Published online Oct 15, 2011. doi: 10.4251/wjgo.v3.i10.144
Revised: September 21, 2011
Accepted: September 28, 2011
Published online: October 15, 2011
Thyroid transcription factor 1 (TTF-1) plays a key role in morphogenesis of the lungs and is expressed in up to 90% of pulmonary small cell carcinomas. This explains why this marker is frequently used in the search for the primary origin of metastatic endocrine tumours. Here we report on a TTF-1 expressing mixed endocrine-exocrine carcinoma of the common bile duct in a patient with pulmonary nodules that did not appear to be neoplastic. TTF-1 positivity in pulmonary and extrapulmonary neuroendocrine tumours is reviewed, and we conclude that TTF-1 expression in neuroendocrine tumours of the small-cell type are not uncommon at extrapulmonary locations. Therefore, immunohistochemistry for TTF-1 in such tumours should be interpreted with caution.
- Citation: Verset L, Arvanitakis M, Loi P, Closset J, Delhaye M, Remmelink M, Demetter P. TTF-1 positive small cell cancers: Don’t think they’re always primary pulmonary! World J Gastrointest Oncol 2011; 3(10): 144-147
- URL: https://www.wjgnet.com/1948-5204/full/v3/i10/144.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v3.i10.144
Thyroid transcription factor 1 (TTF-1) is a homeodomain-containing nuclear transcription factor which belongs to the Nkx2 gene family; it is expressed in the forebrain, thyroid and lung. TTF-1 plays a key role in morphogenesis of the lungs[1] and is expressed in up to 90% of pulmonary small cell carcinoma[2]. This explains why this marker is frequently used to search for the primary origin of metastatic neuroendocrine tumours.
In this article we report the case of a TTF-1 positive mixed endocrine-exocrine tumour of the common bile duct.
A 74-year-old woman was admitted to the Erasme University Hospital because of jaundice, anorexia and a 3-mo history of weight loss (10 kg). In her medical history, we noted hypertension, hysterectomy, ovariectomy and resection of a melanoma of the right forearm. There was no tobacco or alcohol use. Laboratory data showed a cholestatic jaundice with a bilirubin level of 5 mg/dL and an alkaline phosphatase level twice the normal value. The tumour markers carcino-embryonic antigen and CA19.9 were negative. Abdominal magnetic resonance imaging displayed a mass, approximately 2 cm diameter, in front of the pancreatic head with dilation of the main pancreatic duct and of the common bile duct. At endoscopic cholangiopancreatography, a tumoural lesion of the papilla and a distal stenosis of the common bile duct were seen with an upstream dilation. Biopsy of the papilla and brush cytology of the stenosis were inconclusive. In the search for metastases, a chest tomography was performed and two pulmonary nodules were observed, in the right upper and the right lower lobe, respectively. The lower one was hypermetabolic at positron emission tomography-computed tomography (CT). Bronchoscopy excluded any endobronchial lesion; bronchoalveolar fluid lavage, CT-guided fine needle aspiration (FNA) and transbronchial biopsy revealed many macrophages but no neoplastic cells.
Given the absence of histopathologically confirmed metastases, a Whipple resection was performed. Gross examination didn’t reveal any obvious mass; we observed a macrocalcification, and pancreatic tissue surrounding this calcification was indurated. The fresh tissue was formalin-fixed, routinely processed and stained with haematoxylin-eosin.
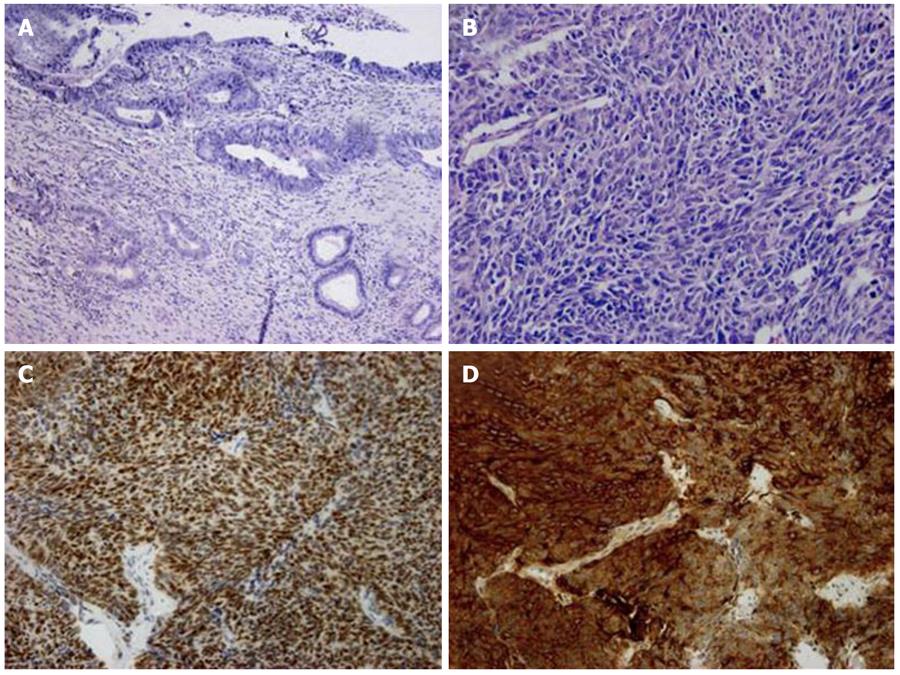
Histologically, the indurated lesion presented two patterns. First, we observed a glandular tumoural pattern arising from the epithelium of the common bile duct which presented high grade dysplasia with nuclear stratification and irregular and hyperchromatic nuclei (Figure 1A). This adenocarcinoma component had invaded the duodenal wall. Another part of the tumour showed an endocrine pattern with neoplastic cells arranged in solid nests and displaying little cytoplasm, irregular nuclei and inconspicuous nucleoli (Figure 1B). This part of the tumour was positive for TTF-1 (Dako, 1:2000) and CD56 (Klinipath, 1:1000) immunohistochemistry (Figure 1C and D). Mitotic figures were frequent and perineural invasion was present. One metastatic lymph node was found in the peripancreatic fat.
Two months after the duodenopancreatectomy, an octreoscan showed multiple hepatic metastases; there was no recruitment of the pulmonary nodules.
Mixed endocrine-exocrine carcinoma (MEEC) is uncommon in the extrahepatic bile duct, representing less than 0.4% of tumours at this location[3]. MEEC is defined as a malignant tumour containing at least 30% of each component; the natural history of MEEC with a small cell cancer component follows the disease progression of this latter[4].
Two histologic subtypes of small cell cancer of the common bile duct exist: a “composite type” which is frequently retrieved in the gallbladder and consists of small cell cancer and adenocarcinoma (as in this case), and a “pure type” which consists only of small cell cancer[5].
Metastatic disease of the pancreas accounts for less than 2% of all pancreatic malignancies[6]. In this case, given the presence of a hypermetabolic pulmonary nodule, a pancreatic metastatic involvement had to be excluded. It could be argued that the positive TTF-1 in our case points to a primary pulmonary origin; the octreoscan was, however, negative in the pulmonary nodules and strongly positive in the hepatic metastases. Moreover, bronchoalveolar fluid lavage, CT-guided FNA and transbronchial biopsy did not reveal any neoplastic cells. Therefore, and because of the dysplastic lesions observed in the common bile duct adjacent to the invasive tumour, we believe that our case is a primary tumour of the common bile duct.
Table 1 summarizes TTF-1 expression in neuroendocrine tumours from pulmonary and extrapulmonary origin[2,7-21]. TTF-1 might be helpful in differentiating pulmonary carcinoid tumours from extrapulmonary carcinoids. Agoff et al[7], Cai et al[8], Du et al[9], Lin et al[11], Oliveira et al[12], Saqi et al[13] and Srivastava et al[14] could not demonstrate TTF-1 expression in extrapulmonary carcinoid tumours while TTF-1 expression varied from 23% to 95% in pulmonary carcinoids[7-14]. Two primary gastric carcinoid tumours were TTF-1 positive, one in the series of Kaufmann et al[10] and one in the series of Cai et al[8].
| Authors | Carcinoid1 | SCC | ||
| P | EP | P | EP | |
| Folpe et al[2] | 27/60 (45) | ND | 20/21 (95) | ND |
| Agoff et al[7] | ND | 0/49 (0) | ND | 7/16 (44) |
| Cai et al[8] | 11/16 (69) | 1/58 (2) | ND | ND |
| Du et al[9] | 15/53 (23) | 0/28 (0) | ND | ND |
| Kaufmann et al[10] | 6/12 (50) | 1/20 (5) | 30/37 (81) | 12/15 (80) |
| Lin et al[11] | 13/30 (43) | 0/95 (0) | 27/30 (90) | ND |
| Oliveira et al[12] | 19/20 (95) | 0/39 (0) | ND | ND |
| Saqi et al[13] | 8/15 (53) | 0/63 (0) | ND | ND |
| Srivastava et al[14] | 7/20 (35) | 0/103 (0) | ND | ND |
| Cheuk et al[15] | ND | ND | 43/52 (82.7) | 21/50 (42) |
| Jones et al[16] | ND | ND | ND | 17/44 (39) |
| Li et al[17] | ND | ND | ND | 9/42 (21) |
| Lu et al[18] | ND | ND | ND | 9/15 (60) |
| McCluggage et al[19] | ND | ND | ND | 11/13 (84) |
| Yun et al[20] | ND | ND | ND | 15/21 (71) |
| Ordonez et al[21] | ND | ND | 27/28 (96) | 4/54 (7) |
| Total | 106/226 (46) | 2/455 (< 1) | 147/168 (87.5) | 105/270 (39) |
With regard to high-grade endocrine tumours (small cell cancers), the situation is, however, quite different. Agoff et al[7], Cheuk et al[15], Jones et al[16], Kaufmann et al[10], Li et al[17], Lu et al[18], McCluggage et al[19] and Yun et al[20] showed TTF-1 expression in 44%, 42%, 39%, 80%, 21%, 60%, 84% and 71% of extrapulmonary small cell cancers, respectively[7,10,15-20]. In the series of Ordonez et al[21], only 7% of extrapulmonary small cell cancers expressed TTF-1. The hypothesis that small cell cancer originates from totipotent stem cells differentiating into various cell types (e.g. TTF-1 positive stem cells for pulmonary small cell cancer)[22] could explain why TTF-1 expression is retrieved in some foregut small cell cancers. However, this hypothesis cannot explain TTF-1 expression in non-foregut endocrine tumours.
In conclusion, we report a MEEC with TTF-1 expressing small cell cancer component of the common bile duct. A literature review indicates that TTF-1 is helpful in discriminating pulmonary from extrapulmonary carcinoid tumors. In searching for the primary origin of high-grade endocrine tumours (small cell cancers), TTF-1 should, however, not be used.
| 1. | Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 336] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 2. | Folpe AL, Gown AM, Lamps LW, Garcia R, Dail DH, Zarbo RJ, Schmidt RA. Thyroid transcription factor-1: immunohistochemical evaluation in pulmonary neuroendocrine tumors. Mod Pathol. 1999;12:5-8. [PubMed] |
| 3. | Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Okamura Y, Maeda A, Matsunaga K, Kanemoto H, Boku N, Furukawa H, Sasaki K, Uesaka K. Small-cell carcinoma in the common bile duct treated with multidisciplinary management. J Hepatobiliary Pancreat Surg. 2009;16:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Reddy S, Wolfgang CL. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009;10:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Agoff SN, Lamps LW, Philip AT, Amin MB, Schmidt RA, True LD, Folpe AL. Thyroid transcription factor-1 is expressed in extrapulmonary small cell carcinomas but not in other extrapulmonary neuroendocrine tumors. Mod Pathol. 2000;13:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Cai YC, Banner B, Glickman J, Odze RD. Cytokeratin 7 and 20 and thyroid transcription factor 1 can help distinguish pulmonary from gastrointestinal carcinoid and pancreatic endocrine tumors. Hum Pathol. 2001;32:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Du EZ, Goldstraw P, Zacharias J, Tiffet O, Craig PJ, Nicholson AG, Weidner N, Yi ES. TTF-1 expression is specific for lung primary in typical and atypical carcinoids: TTF-1-positive carcinoids are predominantly in peripheral location. Hum Pathol. 2004;35:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Kaufmann O, Dietel M. Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology. 2000;36:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Lin X, Saad RS, Luckasevic TM, Silverman JF, Liu Y. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Oliveira AM, Tazelaar HD, Myers JL, Erickson LA, Lloyd RV. Thyroid transcription factor-1 distinguishes metastatic pulmonary from well-differentiated neuroendocrine tumors of other sites. Am J Surg Pathol. 2001;25:815-819. [PubMed] |
| 13. | Saqi A, Alexis D, Remotti F, Bhagat G. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am J Clin Pathol. 2005;123:394-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol. 2009;33:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Cheuk W, Kwan MY, Suster S, Chan JK. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med. 2001;125:228-231. [PubMed] |
| 16. | Jones TD, Kernek KM, Yang XJ, Lopez-Beltran A, MacLennan GT, Eble JN, Lin H, Pan CX, Tretiakova M, Baldridge LA. Thyroid transcription factor 1 expression in small cell carcinoma of the urinary bladder: an immunohistochemical profile of 44 cases. Hum Pathol. 2005;36:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Li AF, Li AC, Hsu CY, Li WY, Hsu HS, Chen JY. Small cell carcinomas in gastrointestinal tract: immunohistochemical and clinicopathological features. J Clin Pathol. 2010;63:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Lu J, Xue LY, Lu N, Zou SM, Liu XY, Wen P. Superficial primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical analysis of 15 cases. Dis Esophagus. 2010;23:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | McCluggage WG, Kennedy K, Busam KJ. An immunohistochemical study of cervical neuroendocrine carcinomas: Neoplasms that are commonly TTF1 positive and which may express CK20 and P63. Am J Surg Pathol. 2010;34:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Yun JP, Zhang MF, Hou JH, Tian QH, Fu J, Liang XM, Wu QL, Rong TH. Primary small cell carcinoma of the esophagus: clinicopathological and immunohistochemical features of 21 cases. BMC Cancer. 2007;7:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Ordóñez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000;24:1217-1223. [PubMed] |
| 22. | Ralston J, Chiriboga L, Nonaka D. MASH1: a useful marker in differentiating pulmonary small cell carcinoma from Merkel cell carcinoma. Mod Pathol. 2008;21:1357-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Peer reviewer: Claudio Sorio, MD, PhD, Department of Pathology, Assistant Professor, University of Verona School of Medicine, General Pathology Section, Strada Le Grazie, 8, 37134 Verona, Italy
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM