEC, a median survival of 34-92 mo with a 5-year survival rate of 67% has been reported[12,20].
RATIONALE OF CYTOREDUCTIVE SURGERY
This therapeutic approach, so complex that it requires a specifically dedicated multidisciplinary team, is based on a rationale and techniques by now well codified. On one hand, CRS allows reduction of the neoplastic mass and, on the other hand, by means of induction of the cell-growth phase, the elimination of the chemoresistant clones and the improvement of the antiblastic perfusion, increasing tumoral chemosensitivity[21,22]. The concept of CRS, different from that more widespread of debulking surgery, forecasts the complete removal of the neoplastic implants with the possibility of leaving residual disease with a maximum cut-off value of 2.5 mm, the optimal target for the employment of HIPEC[23]. For this reason and to evaluate the entity of cytoreduction, we utilized the “Completeness of Cytoreduction Score” (CC score) proposed by Sugarbaker[24]: CC-0: no disease; CC-1: residual disease with size < 2.5 mm; CC-2: residual disease with size included from 2.5 mm to 2.5 cm: CC-3: residual disease with size > 2.5 cm or confluence of many tumoral nodules. While CC-0 and CC-1 are deemed optimal results thanks to the “chemical cytoreduction” performed, CC-2 and CC-3 are defined as incomplete cytoreduction. An accurate surgical technique must be combined with a deep knowledge of the modalities of tumoral dissemination inside the peritoneum. In fact, the dissemination of the PSM occurs by parietal and visceral surfaces and, in particular, in the areas where the digestive tract (rectum-sigma, ileocecal valve and gastric antrum) is fixed to the retroperitoneum and peristalsis is less active. This consideration justifies the fact that a complete cytoreduction can also require multivisceral resections.
RATIONALE OF CHEMOHYPERTHERMIA
The development of chemohyperthermia finds its origins in the necessity to exceed the limits of intraperitoneal chemotherapy performed in normothermia, represented by the low penetration of cytotoxic drugs in the context of the neoplastic tissue[25] and by their lack of homogenous distribution caused by the postoperative adhesions. The essential condition for intraperitoneal treatment is the direct instillation of cytotoxic drugs in the peritoneal cavity, leading to an increase in the contact between chemotherapeutic and peritoneum surfaces due to the peritoneal-plasma barrier[26]. In fact, drugs with a high molecular weight containing hydrophilic groups show a slow rate of movement from the peritoneal cavity into the plasma (peritoneal clearance). This consequently leads to a continuous high concentration gradient of chemotherapeutic drugs between the peritoneal cavity and the plasma compartment, with a more uniform distribution of them throughout the abdominal cavity compared to systemic administration. An additional advantage of intraperitoneal chemotherapy administration is that the blood drainage of the peritoneal surface occurs via the portal vein to the liver, providing a detoxifying first-pass effect and an increased exposure of potential hepatic micrometastases to cytotoxic drugs. Certain drugs are also transported through the lymphatics to the systemic circulation and consequently higher drug concentrations in the lymph than in the plasma are achieved. Also, the association of hyperthermia is based on a strict scientific rationale. In fact, the heat, besides having a recognized direct cytotoxic effect, presents a synergistic activity with some chemotherapeutic agents, allowing the following advantages[27]: a greater intracellular accumulation of drugs, a reduction of the repair of CDDP-DNA complexes, a reduction of intracellular drugs detoxification, a reduction of cellular proliferation, an increase of the apoptotic fraction and a greater tissue penetration. Such mechanisms are further amplified by the chaotic structure of the tumoral vascularity that, being in charge of a reduction of the pH and the glucose and oxygen concentrations, leads to a more sensitive microenvironment to the chemohyperthermic action in respect to healthy tissues[28-30].
TECHNIQUE
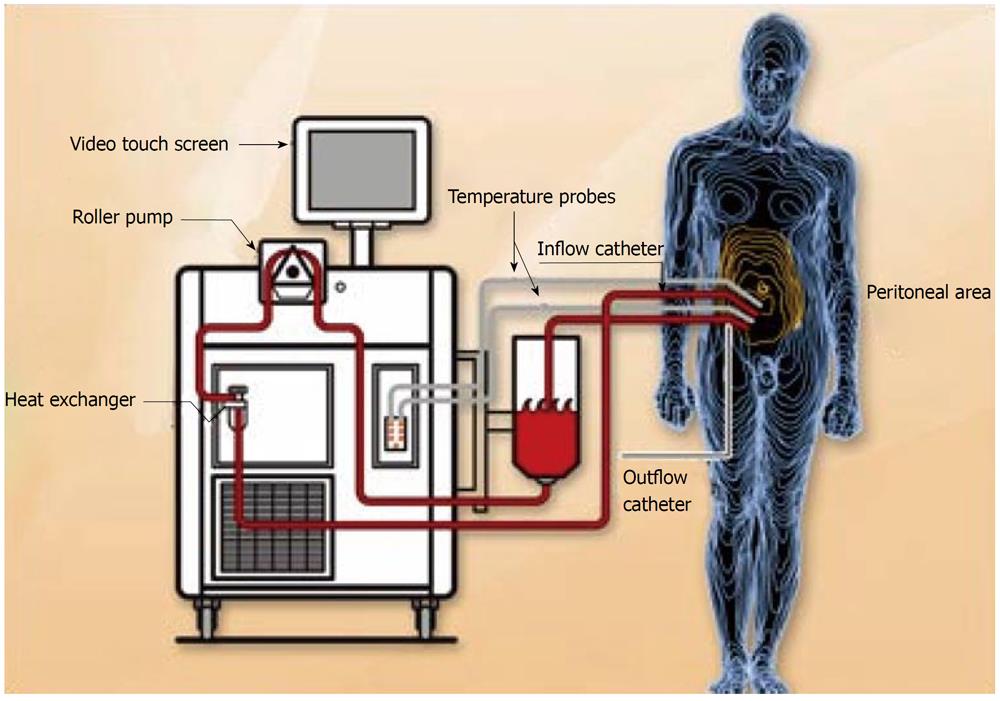
The surgical act requires an accurate phase of preparation: on the operating table, the patient must be placed in the lithotomy position with the legs abducted at 90° and with the buttock folds advanced to the borders of the operating table, a position that affords a wide access to the perineum. The operating table must be able to preserve a good thermal equilibrium or else it is best practice to utilize a cooling-heating blanket on the patient. Moreover, in the preparation of the patient it is important to insert a bladder catheter with three ways that allows hydrodistention of the bladder during the dissection of the pelvic peritoneum, an essential condition to perform this difficult time. A careful abdominal examination is performed through a laparotomy from the xyphoid to the pubic area, making use of a Thompson or Codman self-retaining retractor. The peritonectomy is performed by an electrosurgical scalpel with a spherical electrode of 2-3 mm (ball-tip electrosurgical hand piece) because the peritoneal and visceral resections executed with traditional techniques can scatter a great number of tumoral emboli around inside the abdominal cavity. Furthermore, with the standard dissection it is difficult to obtain a peritoneal surface without cancer cells because only the excision performed with the electrosurgical scalpel leaves a margin of necrosis without vital malignant cells. The electrical burn of the neoplastic and healthy tissue at the level of the resection edges not only reduces the probability of residual disease but also the blood loss. The high-voltage electrosurgical scalpel used on pure cut or spray must be placed on the interface between the peritoneum that must be removed and the healthy tissue. During this type of dissection that leads to tissue carbonization, it is necessary to use a smoke evacuator in order to maintain an optimal vision of the operating field. The aspirator is located 2-3 cm to the dissection field every time that the electrosurgical scalpel is utilized. Cytoreductive surgery is performed through a sequence of manoeuvres well codified[31] that are carried out according to the extent of disease: resection of the greater omentum, right parietal peritonectomy ± resection of right colon; left upper side and left parietal peritonectomy ± splenectomy; right upper side peritonectomy, Glisson’s capsule resection, Morrison pouch peritonectomy; lesser omentum resection, hepatic ileus cytoreduction ± cholecystectomy ± total or partial stomach resection; pelvic peritonectomy ± sigmoid resection ± hysterectomy and bilateral annexectomy; other bowel resections and/or tumoral mass resection; bowel anastomosis. Chemohyperthermia begins at the end of cytoreductive surgery with the placement of four or five drains in the abdominal cavity. We use five drains, two for the infusion of liquid, placed in the right subdiaphragmatic region and in pelvic pouch respectively and three for the effusion of liquid, placed in the left subdiaphragmatic region, in the subhepatic space and in the shallow pelvis respectively. Continuous thermal monitoring is performed by six thermometric probes placed at the level of the upper and lower abdomen, in inflow and outflow and at the level of the rectum and esophagus respectively. There are many procedures for intraperitoneal administration of hyperthermic chemotherapy but those most utilized are the closed and open abdomen techniques. In the first option, the skin edges of the abdominal incision are temporarily closed by a continuous suture[32], while in the second one, the skin edges of the laparotomy are covered by a patch of plastic material and a smoke evacuator is placed under the plastic sheet to clear chemotherapy particles that may be liberated during the procedure[33]. After that, the drains are connected to an extracorporeal circuit and in the open abdominal technique the surgeon performs a manual manipulation of the intra-abdominal contents to assure a homogeneous distribution of both chemotherapeutic agents and heat. Hence, the phase of replenishment of the circuit, defined priming, starts with the liquid chosen, on which optimal composition there is still no consensus. The possibilities most utilized are peritoneal dialysis solution[32], saline solution[34] and a solution composed of a ratio of 2 to 1 by Normosol R to pH 7.4 and a plasma volume expander[35]. The volume of liquid used must be sufficiently copious to guarantee a constant and homogeneous heat in the whole peritoneal cavity, without inducing excessive abdominal distension and thermodilution. For a suitable circuit to work, a volume of about 3-4 L is sufficient for the open abdomen technique while, for the closed abdomen technique, a volume of about 6 L[36] is necessary. Therefore, the heating phase of the perfusion begins, using inflow temperature of approximately 44-46 °C until a intraperitoneal temperature oscillating from 41 to 43 °C is reached when the chemotherapeutic agents are administered (Figure 1). The most commonly used drugs, in mono or poli-chemotherapy, are docetaxel[34], oxaliplatin[12], cisplatin, doxorubicin and mytomicin C[37]. We use cisplatin (25 mg/m² per liter) associated with mytomicin C (3.3 mg/m² per liter) for 60 min in the treatment of colic and gastric carcinomatosis and pseudomyxoma peritonei and cisplatin (43 mg/L) plus doxorubicin (15.25 mg/L) for 90 min in the treatment of the ovarian carcinomatosis and peritoneal mesothelioma. The heated perfusate containing the chemotherapeutic agents is administered in the peritoneal cavity with a medium flow of 600-1000 L/min. At the end of the perfusion, the liquid is quickly drained and, after careful control of the abdominal cavity with particular attention to the possible “suction lesions” of the small intestine, the surgeon proceeds to the definitive closure of the abdominal wall.
Figure 1 Pattern of hyperthermic intraperitoneal chemotherapy.
From the site: http://www. healthinfoispower.wordpress.com.
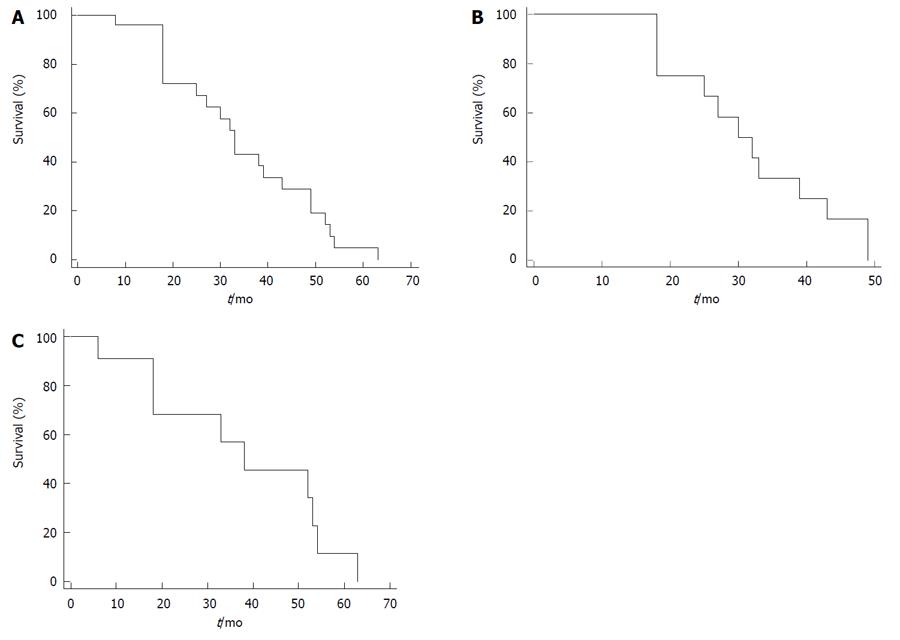
In succession, we report the results that we have obtained at our department (unpublished data). The 1-, 3- and 5-year overall survival rate were 96%, 44% and 5% respectively, with a median survival of 33 mo for the whole sample (Figure 2A); for the gastrointestinal sample, the 1-, 3- and 4-year overall survival rate were 100%, 34% and 17% respectively, with a median survival of 30 mo (Figure 2B) and, finally, for the ovarian sample, the 1-, 3- and 5 year overall survival rate were 86%, 57% and 12% respectively with a median survival of 38 mo (Figure 2C).
Figure 2 Kaplan-Meier curves.
A: Survival probability of patients submitted to cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy; B: Survival probability of gastrointestinal sample; C: Survival probability of ovarian sample.
FUTURE PERSPECTIVES
The peritoneal surface remains an important failure site for patients with abdominal, pelvic or extra abdominal malignancies. During the last two decades, novel therapeutic approaches combining CRS with HIPEC have emerged for peritoneal carcinomatosis patients. This has resulted in remarkable clinical successes in contrast with prior failures. However, there is still a lack of uniformity in the methodology in the interaction and the results between the various operative techniques and intraperitoneal chemotherapies regimens used by various international study groups. In fact, for this reason, a recent international conference was convened and a consensus statement on the appropriate use of CRS and HIPEC was developed and adopted by the Peritoneal Surface Malignancy Group in an attempt to standardize the several procedures for this treatment[37]. However, we maintain that is necessary to perform several RCTs to standardize the technique (open or close abdomen), the chemotherapy regimens, the length of time of HIPEC, the temperature, the flow of the heated perfusate and so on. Furthermore, we maintain that other RCTs should be performed in the future concerning the role of the HIPEC after complete cytoreductive surgery in patients with colorectal cancer (CRS plus HIPEC vs CRS both followed by best systemic therapy), the role of upfront CRS plus HIPEC for long-term survival in selected patients with advanced stage EOC and finally, the role of upfront CRS plus HIPEC for long-term survival in patients with gastric cancer stage III-IV.
Peer reviewers: Takuya Watanabe, MD, PhD, Department of Internal Medicine and Gastroenterology, Medical Hospital, the Nippon Dental University School of Life Dentistry, 1-8 Hamauracho, Chu-o-ku, Niigata 951-8580, Japan; Yo-ichi Yamashita, MD, PhD, Department of Surgery, Hiroshima Red Cross Hospital and Atomic Bomb Survivors Hospital, Senda-machi 1-9-6, Naka-ku, Hiroshima 730-8619, Japan
S- Editor Wang JL L- Editor Roemmele A E- Editor Zhang DN