Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.108650
Revised: May 19, 2025
Accepted: June 4, 2025
Published online: July 15, 2025
Processing time: 85 Days and 21.4 Hours
Intrahepatic cholangiocarcinoma (ICC) is an aggressive malignancy with limited treatment options and a poor prognosis, particularly in unresectable or metastatic cases. Tri-modal strategies combining systemic chemotherapy, targeted therapies, and immune checkpoint inhibitors have demonstrated synergistic effects in converting unresectable ICC to resectable status and improving patient survival.
A 39-year-old male presented with unresectable stage IIIB ICC (cT3N1M0), abdominal pain, and elevated carbohydrate antigen (CA) 19-9 levels. He received tri-modal therapy consisting of gemcitabine-oxaliplatin hepatic arterial infusion chemotherapy (GEMOX-HAIC), lenvatinib (8 mg daily), and toripalimab (160 mg every three weeks). After five cycles, significant tumor shrinkage and normalization of CA19-9 levels enabled a left hepatectomy. Complications, including biliary stenosis and liver abscesses, were managed with biliary stenting and percutaneous drainage, which allowed for the continuation of chemotherapy. Postoperative pathological examination confirmed a pathological complete response. At the last follow-up, the patient had maintained 29 months of disease-free survival post-resection and was continuing postoperative therapy.
This case highlights the potential of a tri-modal therapy combining GEMOX-HAIC, lenvatinib, and toripalimab to convert unresectable ICC to a resectable status, thereby potentially improving patient survival by surgical resection. Further clinical trials investigating this regimen are warranted.
Core Tip: This report details an unresectable advanced intrahepatic cholangiocarcinoma (ICC) patient treated with first-line GEMOX-HAIC, lenvatinib, and toripalimab. This tri-modal therapy led to successful R0 resection and complete pathological remission. The patient achieved 29 months of disease-free survival. This rare case highlights a regimen enabling surgical resection and long-term survival in advanced ICC and discusses multidisciplinary management of severe biliary complications. However, as a single case, generalizability is limited. The regimen's efficacy and safety warrant validation in prospective, multi-center clinical trials.
- Citation: Xie JP, Tang YJ, Fan YW, Huang YZ, Deng M, Zhang TZ, Li Y, Deng G, Tang D. Pathological complete response in advanced intrahepatic cholangiocarcinoma was achieved through tri-modal therapy: A case report and review of literature. World J Gastrointest Oncol 2025; 17(7): 108650
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/108650.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.108650
Intrahepatic cholangiocarcinoma (ICC) is a malignant tumor originating from biliary epithelial cells, accounting for approximately 15% of primary liver cancers. In recent years, the global incidence of ICC has been on an upward trend[1], with an average annual increase of 7.31% in the United States from 2002 to 2014. The prognosis of advanced ICC remains poor, with a median survival of only 5.3 to 8.1 months for inoperable patients. Although standard first-line chemotherapy regimens (e.g., gemcitabine plus cisplatin (GC)[2] or plus oxaliplatin (GEMOX)[3]) are widely used, their efficacy is limited, typically yielding a median overall survival (OS) of less than one year. Given these challenges, studies have explored multimodal strategies combining systemic therapies with locoregional approaches such as HAIC[4]. Additionally, targeted therapies (e.g., lenvatinib) and immune checkpoint inhibitors (ICIs) (e.g., toripalimab) have shown synergistic effects with chemotherapy in advanced ICC, as recently reported by Shi et al[5]. These findings highlight the potential of integrating HAIC with systemic therapies to achieve a better prognosis. Herein, we present a case of advanced-stage ICC treated with a first-line tri-modal regimen comprising GEMOX-HAIC, lenvatinib, and toripalimab. Notably, the patient achieved significant tumor regression, normalization of serum carbohydrate antigen (CA) 19-9 levels, enabling R0 resection with pathological complete response (pCR). To our knowledge, this is among the few reports demonstrating durable recurrence-free survival (29-month post-surgery) with this combination strategy. A literature review of HAIC-based combination therapies for advanced cholangiocarcinoma is included to highlight their clinical potential for this aggressive malignancy.
A 39-year-old male was admitted to our hospital on April 11, 2022, with a 1-month history of upper abdominal pain and distention. A computed tomography (CT) scan performed 5 days prior to admission revealed a large hepatic tumor.
The patient reported upper abdominal discomfort for one month, without chills, fever, nausea, vomiting, or jaundice. He experienced a 5-kg weight loss over this period. A CT scan at a local hospital revealed a large hepatic tumor in the left lobe, consistent with ICC. The patient’s baseline Eastern Cooperative Oncology Group performance status was 1, indicating minimal functional impairment. His baseline weight was 45 kg.
He had a history of chronic hepatitis B for over 10 years but was noncompliant with regular follow-up and nucleotide analogue therapy.
The patient had no reported past medical history relevant to ICC. No family history of cancer or liver disease was noted.
Physical examination showed a soft abdomen with a firm, tender mass in the left upper quadrant, 5 cm below the xiphoid process.
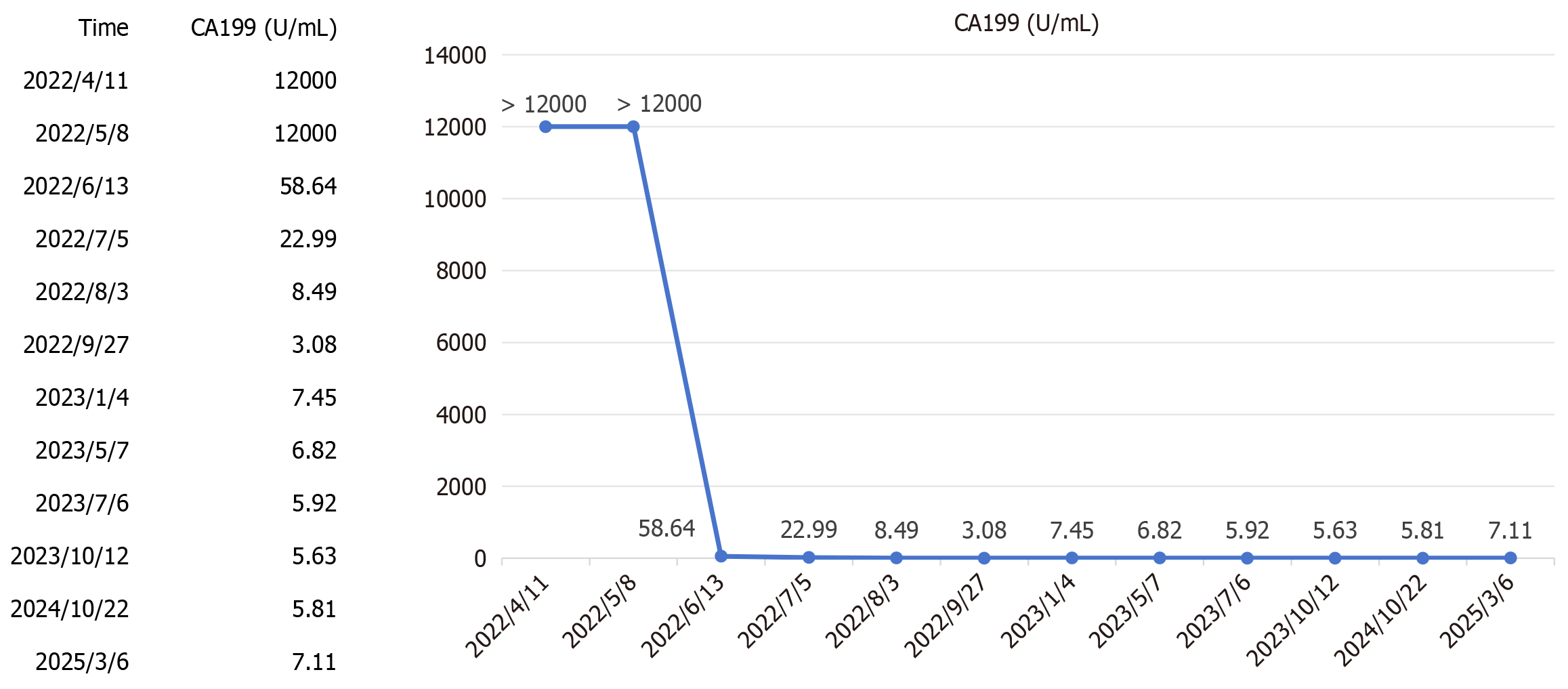
Laboratory tests revealed normal liver function (Child-Pugh Class A, score 5) and a normal complete blood count. Hepatitis B virus (HBV) DNA was undetectable. Tumor markers revealed significantly elevated CA19-9 (> 12000 U/mL), with normal carcinoembryonic antigen and alpha-fetoprotein levels.
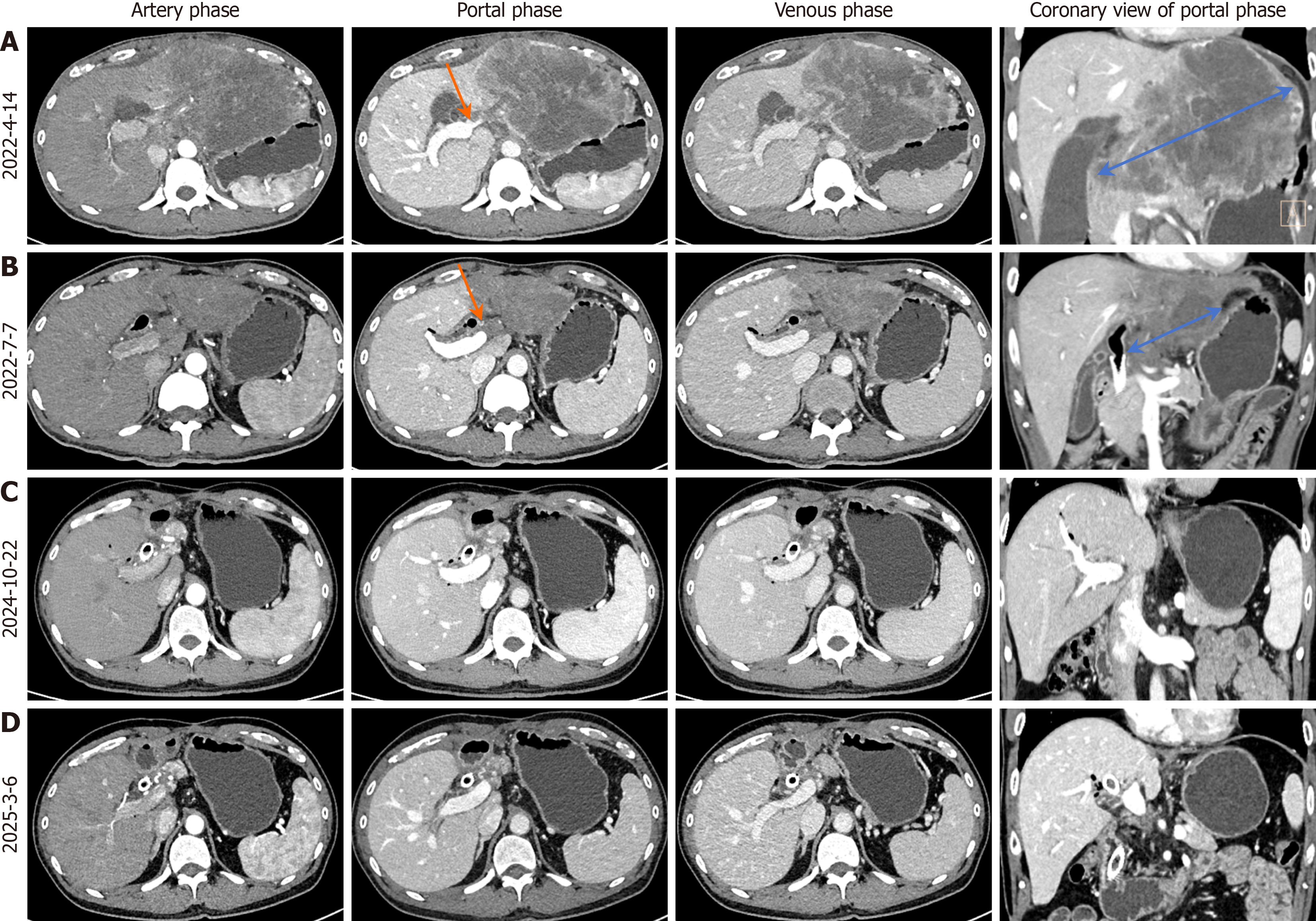
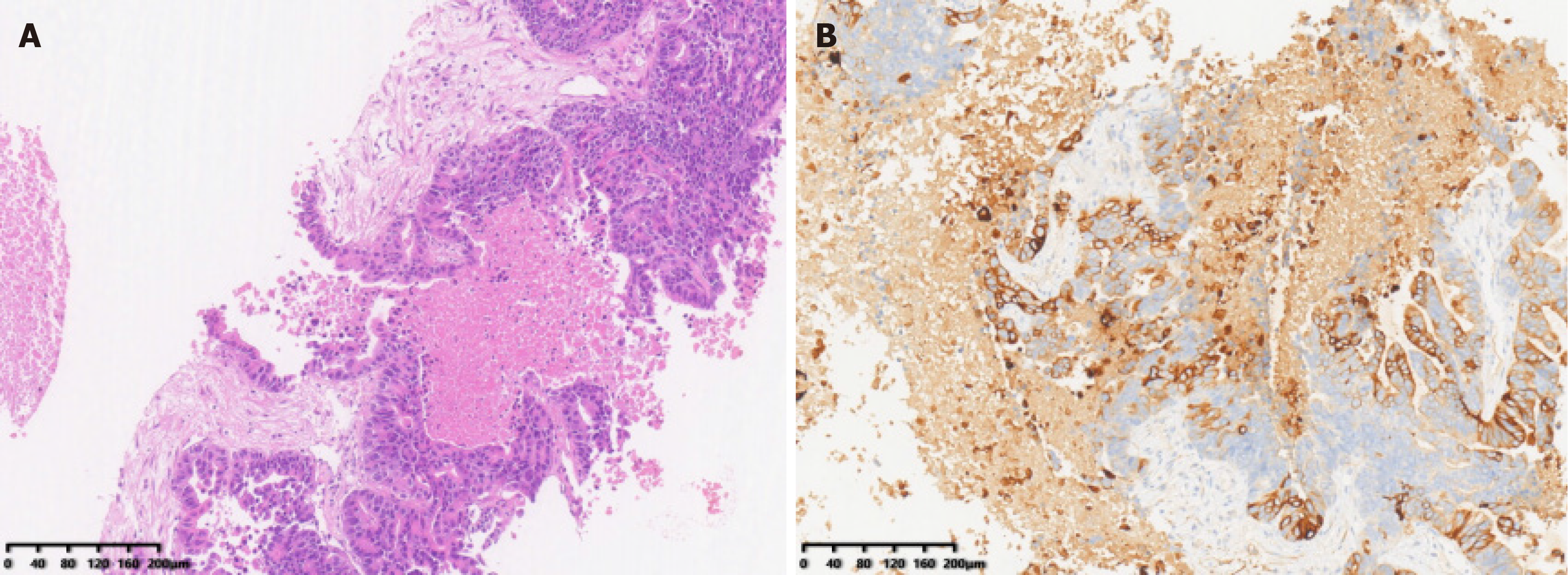
Enhanced CT confirmed a tumor in the left lobe (segment 4), with hepatic hilar lymph node enlargement and a cancerous thrombus in the left portal vein branch (Figure 1A). Ultrasound-guided biopsy confirmed ICC (Figure 2).
After multidisciplinary expert consultation, the patient’s final diagnosis was confirmed, and frontline chemotherapy combining HAIC with targeted and immunotherapy was recommended, enabling R0 surgical resection following conversion therapy.
The patient was diagnosed with stage IIIB (cT3N1M0) ICC, according to the TNM 8th edition.
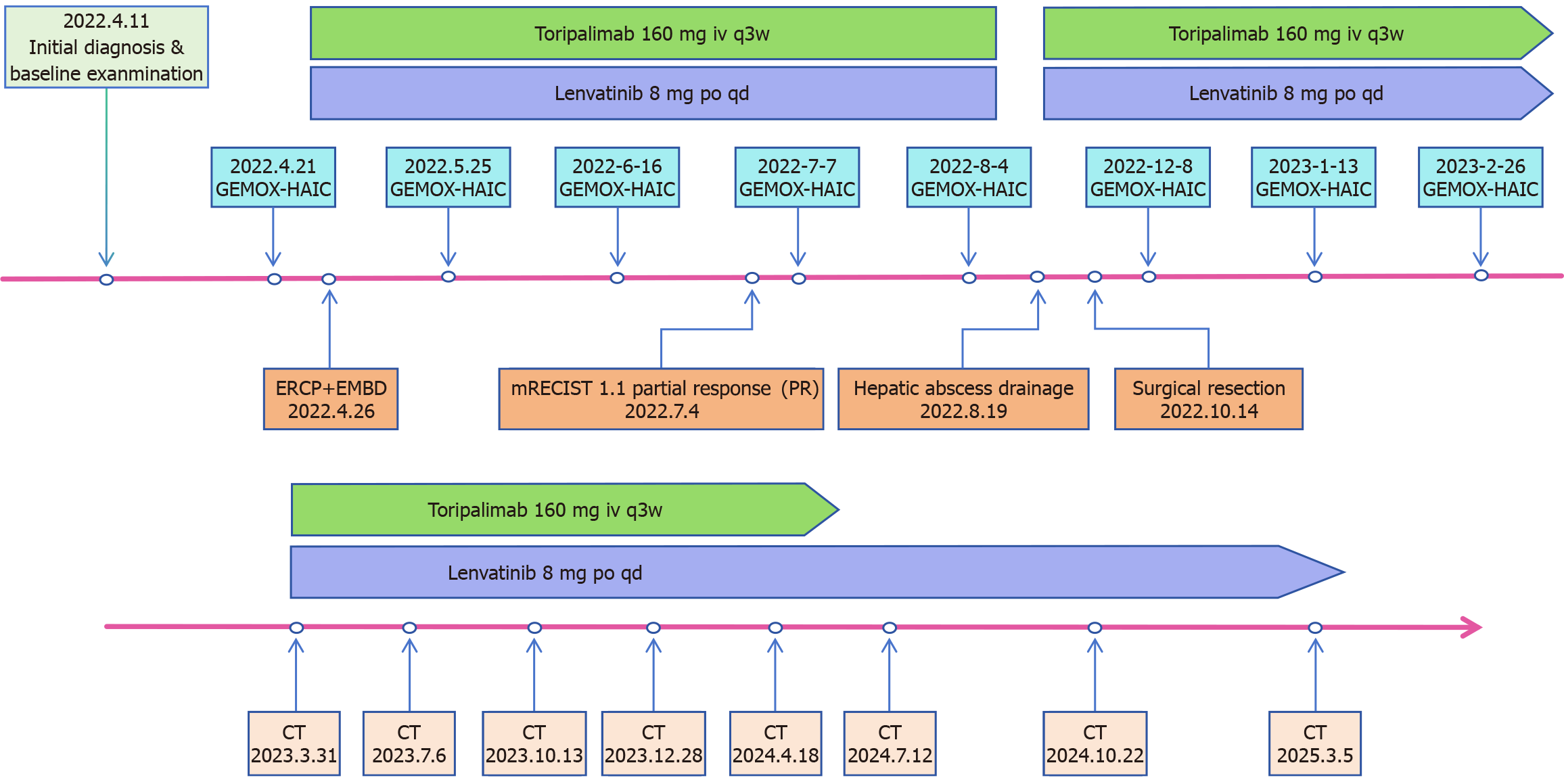
Following a multidisciplinary team (MDT) discussion involving oncologists, surgeons, radiologists, and pathologists, a tri-modal regimen was selected due to the tumor’s advanced stage. The regimen included GEMOX-HAIC, with hepatic arterial infusion of gemcitabine (1.2 g) and oxaliplatin (180 mg) on day 1, followed by intravenous gemcitabine (1.2 g) via a subcutaneous port on day 8, administered every 3 weeks, combined with lenvatinib (8 mg orally daily for a body weight of 45 kg) for targeted therapy and toripalimab (160 mg intravenously every 3 weeks) for immunotherapy. Upon admission, entecavir (0.5 mg daily) was initiated for HBV treatment to prevent reactivation of HBV during chemotherapy. From April 21 to August 4, 2022, the patient completed five cycles. After the third cycle (July 6, 2022), CA19-9 significantly decreased to 22.99 U/mL (Figure 3), and CT showed marked reduction of the hepatic tumor, hilar lymph nodes, and portal vein thrombus (Figure 1B).
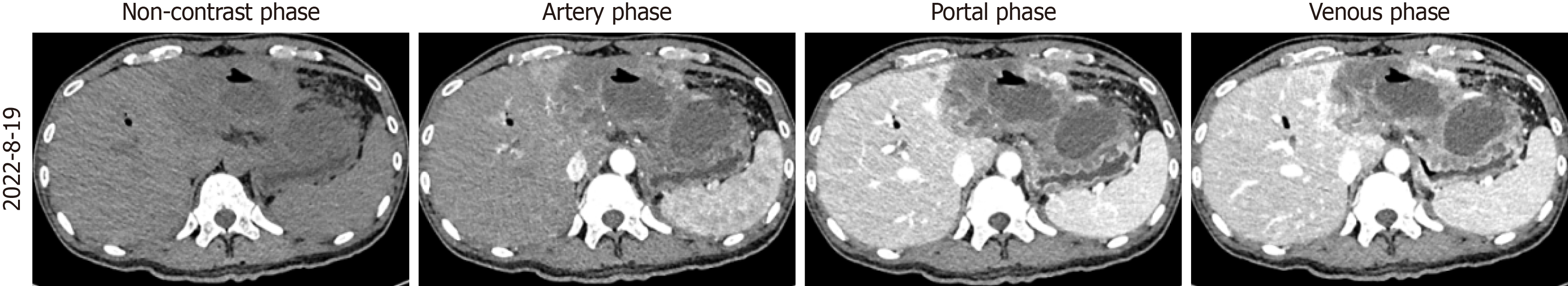
On April 24, 2022, the patient developed obstructive jaundice (total bilirubin: 83.46 μmol/L) secondary to hilar bile duct compression. On April 26, 2022, endoscopic retrograde cholangiopancreatography (ERCP) with biliary metal stent placement was performed, resulting in gradual normalization of bilirubin levels. On August 19, 2022, fever and abdominal pain prompted a CT revealing a liver abscess within the tumor (Figure 4). Blood tests showed a white blood cell count of 18.51 × 109/L, neutrophil percentage of 77.2%. On August 22, ultrasound-guided percutaneous catheter drainage (PCD) and intravenous antibiotics were initiated, leading to clinical recovery. Persistent turbid bile drainage (50-100 mL/day) via the PCD catheter suggested chronic abscess formation. After five cycles, the hepatic tumor decreased in size significantly, and CA19-9 normalized, rendering the tumor resectable. The MDT opted for surgery to address the tumor and chronic abscess.
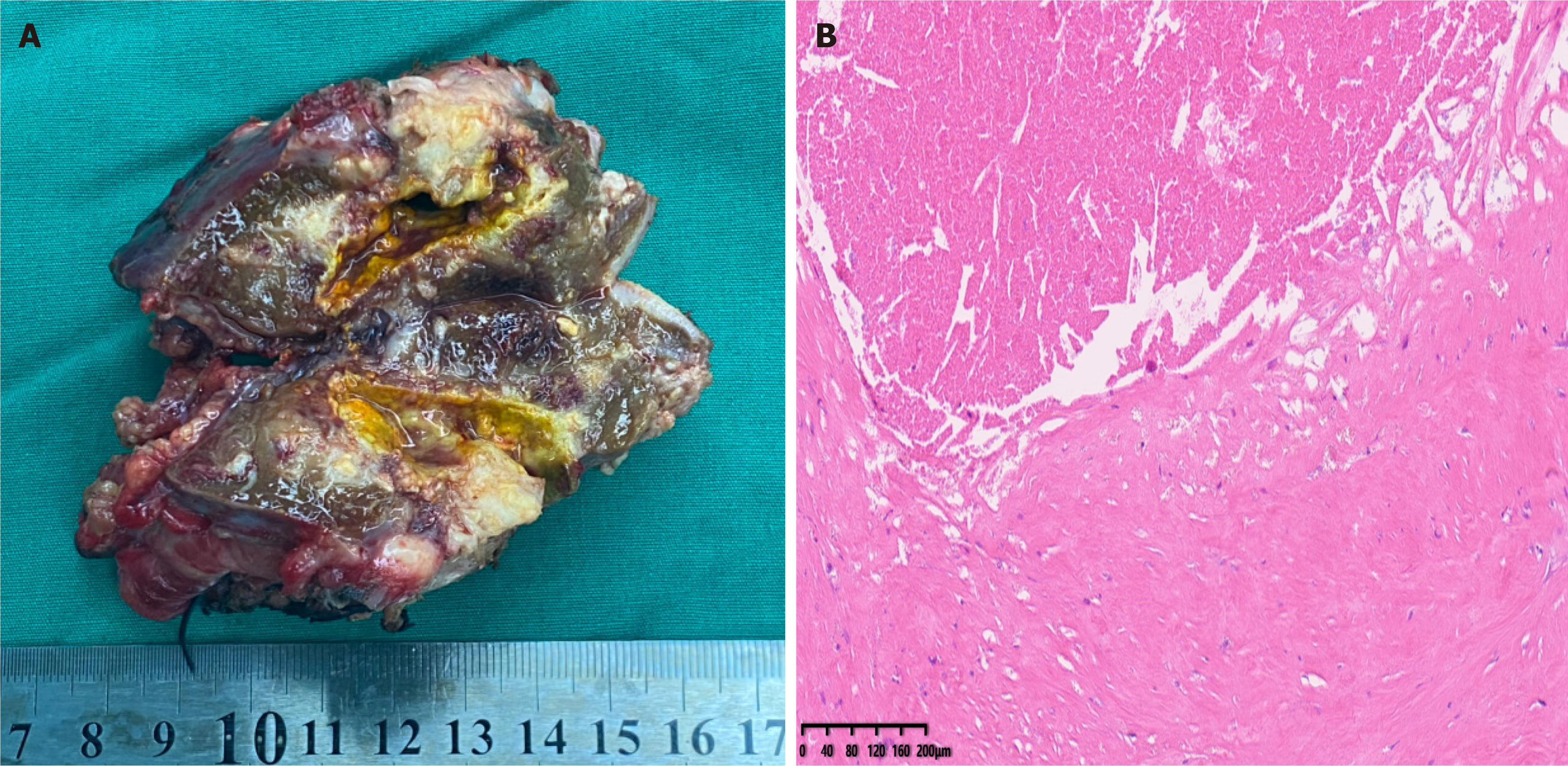
On October 14, 2022, the patient underwent intraoperative ultrasound-guided left hepatectomy (segments II-IV), cholecystectomy, and regional lymph node dissection via a right subcostal incision with midline extension, achieving complete tumor resection. The procedure lasted 5 hours and 20 minutes, with 100 mL blood loss. The resected specimen was illustrated in Figure 5A. Recovery was without complications. Histopathological examination of the resected hepatic tumor revealed extensive necrosis, focal calcification, and fibrous tissue with hyaline degeneration. No viable tumor was observed in the resection margin or within the tumor after sampling of multiple tissue sections, confirming a pCR (Figure 5B). These findings are consistent with an R0 resection.
The patient completed three cycles of GEMOX-HAIC post-surgery. Toripalimab (160 mg every 3 weeks) continued until May 2024, and lenvatinib (8 mg daily) remains ongoing. Regular follow-up included CA19-9 monitoring and quarterly US/CT/MRI. The treatment timeline is shown in Figure 6. Imaging in November 2024 (Figure 1C) and March 2025 (Figure 1D) showed no recurrence, and CA19-9 remained normal, consistent with a complete response (CR).
Radical surgical resection is the preferred treatment for ICC; however, only a small proportion of early or intermittent stage patients are eligible for this procedure[6]. For those with unresectable advanced ICC, systemic therapy is currently the mainstay of treatment. In the ABC-002 study[2], a median OS of 11.7 months was achieved in the GC treatment group compared to 8.1 months in the control group. In recent years, targeted therapy and immunotherapy have significantly advanced the treatment of ICC. Targeted drugs aimed at specific molecular alterations, such as FGFR2 fusion[7,8], IDH1 mutation[9,10], and HER2-positive[11], have exhibited promising clinical efficacy. Nevertheless, these mutations are only present in approximately 10%-15% of patients[8,10,11]. Besides, activation of the tyrosine kinase inhibitor (TKI) signaling pathway and overexpression of vascular endothelial growth factor (VEGF) are common in ICC. As a multi-targeted TKI, lenvatinib has demonstrated potential therapeutic value in the treatment of multiple malignant neoplasms, including ICC[12]. Lenvatinib exerts a potent inhibitory effect on VEGFR1-3 as well as PDGFRα, effectively reducing the tumor’s blood supply and impeding tumor growth and metastasis[13]. Moreover, it also interferes with the downstream signaling within tumor cells by acting on multiple targets, such as FGFR[14], EGFR, RET[15], and other, thereby triggering the apoptosis of tumor cells.
Cancer cells tend to utilize immune checkpoint signaling pathways to elude immune surveillance[16]. ICIs, on the other hand, bring about their anti-tumor impacts by blocking CTLA-4, PD-1, and PD-L1. In recent years, ICIs, such as PD-1/PD-L1 inhibitors, have also shown remarkable efficacy when combined with standard chemotherapy in advanced ICC patients. The TOPAZ-1[17] study randomized 685 patients with advanced biliary tract cancer (BTC, which includes ICC, extrahepatic cholangiocarcinoma (EHC), and gallbladder cancer (GC)) 1:1 to receive durvalumab, plus GC chemotherapy (experimental group) or placebo, plus GC chemotherapy (control group). After a median follow-up of 41.3 months, the results demonstrated a median OS of 12.9 months in the durvalumab arm vs 11.3 months in the control arm (HR 0.74, 95%CI: 0.64-0.86; P = 0.021). This established durvalumab as the first PD-L1 inhibitor to achieve a statistically significant survival benefit in a Phase III trial for advanced BTC. The KEYNOTE-966 Phase III trial validated the TOPAZ-1 findings, confirming that adding pembrolizumab to GC chemotherapy improves survival in advanced BTC. The pembrolizumab combination group achieved a median OS of 12.7 months (95%CI: 11.5-13.6) compared to 10.9 months (95%CI: 9.9-11.6) in the control group (HR = 0.83, 95%CI: 0.72-0.95; one-sided P = 0.0034)[18]. However, these studies included a heterogeneous BTC population. Mody et al[19] highlighted that different BTC subtypes may bear distinct genomic profiles and tumor-immune microenvironments. For example, ICC may have more frequent FGFR2 fusions (14.2%), IDH1 mutations (18.3%), and PBRM1 alterations (16.4%) than GB or EHC, while GB has higher PD-L1 gene expression values (median 0.997) than IHC (median 0.875). This heterogeneity highlights new opportunities for therapeutic development for specific subtypes of BTC.
Moreover, the Chinese Expert Consensus on the Management of ICC (2022 Edition)[20] highlights that for patients in good physical condition, a multimodal regimen combining chemotherapy with sequential immunotherapy and targeted therapy demonstrates relatively high efficacy and conversion rates. For instance, the triple regimen consisting of toripalimab, lenvatinib, and GEMOX is conditionally recommended (Evidence level: 3; Recommendation Strength: Moderate). This recommendation is based on Zhou’s results of the NCT03951597 study[5], which investigated the efficacy of this triple regimen on 30 patients with locally advanced and metastatic ICC. A total of 23 patients achieved partial remission, and one patient achieved CR. The median OS and progression-free survival (PFS) were 22.5 and 10.2 months, respectively, with manageable side effects. In another multicenter retrospective real-world study for advanced cholangiocarcinoma led by Zhu et al[21], this similar regimen (GEMOX, lenvatinib, PD-1) served as an effective and well-tolerated first-line therapy. The median OS was 14.3 months. Notably, only 1.9% (1/53) had grade 4 adverse events (AEs), and no grade 5 AEs were reported. Moreover, 6 out of 53 patients underwent conversion surgery, with 4 achieving a partial response and 2 a CR prior to the operation.
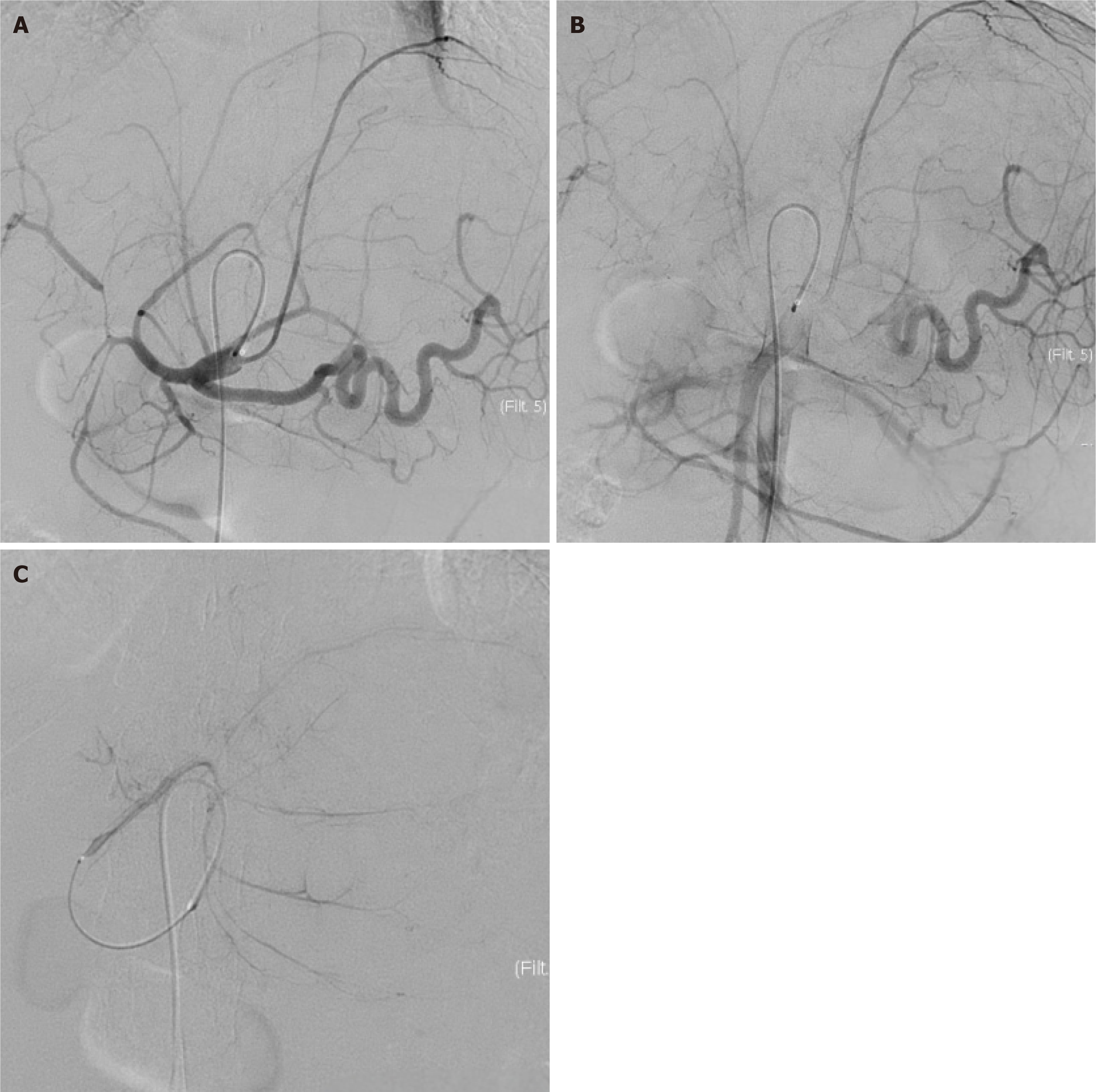
HAIC represents an interventional approach that maximizes locoregional drug exposure by delivering chemotherapeutic agents directly into tumor-feeding arteries. Compared to systemic administration, 5-FU administered via HAIC achieves an estimated 400-fold increase in tumor exposure while mitigating systemic toxicity[22]. Drugs are widely distributed throughout the liver, effectively eliminating small metastases. These pharmacokinetic advantages are particularly relevant for hepatic malignancies. For advanced-stage hepatocellular carcinoma, FOLFOX-HAIC shows advantages when combined with TKIs and immunotherapy, offering longer OS, PFS, and resection rates[23]. Unlike hepatocellular carcinoma, ICC is typically not hypervascular. Nonetheless, as shown in this patient’s pre-chemotherapy hepatic arteriography (Figure 7) and other reports[24], its main blood supply remains the hepatic artery. Emerging ICC-specific data support HAIC’s potential in multimodal frameworks, generally achieving superior objective response rates (ORR; 35.5%-55%) and OS (15.8-20.8 months) compared to systemic chemotherapy alone (ORR 14.5%-21.2%, mOS 3.7-17.8 months)[25-29]. However, this conclusion apparently requires validation through larger-scale prospective randomized controlled trials in the future, due to variations in methodology and the inherent limitations of small-sample retrospective studies. Besides, not all HAIC-based regimens have shown superior efficacy, especially as second-line therapy. For instance, Huang et al[30] reported a modest ORR of 22.2% with FOLFIRI-HAIC in a real-world study of nine ICC patients, with a median OS of 8 months. These findings highlight the variability in HAIC outcomes and the need for careful patient selection. Table 1[24-28,30-42] summarizes the study design and outcomes of HAIC-containing regimens for cholangiocarcinoma in recent years. FOLFOX-HAIC is the most frequently used regimen, while GEMOX-HAIC is also promising. The reported treatment-related AEs (TrAEs) of HAIC-containing regimens are manageable, with grade 3-4 AEs primarily including hepatic toxicity, myelosuppression, and abdominal pain, affecting only a small proportion of patients. In one retrospective study, Ghiringhelli et al[4] reported GEMOX-HAIC as a second-line treatment for 12 cases of unresectable locally advanced ICC, achieving a 91% disease control rate and a median OS of 20.3 months, enabling curative-intent treatments (R0 surgery in 2 patients and stereotactic radiotherapy in 3) with manageable toxicity in patients refractory to prior systemic GEMOX. In a prospective trial of 38 patients with unresectable ICC treated with 5-FU-HAIC combined with systemic GEMOX, an ORR of 58% was achieved, and 10.5% (4/38) underwent conversion surgery[32]. Notably, Lin et al[31] observed enhanced HAIC efficacy in solitary large ICC lesions (> 5 cm), with preoperative CT angiography facilitating precise catheterization of dominant tumor-feeding arteries. This anatomical selectivity may explain the higher conversion rates observed in such cases. Our case report presents a large, advanced-stage ICC treated with GEMOX-HAIC combined with lenvatinib and toripalimab. Following significant tumor regression and tumor marker normalization, the patient underwent tumor resection with histopathological confirmation of complete remission. At the 29-month follow-up, sustained disease-free survival was maintained (Figure 6). This case is among the few reports demonstrating durable recurrence-free survival (29-month post-surgery) with this combination strategy, highlighting its clinical potential for late-stage cholangiocarcinoma.
| Ref. | Year | Study design | Sample size | Patients | Arms | mOS (m) | ORR (%) | DCR (%) | Conversion rate | TrAEs (Grade 3-4) |
| Zheng et al[25] | 2024 | Retrospective | 202 | ICC | Arm 1: FOLFOX-HAIC +TKI+ PD(L)-1; Arm 2: GC /GEMOX | 20.8 vs 14.8 | 35.5 vs 14.5 | 77.6 vs 63.2 | NA | Elevated AST 145% vs 7.9%; Thrombocytopenia 118% vs 15.8%; Neutropenia 145% vs 14.5%; Abdominal pain 7.9% vs 1.3% |
| Lin et al[31] | 2024 | Retrospective | 141 | ICC | Arm 1: FOLFOX-HAIC+LEN+PD(L)-1; Arm 2: GEMOX/GC +PD(L)-1; Arm 3: GEMOX/GC | NR vs NR vs 21.8 | 50 vs 18.4 vs 6.0 | 88.1 vs 73.5 vs 52.0 | 9.5% (4/42) vs 2.0% (1/49) vs 0.0% (0/50) | Elevated ALT 48% vs 2.0% vs 4.0%; Elevated AST 48% vs 2.0% vs 2.0%; Neutropenia 24% vs 8.2% vs 10.0%; Vomit 0% vs 12.2% vs 14.0% |
| Li et al[26] | 2024 | Retrospective | 179 | ICC | Arm 1: FOLFOX -HAIC + SYS; Arm 2: SYS | 15.8 vs 12.7 | 55 vs 27 | 84 vs 83 | NA | Increased ALT or AST levels; 7.2%; thrombocytopenia 48% |
| Lin et al[27] | 2024 | Retrospective | 90 | ICC | Arm 1: GEMOX; HAIC + LEN + PD-1; Arm 2: GC | 16.8 vs 11.0 | 43.1 vs 20.5 | 90.2 vs 69.2 | NA | Stomach ache 7.8% vs 2.6%; GI bleeding 5.9% vs 2.6%; Hyperbilirubinemia 59% vs 0%; Leukopenia 20% vs 10.3% |
| Zhao et al[32] | 2024 | Retrospective | 59 | ICC | Arm 1: FOLFOX-HAIC + LEN + Durvalumab; Arm 2: GC + Durvalumab | 15.8 vs 9.6 | 76.9.% vs 17.4% (mRECIST) | 92.3% vs 56.5% (mRECIST) | NA | Elevated ALT 129% vs 3.6%; Anemia 65% vs 3.6%; Thrombocytopenia 97% vs 7.1%; Abdominal pain 3.2% vs 10.7% |
| Huang et al[33] | 2024 | Retrospective | 46 | ICC | FOLFOX-HAIC + LEN + PD-1 | 16.77 | 47.8 | 91.3 | 6.5% (3/46) | Neutropenia 217%; Thrombocytopenia 174%; Elevated ALT 130%; Elevated AST 87% |
| Li et al[34] | 2024 | Retrospective | 34 | Hilar CCA | mFOLFOX7-HAIC+ Capecitabine + Camrelizumab | 20.0 | 61.8 | 97.0 | NA | Fatigue 59%; Rash 2.9% |
| Song et al[35] | 2024 | Retrospective | 28 | ICC | mFOLFOX6-HAIC + Toripalimab + Surufatinib | NR | 58 | 79 | NA | Abdominal pain 7.2%; Leukopenia 72% Thrombocytopenia 7.2% |
| Zhao et al[36] | 2024 | Retrospective | 28 | ICC | FOLFOX-HAIC + LEN + Durvalumab | 17.9 | 65.2 (mRECIST) | 91.31 (mRECIST) | 10.7% (3/28) | Elevated ALT 143; Thrombocytopenia 107%; Anemia 71%; Abdominal pain 7.1% |
| Ni et al[37] | 2024 | Retrospective | 21 | ICC | GEMOX-HAIC + GEM + LEN + PD-1 | 19.5 | 52.3 | 76.1 | 19% (4/21) | Hypertension 28.6%; Hand-foot syndrome 19%; Myelosuppression 14.3% |
| Yang et al[28] | 2023 | Retrospective | 146 | ICC | Arm 1: mFOLFOX-HAIC; Arm 2: GC or GEMOX | 18.0 vs 17.8 | 45.3 vs 21.1 (mRECIST) | 88 vs 88.7 (mRECIST) | NA | Abdominal pain 4% vs 0%; Vomit 1.3% vs 11.2%; Neutropenia 13% vs 5.6%; Elevated AST 27% vs 2.8% |
| Wei et al[38] | 2023 | Retrospective | 55 | CCA | Arm 1: FOLFOX-HAIC+LEN+PD-1; Arm 2: FOLFOX-HAIC+LEN | 16.0 vs 11.0 | 28.6 vs 20.0 | 80 vs 65 | HAIC+LEN+PD-1 arm: 17.1% (6/35) vs HAIC+LEN arm: 10% (2/20) | Hypertension 42.9% vs 45%; Immune-mediated pneumonia 86% vs 0%; Hand-foot syndrome 86% vs 20%; GI bleeding 5.7 vs 5% |
| Wang et al[39] | 2023 | Retrospective | 41 | CCA | Arm 1: FOLFOX-HAIC + LEN; Arm 2: FOLFOX-HAIC | 32 vs 10 | 15.8 vs 13.6 | 84.2 vs 63.6 | HAIC + LEN arm 21.0% (4/19) vs HAIC arm 13.6% (3/22) | Hypertension Grade 157% vs 0%; Fever 10.5% vs 0%; Thrombocytopenia 105% vs 4.5%; Leukocytopenia 105% vs 4.5% |
| Zhang et al[40] | 2022 | Retrospective | 58 | ICC | Arm 1: GEMOX-HAIC + TKI + PD-1; Arm 2: TACE + TKI + PD-1 | NR | 61.5 vs 21.1 (mRECIST) | 82.1 vs 36.8 (mRECIST) | 15.4% (6/39) vs 0% (0/19) | Elevated AST 154%; Nausea/vomiting 12.8%; Hyperbilirubinemia 128%; Neutropenia 103% |
| Ishii et al[24] | 2022 | Retrospective | 42 | ICC | Arm 1: GEM-FP-HAIC (no prior chemo); Arm 2: GEM-FP-HAIC (post-GC); Arm 3: SC (GC-refractory) | 20.6 vs 6.3 vs 3.7 | 37.5 vs 0 vs NA | 75 vs 80 vs NA | NA | Anemia 333%; Thrombocytopenia 222%; leukopenia 56%; Hyperbilirubinemia 56% |
| Huang et al[30] | 2022 | Real-World Study | 9 | ICC | FOLFIRI-HAIC | 8 | 22.2 | 55.5 | NA | Ascites 22.2%; Hyperbilirubinemia 222%; Elevated ALT or AST 222%; Vomit 11.1% |
| Cai et al[41] | 2021 | Retrospective | 126 | ICC | Arm 1: mFOLFOX -HAIC; Arm 2: TACE | 19.6 vs 10.8 | NA | NA | NA | Myelosuppression 24.6%; Vomit: 10.5% |
| Kasai et al[42] | 2014 | Prospective | 20 | ICC | 5-FU-HAIC+ PEG-IFNα-2b | 14.6 | 60.0 | 90.0 | 5% (1/20) | Leukopenia 10%; Thrombocytopenia 10%; Anemia 10% |
Unlike hepatocellular carcinoma, cholangiocarcinoma is an aggressive malignancy that readily metastasizes to the hepatoduodenal ligament and retroperitoneal lymph nodes. The tumor frequently invades or compresses the hilar bile duct and its branches, often leading to biliary stenosis, cholangitis, and pyogenic liver abscesses. These sequelae not only compromise patients' functional status but may also hinder the continuation of anti-tumor therapies. In this case, the patient developed hilar biliary stenosis due to tumor invasion. The symptoms were alleviated through ERCP-guided metal stent placement. Subsequently, the patient developed cholangitis and a pyogenic abscess in the necrotic, previous cancerous hepatic segments, treated with abscess drainage and antibiotic therapy. Definitive management was ultimately achieved by the surgical resection of the lesion. This clinical trajectory underscores the importance of multidisciplinary coordination in ICC management. The joint efforts of surgeons, oncologists, endoscopists, radiologists, and pathologists are crucial to develop an optimal treatment plan, manage refractory biliary complications and other TrAEs, and improve the patient's survival and quality of life.
This case report highlights the potential of a tri-modal regimen combining GEMOX-HAIC, lenvatinib, toripalimab, and surgical resection in achieving a pCR and 29-month relapse-free survival in a patient with initially unresectable ICC. It also underscores the role of multidisciplinary management in addressing biliary complications during treatment, offering insights for managing this challenging malignancy. However, the findings of a single case may reflect individual variability, and their generalizability to a broader ICC population remains limited. Further validation through prospective, multi-center, randomized controlled clinical trials might be warranted to assess the efficacy and safety of this therapeutic approach.
| 1. | Ilyas SI, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1070] [Cited by in RCA: 1010] [Article Influence: 77.7] [Reference Citation Analysis (6)] |
| 2. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3317] [Article Influence: 207.3] [Reference Citation Analysis (15)] |
| 3. | Fiteni F, Nguyen T, Vernerey D, Paillard MJ, Kim S, Demarchi M, Fein F, Borg C, Bonnetain F, Pivot X. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med. 2014;3:1502-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Ghiringhelli F, Lorgis V, Vincent J, Ladoire S, Guiu B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: preliminary experience. Chemotherapy. 2013;59:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, Chen Y, Yang GH, Lu JC, Meng XL, Wang XY, Sun L, Ge NL, Huang XW, Qiu SJ, Yang XR, Gao Q, He YF, Xu Y, Sun J, Ren ZG, Fan J, Zhou J. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther. 2023;8:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 6. | Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, Youssef BA M, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507-17; discussion 517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 981] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 7. | Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S, Macarulla T, El-Khoueiry A, Kelley RK, Borbath I, Choo SP, Oh DY, Philip PA, Chen LT, Reungwetwattana T, Van Cutsem E, Yeh KH, Ciombor K, Finn RS, Patel A, Sen S, Porter D, Isaacs R, Zhu AX, Abou-Alfa GK, Bekaii-Saab T. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 2018;36:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 535] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 8. | Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Ji T, Lihou CF, Zhen H, Féliz L, Vogel A. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 1173] [Article Influence: 195.5] [Reference Citation Analysis (0)] |
| 9. | Lowery MA, Burris HA 3rd, Janku F, Shroff RT, Cleary JM, Azad NS, Goyal L, Maher EA, Gore L, Hollebecque A, Beeram M, Trent JC, Jiang L, Fan B, Aguado-Fraile E, Choe S, Wu B, Gliser C, Agresta SV, Pandya SS, Zhu AX, Abou-Alfa GK. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 10. | Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 792] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 11. | Lee CK, Chon HJ, Cheon J, Lee MA, Im HS, Jang JS, Kim MH, Park S, Kang B, Hong M, Kim JW, Park HS, Kang MJ, Park YN, Choi HJ. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol Hepatol. 2023;8:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Yang X, Wang D, Yang X, Wang Y, Long J, Zhou J, Lu Z, Mao Y, Sang X, Guan M, Zhao H. Lenvatinib Beyond First-Line Therapy in Patients With Advanced Biliary Tract Carcinoma. Front Oncol. 2022;12:785535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A, Hoshi SS, Mimura F, Haneda T, Fukuda Y, Kamata JI, Takahashi K, Matsukura M, Wakabayashi T, Asada M, Nomoto KI, Watanabe T, Dezso Z, Yoshimatsu K, Funahashi Y, Tsuruoka A. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 391] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 14. | Ogasawara S, Mihara Y, Kondo R, Kusano H, Akiba J, Yano H. Antiproliferative Effect of Lenvatinib on Human Liver Cancer Cell Lines In Vitro and In Vivo. Anticancer Res. 2019;39:5973-5982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Liu YC, Huang BH, Chung JG, Liu WL, Hsu FT, Lin SS. Lenvatinib Inhibits AKT/NF-κB Signaling and Induces Apoptosis Through Extrinsic/Intrinsic Pathways in Non-small Cell Lung Cancer. Anticancer Res. 2021;41:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. 2018;96:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 17. | Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Ah Lee M, Kitano M, Burris H, Bouattour M, Tanasanvimon S, McNamara MG, Zaucha R, Avallone A, Tan B, Cundom J, Lee CK, Takahashi H, Ikeda M, Chen JS, Wang J, Makowsky M, Rokutanda N, He P, Kurland JF, Cohen G, Valle JW. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022;1:EVIDoa2200015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 682] [Article Influence: 170.5] [Reference Citation Analysis (1)] |
| 18. | Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 648] [Article Influence: 216.0] [Reference Citation Analysis (0)] |
| 19. | Mody K, Jain P, El-Refai SM, Azad NS, Zabransky DJ, Baretti M, Shroff RT, Kelley RK, El-Khouiery AB, Hockenberry AJ, Lau D, Lesinski GB, Yarchoan M. Clinical, Genomic, and Transcriptomic Data Profiling of Biliary Tract Cancer Reveals Subtype-Specific Immune Signatures. JCO Precis Oncol. 2022;6:e2100510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Chinese Society of Liver Cancer Cholangiocarcinoma Cooperative Group. [Chinese expert consensus on management of intrahepatic cholangiocarcinoma (2022 edition)]. Zhonghua Xiaohua Waike Zazhi. 2022;21:1269-1301. [DOI] [Full Text] |
| 21. | Zhu C, Li H, Yang X, Wang S, Wang Y, Zhang N, Wang Y, Xue J, Zhang L, Ning C, Yang X, Xun Z, Chao J, Long J, Sang X, Zhu Z, Zhao H. Efficacy, safety, and prognostic factors of PD-1 inhibitors combined with lenvatinib and Gemox chemotherapy as first-line treatment in advanced intrahepatic cholangiocarcinoma: a multicenter real-world study. Cancer Immunol Immunother. 2023;72:2949-2960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Kingham TP, D'Angelica M, Kemeny NE. Role of intra-arterial hepatic chemotherapy in the treatment of colorectal cancer metastases. J Surg Oncol. 2010;102:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Si T, Huang Z, Khorsandi SE, Ma Y, Heaton N. Hepatic arterial infusion chemotherapy versus transarterial chemoembolization for unresectable hepatocellular carcinoma: A systematic review with meta-analysis. Front Bioeng Biotechnol. 2022;10:1010824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 24. | Ishii M, Itano O, Morinaga J, Shirakawa H, Itano S. Potential efficacy of hepatic arterial infusion chemotherapy using gemcitabine, cisplatin, and 5-fluorouracil for intrahepatic cholangiocarcinoma. PLoS One. 2022;17:e0266707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 25. | Zheng Z, Wang J, Wu T, He M, Pan Y, Wang J, Chen J, Hu D, Xu L, Zhang Y, Chen M, Zhou Z. Hepatic arterial infusion chemotherapy plus targeted therapy and immunotherapy versus systemic chemotherapy for advanced intrahepatic cholangiocarcinoma: a retrospective cohort study. Int J Surg. 2025;111:1552-1557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Li Z, Xu R, Chang X, Sun P. Systemic Chemotherapy with or without Hepatic Arterial Infusion Chemotherapy for Intrahepatic Cholangiocarcinoma with Extrahepatic Oligometastasis: A Propensity Score-Matched Analysis. J Vasc Interv Radiol. 2024;35:416-427.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Lin Z, Zou X, Hu X, Huang D, Chen Y, Lin J, Li X, Zhang J. Efficacy analysis of HAIC combined with lenvatinib plus PD1 inhibitor vs. first-line systemic chemotherapy for advanced intrahepatic cholangiocarcinoma. Sci Rep. 2024;14:23961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Yang Z, Fu Y, Wu W, Hu Z, Pan Y, Wang J, Chen J, Hu D, Zhou Z, Chen M, Zhang Y. Comparison of hepatic arterial infusion chemotherapy with mFOLFOX vs. first-line systemic chemotherapy in patients with unresectable intrahepatic cholangiocarcinoma. Front Pharmacol. 2023;14:1234342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 29. | Cercek A, Boerner T, Tan BR, Chou JF, Gönen M, Boucher TM, Hauser HF, Do RKG, Lowery MA, Harding JJ, Varghese AM, Reidy-Lagunes D, Saltz L, Schultz N, Kingham TP, D'Angelica MI, DeMatteo RP, Drebin JA, Allen PJ, Balachandran VP, Lim KH, Sanchez-Vega F, Vachharajani N, Majella Doyle MB, Fields RC, Hawkins WG, Strasberg SM, Chapman WC, Diaz LA Jr, Kemeny NE, Jarnagin WR. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination With Systemic Gemcitabine and Oxaliplatin in Patients With Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020;6:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | Huang P, Huang X, Zhou Y, Yang G, Sun Q, Shi G, Chen Y. The Efficacy and Safety of Hepatic Arterial Infusion Chemotherapy Based on FOLFIRI for Advanced Intrahepatic Cholangiocarcinoma as Second-Line and Successive Treatment: A Real-World Study. Can J Gastroenterol Hepatol. 2022;2022:9680933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Lin YS, Li S, Yang X, Guo RP, Huang YH, Bai KH, Weng J, Yun JP. First-line hepatic arterial infusion chemotherapy plus lenvatinib and PD-(L)1 inhibitors versus systemic chemotherapy alone or with PD-(L)1 inhibitors in unresectable intrahepatic cholangiocarcinoma. J Cancer Res Clin Oncol. 2024;150:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 32. | Zhao R, Zhou J, Xiong X, Wang Q, Liu C, Wei W, Li S, Guo R. Hepatic arterial infusion chemotherapy in combination with lenvatinib and durvalumab versus standard first-line treatment gemcitabine and cisplatin plus durvalumab in advanced intrahepatic cholangiocarcinoma. Am J Cancer Res. 2024;14:4922-4934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Huang Y, Du Z, Kan A, He M, Li H, Lai Z, Wen D, Huang L, Li Q, Xu L, Shi M. Clinical and biomarker analyses of hepatic arterial infusion chemotherapy plus lenvatinib and PD-1 inhibitor for patients with advanced intrahepatic cholangiocarcinoma. Front Immunol. 2024;15:1260191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 34. | Li L, Liu S, Wang Q, Wang Y, Yu G. Hepatic artery infusion chemotherapy with systemic capecitabine and camrelizumab for treating unresectable hilar cholangiocarcinoma: An initial investigation of efficacy and safety. J Cancer Res Ther. 2024;20:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Song S, Liu Y, Ren Y, Zheng C, Liang B. Hepatic arterial infusion chemotherapy combined with toripalimab and surufatinib for the treatment of advanced intrahepatic cholangiocarcinoma. Diagn Interv Radiol. 2025;31:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Zhao R, Zhou J, Miao Z, Xiong X, Wei W, Li S, Guo R. Efficacy and safety of lenvatinib plus durvalumab combined with hepatic arterial infusion chemotherapy for unresectable intrahepatic cholangiocarcinoma. Front Immunol. 2024;15:1397827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Ni JY, Sun HL, Guo GF, Zhou X, Wei JX, Xu LF. Hepatic arterial infusion of GEMOX plus systemic gemcitabine chemotherapy combined with lenvatinib and PD-1 inhibitor in large unresectable intrahepatic cholangiocarcinoma. Int Immunopharmacol. 2024;140:112872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 38. | Wei Z, Wang Y, Wu B, Liu Y, Wang Y, Ren Z, Yang X, Chen Q, Zhang Y. Hepatic arterial infusion chemotherapy plus lenvatinib with or without programmed cell death protein-1 inhibitors for advanced cholangiocarcinoma. Front Immunol. 2023;14:1235724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 39. | Wang Y, Wei Z, Zhang Z, Xu J, Wang Y, Chen Q, Zhang Y. Hepatic arterial infusion chemotherapy with or without lenvatinib for unresectable cholangiocarcinoma: a single-center retrospective study. Hepat Oncol. 2023;10:HEP49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 40. | Zhang N, Yu BR, Wang YX, Zhao YM, Zhou JM, Wang M, Wang LR, Lin ZH, Zhang T, Wang L. Clinical outcomes of hepatic arterial infusion chemotherapy combined with tyrosine kinase inhibitors and anti-PD-1 immunotherapy for unresectable intrahepatic cholangiocarcinoma. J Dig Dis. 2022;23:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 41. | Cai Z, He C, Zhao C, Lin X. Survival Comparisons of Hepatic Arterial Infusion Chemotherapy With mFOLFOX and Transarterial Chemoembolization in Patients With Unresectable Intrahepatic Cholangiocarcinoma. Front Oncol. 2021;11:611118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Kasai K, Kooka Y, Suzuki Y, Suzuki A, Oikawa T, Ushio A, Kasai Y, Sawara K, Miyamoto Y, Oikawa K, Takikawa Y. Efficacy of hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic pegylated interferon α-2b for advanced intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2014;21:3638-3645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |