Published online Feb 15, 2026. doi: 10.4251/wjgo.v18.i2.113494
Revised: November 16, 2025
Accepted: December 25, 2025
Published online: February 15, 2026
Processing time: 160 Days and 16.8 Hours
We report an exceptionally rare case of a solitary esophageal metastasis occurring in the tenth year after curative resection of stage I (pT2N0M0) rectal adenocarcinoma. This represents one of the longest reported intervals to esophageal meta
A 42-year-old male underwent curative resection for rectal adenocarcinoma (pT2N0M0, stage I) in 2015. Ten years later (2025), he presented with progressive dysphagia. Imaging and endoscopy revealed a mid-esophageal tumor with me
This case underscores the risk of early tumor recurrence or metastasis beyond standard follow-up windows thus long-term follow-up is necessary.
Core Tip: We report a case of a solitary esophageal metastasis occurring in the tenth year after curative resection of stage I rectal adenocarcinoma, representing one of the longest intervals for rectal metastasis to the esophagus. It emphasizes that even early-stage tumors can metastasize to atypical sites after years. Furthermore, we discuss the role of immunohistochemistry in diagnosis and the potential value of radiotherapy combined with systemic treatment.
- Citation: Zhang Y, Li ZX, Ma DY, Liu F. Solitary esophageal metastasis ten years after curative resection of stage I rectal adenocarcinoma: A case report. World J Gastrointest Oncol 2026; 18(2): 113494
- URL: https://www.wjgnet.com/1948-5204/full/v18/i2/113494.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i2.113494
Autopsy series show esophageal metastases most often originate from lung, breast, or gastric primaries[1]. Metastatic involvement of the esophagus from colorectal carcinoma (CRC) is exceptionally uncommon[1]. Clinically, esophageal metastases often present with dysphagia and can mimic a primary esophageal cancer[2]. Metastatic lesions typically lie in the submucosal layer with intact overlying mucosa, rendering endoscopic biopsies potentially non-diagnostic and the lesion indistinguishable from a primary tumor[1,2]. Stage I CRC generally carries an excellent prognosis, with only about 10% of patients developing any recurrence within five years[3]. In one large series of stage I colon cancer, the vast majority of relapses occurred within the first eight years after surgery[4]. We report a case of an ultra-late solitary esophageal metastasis occurring in the tenth year after curative resection of stage I rectal adenocarcinoma, which highlights the unusual latency and diagnostic challenge, expands the recognized metastatic spectrum of CRC and underscores the need for long-term vigilance even after resection of early-stage disease.
A 42-year-old male, who had completed two cycles of immunochemotherapy for primary esophageal adenocarcinoma, presented with aggravated dysphagia for 4 days and was subsequently referred to the multidisciplinary team (MDT) by the thoracic surgeon.
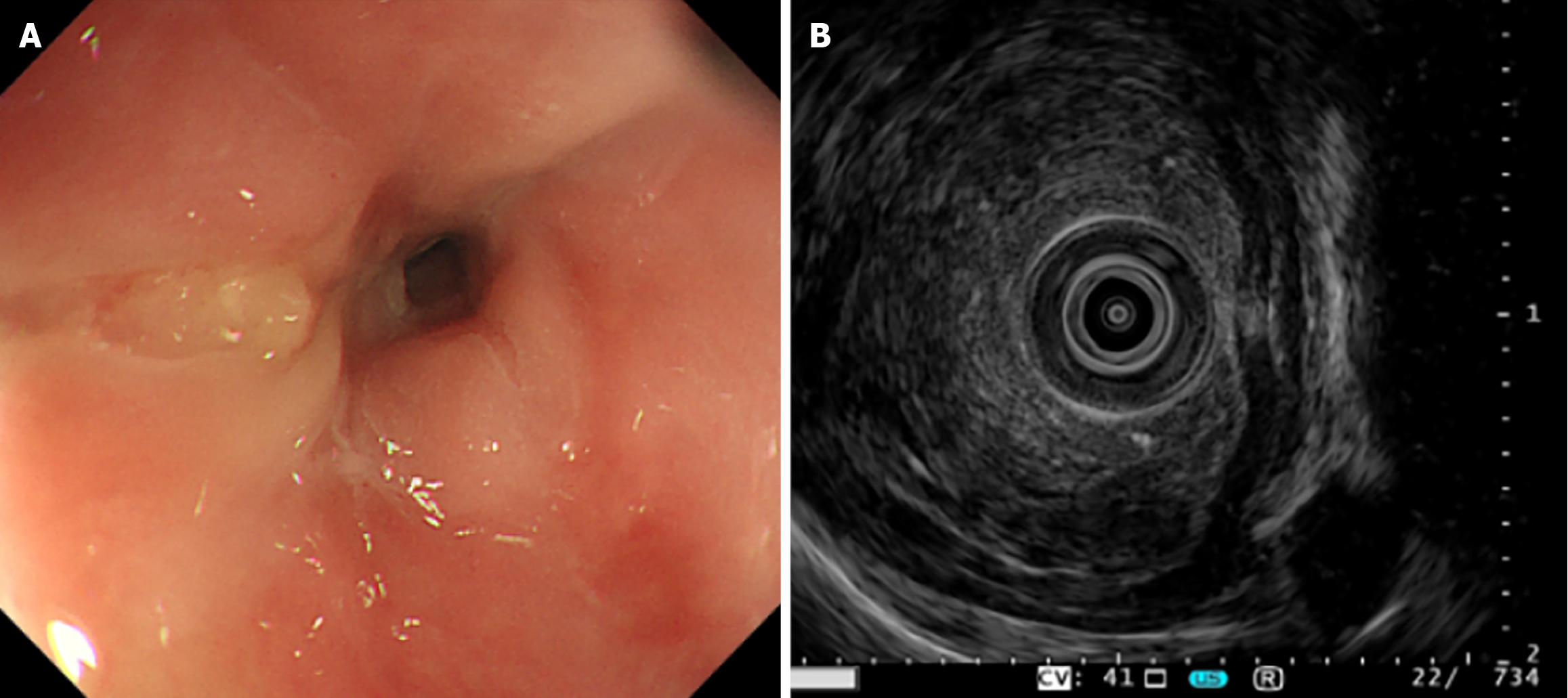
One month ago, the patient presented to the thoracic surgery department with progressive dysphagia for three weeks (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Oesophageal Cancer Module 18 (EORTC QLQ-OES18) dysphagia scale score: 40)[5] on February 14, 2025. Chest computed tomography (CT) scan indicated a neoplastic lesion in the esophagus. Both white-light endoscopy (Figure 1A) and the endoscopic ul
A treatment plan of neoadjuvant immunochemotherapy (nedaplatin 120 mg, nab-paclitaxel 300 mg, tislelizumab 200 mg, q3w) followed by radical surgery was initiated. After two cycles of neoadjuvant therapy, the patient experienced significant worsening of dysphagia (EORTC QLQ-OES18 dysphagia scale score: 90). Surgical resection was deemed unfeasible, prompting referral to the MDT on March 15, 2025.
The patient underwent laparoscopic low anterior resection for rectal cancer in 2015. Postoperative pathology confirmed moderately differentiated rectal adenocarcinoma (lymphovascular invasion absent, perineural invasion absent, pT2N0M0, Stage I, according to the UICC 7th edition). Standard postoperative surveillance, including annual thoracoabdominal CT and endoscopy, was performed. The most recent surveillance examination was on October 28, 2024, showing no abnormalities.
The patient denied notable family history.
A well-healed surgical scar, approximately 3 cm in length, was observed in the right lower abdominal quadrant.
Serum tumor markers were elevated: Carbohydrate antigen (CA) 50: 22.65 IU/mL, CA-242: 87.41 IU/mL, serum car
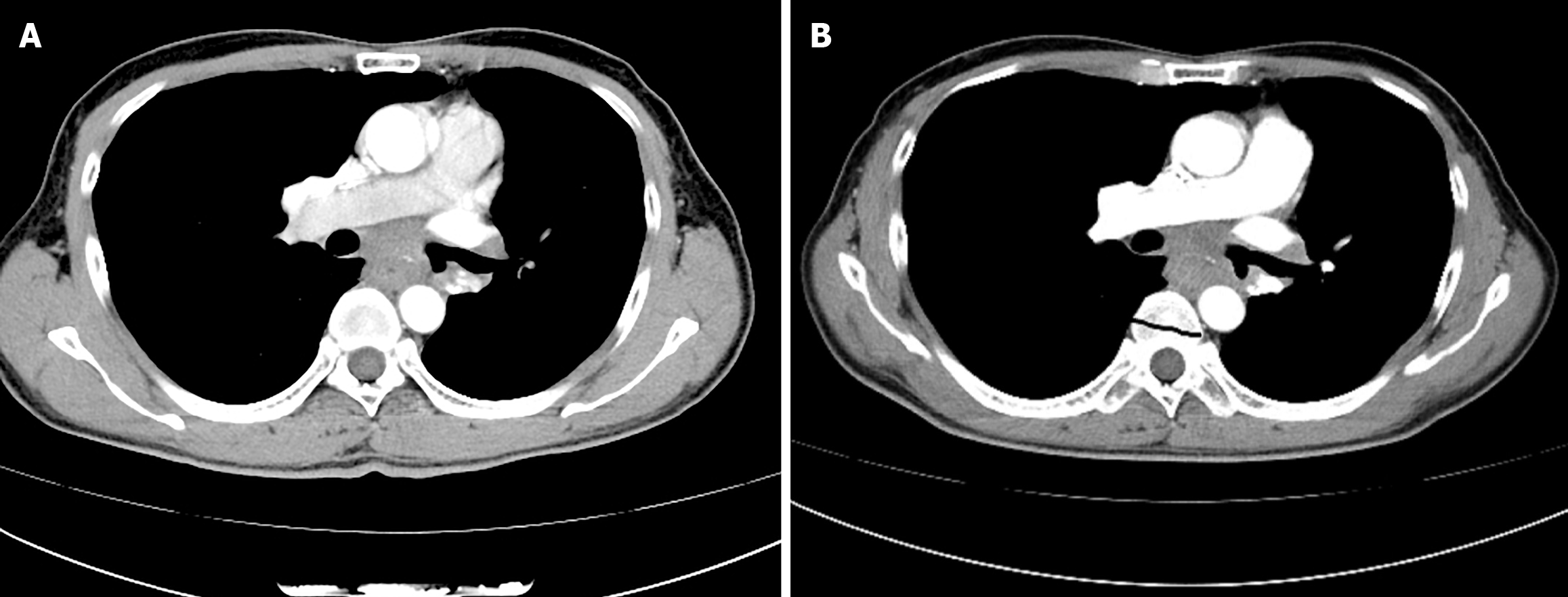
The chest CT result on February 14, 2025 revealed irregular wall thickening in the mid-thoracic esophagus, approximately at T6-T8 level, and subcarinal lymphadenopathy (Figure 3A). The chest CT result on March 15, 2025 indicated that the volume of the esophageal tumor had increased, causing the esophageal lumen to narrow (Figure 3B).
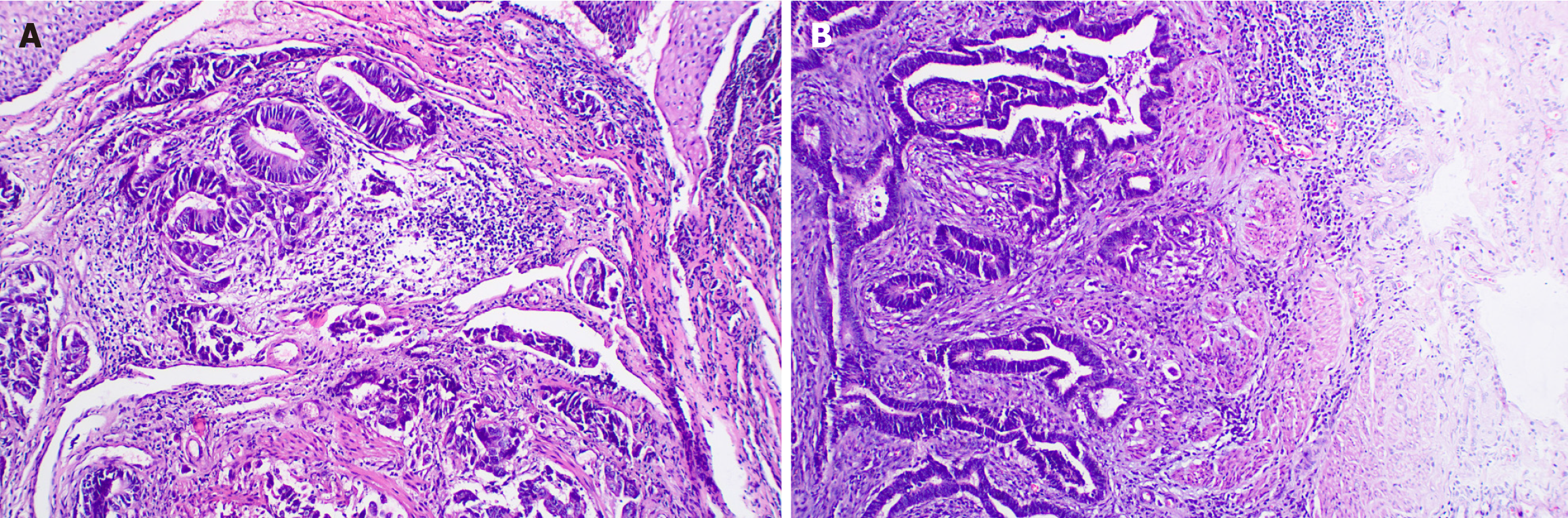
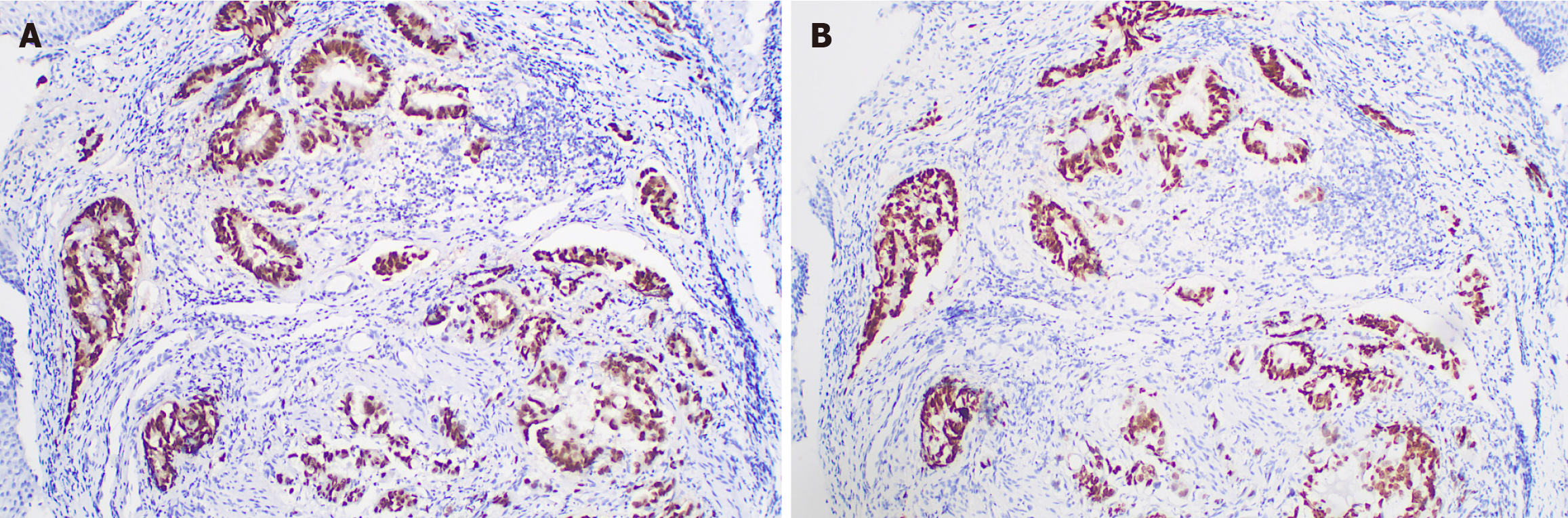
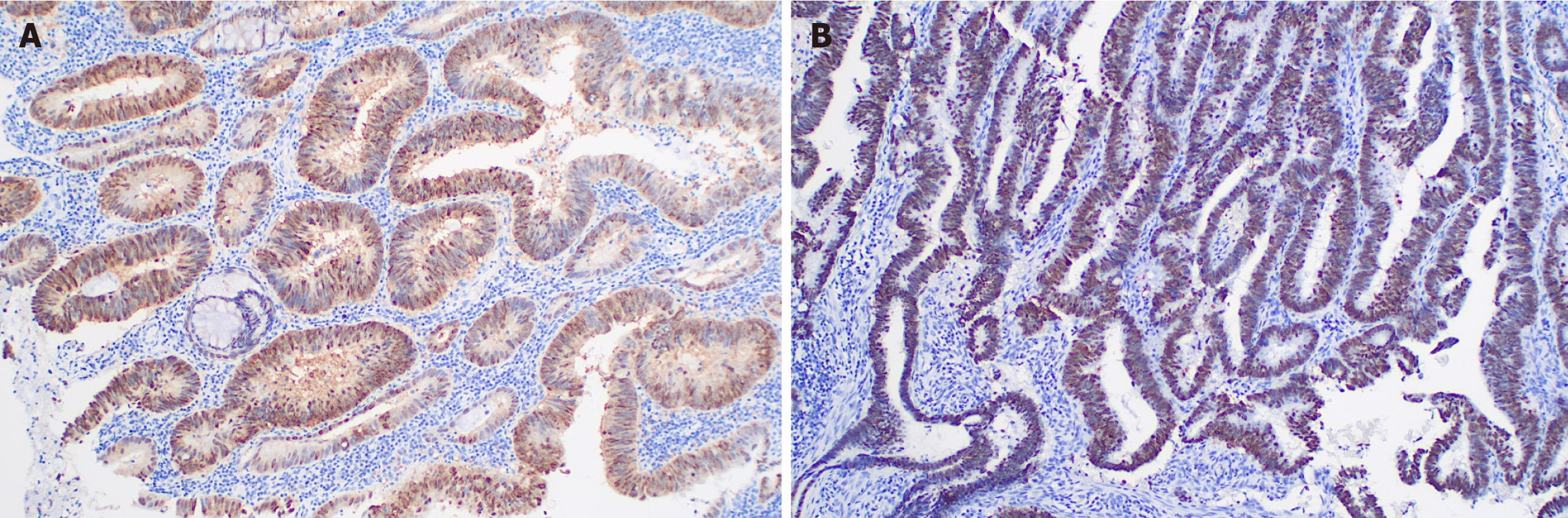
The radiation oncologist initiated an MDT discussion. The MDT pointed out that the patient's neoadjuvant treatment plan was not standard, but considering the history of rectal adenocarcinoma, a thorough re-evaluation of the esophageal pathology specimen (Figure 4A) and rectal cancer specimen (Figure 4B) was undertaken. The immunohistochemistry (IHC) results of the esophageal lesion showed: CDX2(+) (Figure 5A), CK7(-), CK20(+), SATB2(+) (Figure 5B), MSH2(+), MSH6(+), MLH1(+), PMS2(+), Ki67 index (approximately 40%), P53 (mutant pattern), HER-2(-), findings highly sug
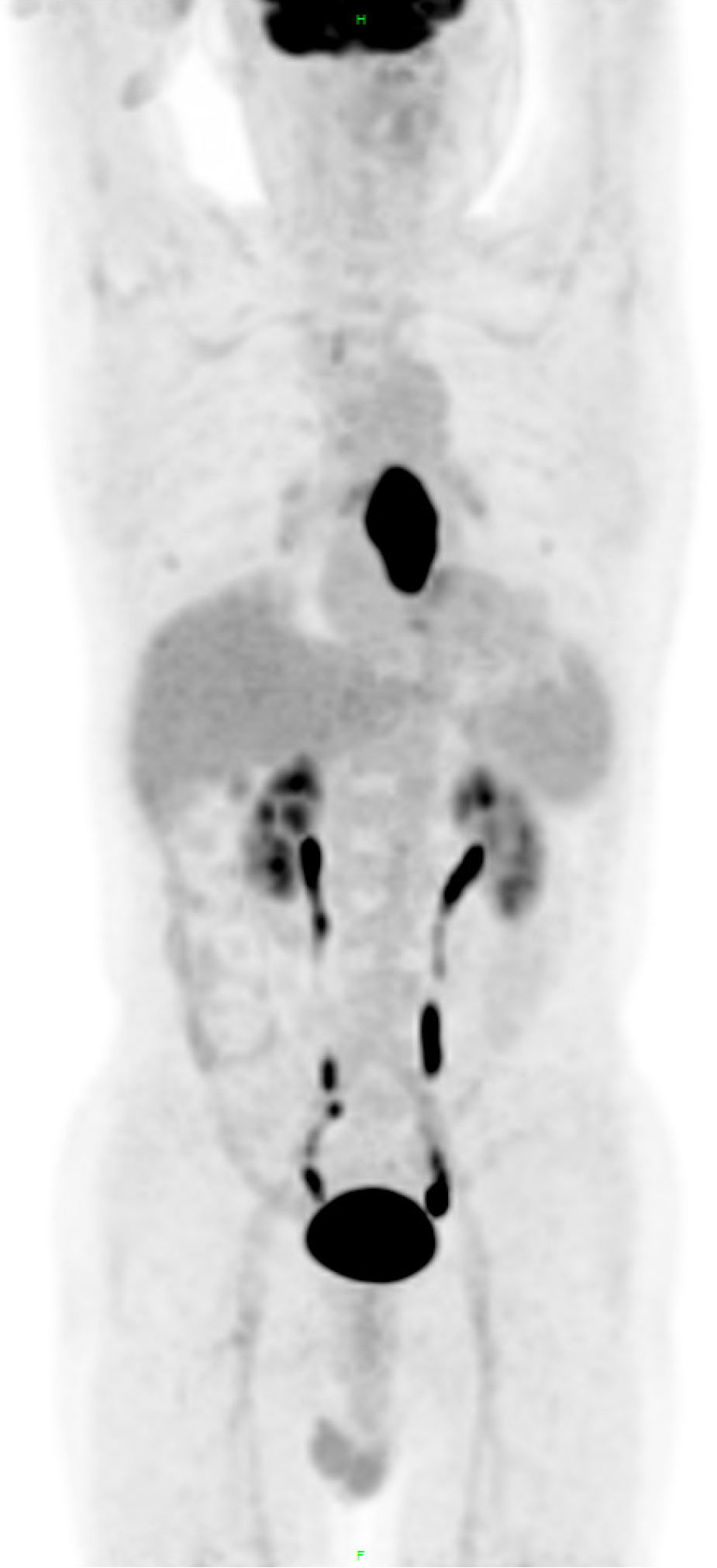
Given the inaccuracy of the previous treatment plan, the patient immediately underwent CT scans of the neck, chest and abdomen, magnetic resonance imaging of the head and pelvis, radionuclide bone imaging, and colonoscopy, in order to determine whether new lesions had appeared among the past two months (March 22, 2025, Imaging Center and Endoscopy Center of the Affiliated Hospital of NSMC). All results supported a solitary esophageal metastasis without local recurrence of the rectum or distant metastasis. The final diagnosis was revised to a solitary esophageal metastasis from rectal adenocarcinoma (RAS wild-type).
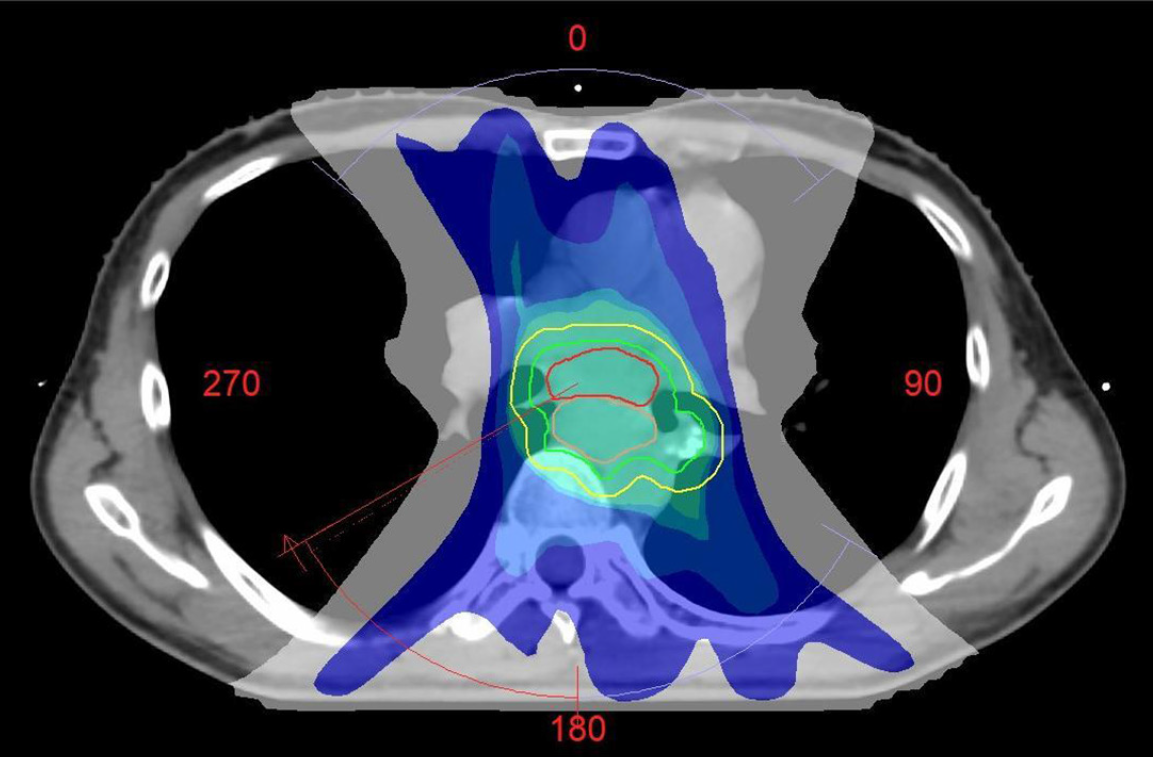
The MDT immediately adjusted the treatment plan for metastatic CRC. Adding cetuximab to chemotherapy including capecitabine and oxaliplatin (CAPOX) in RAS wild-type metastatic CRC has been shown to significantly increase response rates and prolong survival. In order to quickly alleviate the patient's dysphagia, simultaneous radiotherapy was performed on the solitary metastatic lesion, which enabled the local tumor to shrink to the greatest extent and relieved the symptom. Concurrent oral administration of capecitabine has also been proven to be an effective radiosensitizing component. On March 24, 2025, the patient received involved-field radiotherapy using volumetric modulated arc therapy. The planning target volume, encompassing the esophageal metastasis and involved mediastinal lymph nodes, was prescribed a total dose of 50.4 Gy in 28 fractions (1.8 Gy per fraction), delivered five days per week (Figure 7). The doses to surrounding organs at risk were rigorously constrained within accepted tolerance limits to minimize toxicity, as detailed below: Lungs: The mean dose (Dmean) was kept below 8.05 Gy, with the volume of lungs receiving 20 Gy below 9.01% and 5 Gy below 48.14%; Spinal cord: The maximum dose was strictly limited to below 41.46 Gy; Heart: The Dmean was kept below 21.96 Gy, with the volume of heart receiving 30 Gy below 18.63% and 40 Gy below 7.11%. CAPOX plus cetuximab was administered for six 3-week cycles: Capecitabine 1000 mg/m² bid on days 1-14, oxaliplatin 130 mg/m² on day 1, and cetuximab 400 mg/m² loading dose followed by 250 mg/m² weekly.
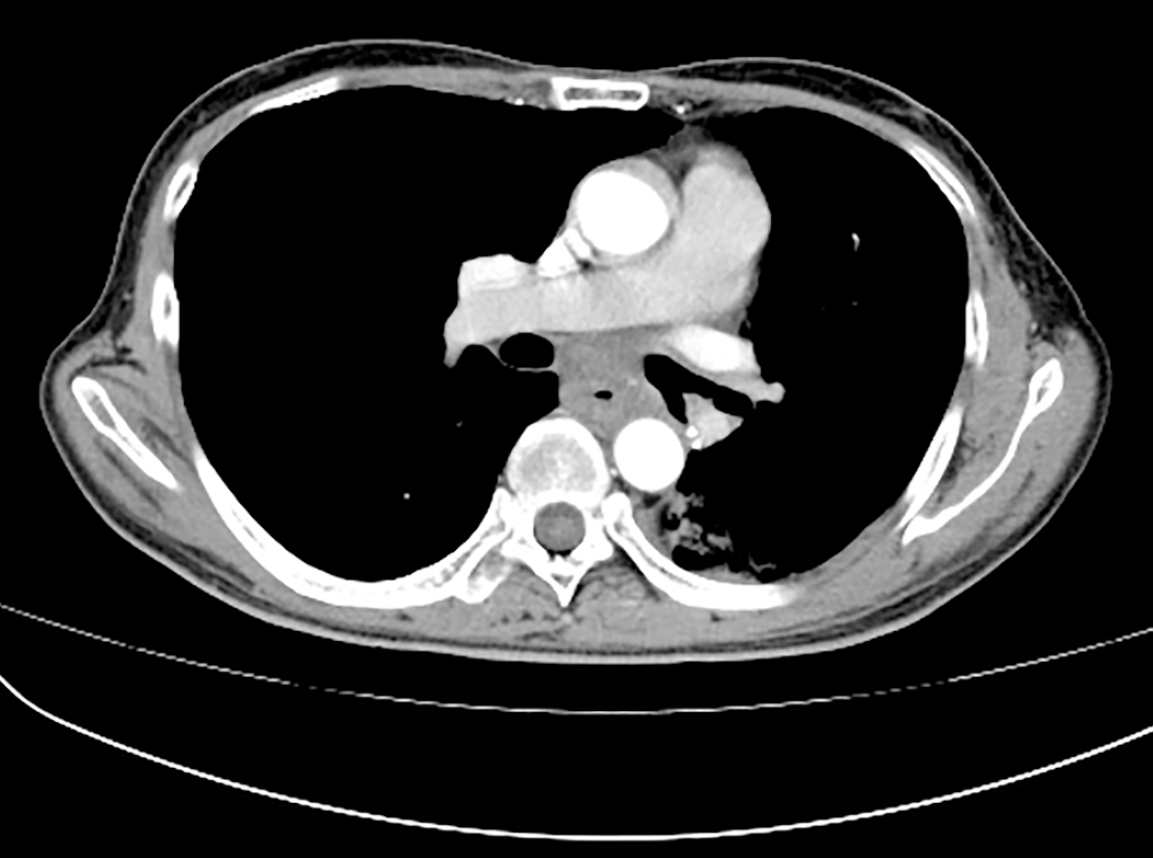
Dysphagia improved significantly within one week after radiotherapy initiation (EORTC QLQ-OES18 dysphagia scale score: 30). Three weeks after radiotherapy initiation, the patient experienced grade one bone marrow suppression with WBC 3.23 × 109 /L (according to Common Terminology Criteria for Adverse Events Version 5.0). The patient was treated symptomatically and recovered. Additionally, no more adverse reactions were observed during the treatment period. One month after radiotherapy completion, dysphagia had nearly completely resolved (EORTC QLQ-OES18 dysphagia scale score: 10). On August 1, 2025, three-month follow-up CT imaging after radiotherapy completion demonstrated a partial response (Figure 8). Serum tumor markers showed substantial decreases: CA-50: 19.15 IU/mL, CA-242: 10.40
PubMed, EMBASE, and Web of Science databases were systematically queried on November 14, 2025, using the following search terms: (“colorectal” OR “colon” OR “rectal”) AND (“esophagus” OR “esophageal” OR “oesophageal”) AND (“metastasis” OR “metastases” OR “secondary” OR “metastatic”). No language or date restrictions were applied. Titles and abstracts were screened. Reports describing metastasis to the esophagus originating from a colorectal or rectal primary were included. Data extracted from each report included: Interval between the primary tumor and esophageal metastasis, primary tumor site and stage, location and characteristics of the esophageal lesion, diagnostic method, treatment, and outcome (Table 1). Based on these data[2,6-12], our case, occurring in the tenth year after curative resection of stage I rectal adenocarcinoma, represents an ultra-late and exceptionally rare presentation, and can be considered one of the longest reported intervals of esophageal metastasis from CRC.
| No. | Ref. | Interval | Primary site/stage | Esophageal site/pattern | Diagnostic method | Treatment | Outcome |
| 1 | Kagaya et al[6] | 14 months | Cecal adenocarcinoma/stage IV | Lower esophagus/submucosal tumor | Histology | Palliative stent + bypass surgery | Well 6 months after surgery |
| 2 | Watanabe et al[7] | 3 years | Rectal adenocarcinoma/stage IIIB | Mid-thoracic esophagus/submucosal tumor | Biopsy + IHC | FOLFOX + bevacizumab | Died 16 months after recurrence |
| 3 | Sönmez et al[8] | Synchronous | Rectal adenocarcinoma/stage IV | Polypoid esophageal lesion | Biopsy + IHC | Plan to surgery after radiation radiotherapy to the rectum | NR |
| 4 | Tanaka et al[2] | 1 year | Cecal signet-ring cell carcinoma/stage I | Multiple longitudinal submucosal tumors | Biopsy + IHC | FOLFOX + bevacizumab | Died 1 year and 5 months after surgery |
| 5 | Thomasset et al[9] | 1 year | Sigmoid carcinoma/Dukes C | Lower esophagus/polypoidal lesion | Histology | 5fluorouracil + folinic acid | Died six months after metastases |
| 6 | Bhurgri et al[10] | 3 years | Sigmoid colon carcer/stage IIB | Esophageal lesion | Biopsy + IHC | Gastrostomy tube + FOLFOX | NR |
| 7 | Vashi et al[11] | 4 years | Colon carcinoma/stage III | Gastroesophageal junction and mid esophagus/ multiple nodules | Histological and immunostains | Irinotecan + cetuximab | NR |
| 8 | Lohsiriwat et al[12] | 1 year | Rectal carcinoma/stage IV | Proximal esophagus/ a 4-mm sessile polyp | Biopsy | Supportive therapy | Died 2 months later |
| 9 | This study | 10 years | Rectal adenocarcinoma/stage I | Mid-thoracic esophagus/submucosal involvement | Biopsy + IHC + KNPB gene test | VMAT + CAPOX + cetuximab | Partial response at 3 months |
Metastatic involvement of the esophagus is uncommon (0.3%-6.1% in autopsy series), most commonly originating from breast or lung cancers[1]. Esophageal metastasis from CRC is exceedingly rare[13]. Proposed mechanisms include hematogenous spread, lymphatic dissemination (including retrograde flow), or direct invasion[14]. Clinical presentation, typically dysphagia, and radiological/endoscopic findings such as circumferential wall thickening or luminal narrowing are often indistinguishable from primary esophageal cancer[1,14]. Biopsies may be inconclusive due to submucosal tumor deposits, which represent a common growth pattern in metastases[14]. In this case, the patient's PET-CT and endoscopic examinations indicated a solitary esophageal lesion, so the thoracic surgeon misdiagnosed it as primary esophageal adenocarcinoma. Thanks to the MDT discussion, the comparative IHC results supported that the esophageal lesion originated from the rectum. Therefore, in patients presenting with esophageal tumors with a prior history of malignancy, clinical vigilance is warranted. Appropriate IHC or genetic testing is crucial for accurate diagnosis[15,16]. However, it must be noted that while IHC profiles are highly indicative, they do not constitute definitive proof of clonal origin. The most conclusive evidence would be the demonstration of identical somatic mutation patterns via next-generation se
This patient remained disease-free for ten years under regular surveillance, yet developed the esophageal metastasis merely three months after an unremarkable follow-up. This decade-long interval strongly suggests tumor dormancy: A state where disseminated tumor cells (DTCs) or circulating tumor cells/DNA (CTCs/ctDNA) persist in a quiescent state before reactivation[17]. At the time of primary tumor resection, DTCs may have already disseminated via vascular/Lymphatic routes to distant sites (e.g., bone marrow, lung, liver) but remained dormant[17-19]. Mechanistically, tumor dormancy can be sustained by angiogenic suppression, where DTCs remain avascular and unable to expand until neovascularization is triggered, and by immune editing, in which cytotoxic immune surveillance maintains equilibrium by eliminating proliferative clones[17,20]. Studies have shown that non-metastatic CRC can still occur late recurrence and secondary primary cancers within five to ten years after curative treatment[21]. The ten-year latency here far exceeds the typical five-year high-risk window for CRC recurrence, indicating that even after curative resection of early-stage disease, the risk of ultra-late metastasis persists[21]. ctDNA-based minimal residual disease (MRD) assays permit sensitive detection of molecular relapse prior to radiographic or clinical progression[22]. Studies find that ctDNA positivity strongly predicts recurrence and survival in resectable CRC, and serial monitoring increases detection yield[22]. This underscores the potential value of ongoing research into biomarkers like ctDNA for monitoring MRD and predicting ultra-late recurrence[17,22].
Due to the rarity of esophageal metastases, treatment consensus is lacking[13]. Approaches often involve local resection or stenting for palliation[11]. Employing an MDT strategy, we opted for a combined modality approach fo
This case of an extremely late solitary esophageal metastasis exemplifies the unpredictable spatiotemporal dynamics of tumor dormancy and recurrence. Even beyond standard follow-up windows, early-stage tumors still have the possibility of metastasizing to atypical sites, which should draw the attention of clinicians. The development of relevant biomarkers for long-term monitoring of the potential for tumor recurrence and metastasis is valuable. Furthermore, the case demonstrates the critical role of MDT review, IHC, and molecular profiling in diagnosing atypical metastases, and the efficacy of a tailored combined-modality approach integrating radiotherapy and targeted therapy.
The authors thank the patient for his kind consent for the publication of this case report and accompanying images.
| 1. | Mizobuchi S, Tachimori Y, Kato H, Watanabe H, Nakanishi Y, Ochiai A. Metastatic esophageal tumors from distant primary lesions: report of three esophagectomies and study of 1835 autopsy cases. Jpn J Clin Oncol. 1997;27:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Tanaka Y, Chino O, Kajiwara H, Hanashi T, Nakamura T, Makuuchi H. A rare case of esophageal metastasis from signet-ring cell carcinoma of the cecum. Surg Case Rep. 2023;9:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Wu Q, Chen P, Shu C, Chen L, Jin Z, Huang J, Wang X, Li X, Wei M, Yang T, Deng X, Wu A, He Y, Wang Z. Survival outcomes of stage I colorectal cancer: development and validation of the ACEPLY model using two prospective cohorts. BMC Med. 2023;21:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 4. | Turri G, Martinelli L, Rega D, Tamini N, Paiano L, Deidda S, Bao Q, Lorenzon L, De Luca R, Foppa C, Mari V, Taffurelli G, Picciariello A, Marsanic P, Siragusa L, Bagolini F, Nascimbeni R, Rizzo G, Vertaldi S, Zuolo M, Bianchi G, Rorato LM, Reddavid R, Gallo G, Crepaz L, Di Leo A, Trompetto M, Potenza E, Santarelli M, de'Angelis N, Ciarleglio F, Milone M, Coco C, Tiberio GA, Anania G, Sica GS, Muratore A, Altomare DF, Montroni I, De Luca M, Spinelli A, Simone M, Persiani R, Spolverato G, Restivo A, de Manzini N, Braga M, Delrio P, Verlato G, Pedrazzani C. Predictors of Recurrence After Curative Surgery for Stage I Colon Cancer: Retrospective Cohort Analysis of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Ann Surg Open. 2024;5:e510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11915] [Article Influence: 361.1] [Reference Citation Analysis (0)] |
| 6. | Kagaya H, Kitayama J, Hidemura A, Kaisaki S, Ishigami H, Takei J, Kanazawa T, Nagawa H. Metastatic esophageal tumor from cecal carcinoma. Jpn J Clin Oncol. 2007;37:628-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Watanabe S, Takashima A, Taniguchi H, Tanaka Y, Nakamura S, Okita N, Honma Y, Iwasa S, Kato K, Hamaguchi T, Boku N. Esophageal Metastasis from Rectal Cancer Successfully Treated with Fluorouracil-Based Chemotherapy with Bevacizumab: A Case Report and Review of the Literature. Case Rep Oncol. 2017;10:407-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Sönmez GM, Aytekin A, Erten R, Aldemir MN, Sakin A, Esen R. Multiple Primary Synchronous Gastric, Esophageal, and Rectal Cancer and Isolated Esophageal Metastasis from Rectal Cancer: Case Report. J Oncol Sci. 2021;7:159-162. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Thomasset SC, Garcea G, Berry DP. Oesophageal metastasis from colorectal cancer. Case Rep Gastroenterol. 2008;2:40-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Bhurgri H, Samiullah S, Dave V, Lenza C. Hard to swallow: colonic adenocarcinoma in esophagus. Gastrointest Endosc. 2014;79:696-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Vashi PG, Gupta D, Tan B. Colon carcinoma with unusual metastasis to the esophagus manifesting as multiple nodules and Dysphagia: management with systemic chemotherapy. Case Rep Gastroenterol. 2012;6:484-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Lohsiriwat V, Boonnuch W, Suttinont P. Esophageal metastasis from rectal carcinoma. J Clin Gastroenterol. 2005;39:744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Editor EDCS, Bourlon MT, Bolaños LM, Castro-Alonso FJ, Benitez BM. What Are the Most Common Esophageal Metastases. Oncology (Williston Park). 2019;33:687520. [PubMed] |
| 14. | Simchuk EJ, Low DE. Direct esophageal metastasis from a distant primary tumor is a submucosal process: a review of six cases. Dis Esophagus. 2001;14:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Elnady MS, Eltatawy FA, Nosseir AG, Zamzam YA, El-Guindya DM. Diagnostic accuracy of SATB2 in identifying primary and metastatic colorectal carcinoma: a comparative immunohistochemical study. Ecancermedicalscience. 2022;16:1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Brannon AR, Vakiani E, Sylvester BE, Scott SN, McDermott G, Shah RH, Kania K, Viale A, Oschwald DM, Vacic V, Emde AK, Cercek A, Yaeger R, Kemeny NE, Saltz LB, Shia J, D'Angelica MI, Weiser MR, Solit DB, Berger MF. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014;15:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 17. | Liu R, Zhao Y, Su S, Kwabil A, Njoku PC, Yu H, Li X. Unveiling cancer dormancy: Intrinsic mechanisms and extrinsic forces. Cancer Lett. 2024;591:216899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 18. | Janssens JF, T'Syen M, Verhaegen S, Spaepen K, Verbeeck G. Ultra-late recurrences of gastro-intestinal carcinoma after primary resection: the mechanism of dormancy. Acta Gastroenterol Belg. 2013;76:251-254. [PubMed] |
| 19. | Linde N, Fluegen G, Aguirre-Ghiso JA. The Relationship Between Dormant Cancer Cells and Their Microenvironment. Adv Cancer Res. 2016;132:45-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 20. | Collignon E. Unveiling the role of cellular dormancy in cancer progression and recurrence. Curr Opin Oncol. 2024;36:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Nors J, Gotschalck KA, Erichsen R, Andersen CL. Incidence of late recurrence and second primary cancers 5-10 years after non-metastatic colorectal cancer. Int J Cancer. 2024;154:1890-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Nakamura Y, Watanabe J, Akazawa N, Hirata K, Kataoka K, Yokota M, Kato K, Kotaka M, Kagawa Y, Yeh KH, Mishima S, Yukami H, Ando K, Miyo M, Misumi T, Yamazaki K, Ebi H, Okita K, Hamabe A, Sokuoka H, Kobayashi S, Laliotis G, Aushev VN, Sharma S, Jurdi A, Liu MC, Aleshin A, Rabinowitz M, Bando H, Taniguchi H, Takemasa I, Kato T, Kotani D, Mori M, Yoshino T, Oki E. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat Med. 2024;30:3272-3283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 99] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 23. | Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne CH. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 436] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 24. | Murray LJ, Din OS, Kumar VS, Dixon LM, Wadsley JC. Palliative radiotherapy in patients with esophageal carcinoma: A retrospective review. Pract Radiat Oncol. 2012;2:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Rosati LM, Kummerlowe MN, Poling J, Hacker-Prietz A, Narang AK, Shin EJ, Le DT, Fishman EK, Hruban RH, Yang SC, Weiss MJ, Herman JM. A rare case of esophageal metastasis from pancreatic ductal adenocarcinoma: a case report and literature review. Oncotarget. 2017;8:100942-100950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/