Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.108274
Revised: May 1, 2025
Accepted: May 29, 2025
Published online: July 15, 2025
Processing time: 96 Days and 7 Hours
Pancreatic cancer (PC) is an aggressive malignancy. As a member of the BTN/BTNL family, BTNL9 has been identified as a tumor suppressor in breast cancer, lung adenocarcinoma, and colon cancer; however, its role and underlying mechanisms in PC remain to be elucidated.
To investigate the role of BTNL9 in the pathogenesis and development of PC.
The difference of BTNL9 expression in cancer and adjacent normal tissues was analyzed by RNA sequencing data from a public database and tissue microarray detection. The relationship between BTNL9 expression and the prognosis of patients was also studied. The effects of BTNL9 on proliferation, metastasis, and cell cycle of PC cells were investigated by phenotypic experiments. The me
The mRNA and protein levels of BTNL9 in PC tissues were downregulated compared with normal tissues. Based on survival data from The Cancer Genome Atlas and tissue microarray, BTNL9 was an independent influencing factor for overall survival, and its low expression predicted a shortened overall survival of patients. In vitro, BTNL9 could inhibit cell proliferation and metastasis in both PANC-1 and MIA PaCa-2 cells and induce cell cycle arrest in G2/M phases. Downregulation of BTNL9 could activate the cell cycle signaling pathway. Furthermore, overexpression of BTNL9 could significantly inhibit the expression of cell division cycle 20 (CDC20). Rescue experiments demonstrated that overexpression of CDC20 reversed the effect of BTNL9 on the proliferation, metastasis, and cell cycle of PC cells.
The expression of BTNL9 was downregulated in PC, and it has the prediction ability for prognosis. Functionally, BTNL9 exerted an anti-cancer effect by suppressing downstream CDC20 expression in PC.
Core Tip: The present study reported the downregulated expression of BTNL9 in pancreatic cancer and correlated with a poorer prognosis. Pancreatic cancer cells overexpressing BTNL9 exhibited cell cycle arrest at G2/M, accompanied by a reduction in their proliferative and metastatic capacity. BTNL9 regulated the cell cycle by inhibiting cell division cycle 20.
- Citation: Xiao M, Luo ZY, Yu AR, Xu K, Zhou W. BTNL9 exerts anti-cancer effects by inhibiting CDC20 to induce G2/M arrest in pancreatic cancer. World J Gastrointest Oncol 2025; 17(7): 108274
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/108274.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.108274
Pancreatic cancer (PC) is an aggressive malignancy that occurs most frequently in countries with a high human development index and always has a poor prognosis, resulting in approximately 467000 deaths worldwide each year[1]. The primary cause of the heightened mortality rate associated with PC stems from the challenges pertaining to its early detection and the dearth of effective therapeutic agents. A majority of patients are diagnosed at an advanced stage, which limits their potential to benefit significantly from any identified effective targets. The median progression-free survival of first-line chemotherapy regimens is only 6.3 months, and the median overall survival (OS) is less than 1 year[2,3]. In order to improve the prognosis of patients with PC, further elucidation of the pathogenesis of PC, discovery of diagnostic and prognostic markers, and analysis of effective therapeutic targets are the main directions of future exploration.
BTNL9 belongs to the BTN/BTNL family, which is a member of the immunoglobulin superfamily and has significant homology and similar structural characteristics with B7-like molecules that regulate the immune response of T cells. Therefore, the BTN/BTNL family has been hypothesized to play a role in inflammation and cancer by regulating T cell activation[4,5]. As a member of the BTN/BTNL family, BTNL9 is downregulated in tissues of breast cancer, colon cancer, lung adenocarcinoma, and uveal melanoma and could inhibit the malignant phenotype of breast cancer and uveal melanoma cells[6-10]. The high expression of BTNL9 is also associated with good prognosis in patients with breast cancer and uveal melanoma[8,10]. BTNL9 play the role of a tumor suppressor gene in a variety of cancers, but its role in PC has not been investigated. Therefore, this study aimed to explore whether BTNL9 affects the phenotype of PC and the mechanism of its role in the occurrence and development of PC.
Human PC tissue samples were obtained from the First Affiliated Hospital of Chengdu Medical College and were all pathologically diagnosed with PC in the past. The study was conducted with the inclusion of all patients who demonstrated their awareness and provided their informed consent. The study was approved by the Ethics Committee of the First Affiliated Hospital of Chengdu Medical College (2022CYFYIRB-SQ-22). The human PC cell lines PANC-1, BxPC-3, Capan-1, and MIA PaCa-2 were purchased from the American Type Culture Collection. The cells were maintained at 37 °C and 5%CO2 in a humidified incubator.
The protein expression levels of the human PC tissue microarray (TMA) (HPanA180Su10, Outdo Biotech) and collected tissues were detected by routine immunohistochemistry (IHC). The human TMA used has been approved by the Ethics Committee of Shanghai Outdo Biotech Company (YB M-05-01) and the First Affiliated Hospital of Chengdu Medical College (2022CYFYIRB-SQ-22). After hydration, penetration, and sealing, the primary antibody was used to incubate at 4 °C overnight, and the secondary antibody was then used to incubate at room temperature for 1 h. Finally, the diaminobenzidine color reaction and observation after sealing was carried out. The IHC staining was quantified by the H-score method. The final H-score was calculated as the sum of the products of staining intensity and the corresponding percentage of positive cells. The stain score ranges from 0 to 300. Low expression was defined as a score of < 100, and high expression was defined as a score of 100-300.
Overexpression plasmid of BTNL9 and CDC20 were packaged by lentivirus, which was synthesized by Genechem (Shanghai, China) using the vectors Ubi-MCS-3FLAG-CBh-gcGFP-IRES-puromycin and CMV-MCS-3FLAG-EF1a-mCherry-T2A-neomycin, respectively. All positive clones were validated by sequencing. After lentivirus and co-transfection agents were added to the cells for 48 h, puromycin or neomycin was used continuously for 7 days, and the stable cells were screened.
In the CCK8 experiment cells were seeded into 96-well plates, and cell proliferation was assayed at 0 h, 24 h, 48 h, and 72 h using the CCK8 (K1018, Apexbio). Absorbance was detected by a microplate reader (BioTek, United States) at 450 nM. EdU assay was implemented with an EdU kit (C10310-1, RiboBio). Cells were incubated with EdU, formaldehyde was added for fixation, glycine was added for incubation, Apollo was added for reactant reaction, and Hoechst was added for nucleation. In clone formation assays cells were plated into 6-well plates. Cells were fixed with 4% paraformaldehyde and then stained with crystal violet after cultured for 2 weeks.
Cell migration and invasion were assessed using the transwell chamber (14341, Labselect). Cells suspended in serum-free medium were planted in the upper chamber, and the lower chamber was filled with complete medium. The cells were fixed and stained after 24-48 h. In the invasion experiment 1 mg/mL of Matrigel® Invasion Chambers (354480, Corning) (approximately 80 μL/chamber) was added in the center of the chamber, and the chamber was placed in an incubator at 37 °C for overnight fusion. The method of cell cycle detection was as follows: Cells were fixed with 70% ethanol at 4 °C overnight, stained with a cell cycle detection kit (KGA512, KeyGen BioTech), and analyzed with flow cytometry (Agilent, United States) and NovoExpress software (Agilent, United States).
Total RNA was extracted by the Trizol method according to the instructions of the total RNA extraction kit (R1200, Solarbio). mRNA reverse transcription was performed using the PrimeScript™ RT Reagent Kit (KR116-02, TianGen). Reverse transcription quantitative PCR (RT-qPCR) was performed using the SYBR 10 μL reaction system, and the reaction system was configured using the kit (1725272, Bio-Rad) for onboard detection. The CFX96TM Real-Time PCR System (Bio-Rad, United States) was applied to analyze the samples. The primers used in this study are shown in Table 1.
| Method | Name | Sequence (5’-3’) |
| qPCR | GAPDH-F | GGAGTCAACGGATTTGGT |
| qPCR | GAPDH-R | GTGATGGGATTTCCATTGAT |
| qPCR | BTNL9-F | ATGGTGGACCTCTCAGTCTCC |
| qPCR | BTNL9-R | GCCAGGATGGGATACTCAGG |
| qPCR | MYC-F | GGCTCCTGGCAAAAGGTCA |
| qPCR | MYC-R | CTGCGTAGTTGTGCTGATGT |
| qPCR | CDC20-F | GACCACTCCTAGCAAACCTGG |
| qPCR | CDC20-R | GGGCGTCTGGCTGTTTTCA |
| qPCR | E2F1-F | ACGCTATGAGACCTCACTGAA |
| qPCR | E2F1-R | TCCTGGGTCAACCCCTCAAG |
| qPCR | CDKA5-F | GACGCCAGAGACTTGGAAATG |
| qPCR | CDKA5-R | GGACCTCGGTGAGTTTGGAG |
| qPCR | CDKN1C-F | GCGGCGATCAAGAAGCTGT |
| qPCR | CDKN1C-R | GCTTGGCGAAGAAATCGGAGA |
| qPCR | PTTG1-F | ACCCGTGTGGTTGCTAAGG |
| qPCR | PTTG1-R | ACGTGGTGTTGAAACTTGAGAT |
| qPCR | CDK1-F | GGATGTGCTTATGCAGGATTCC |
| qPCR | CDK1-R | CATGTACTGACCAGGAGGGATAG |
| qPCR | GADD45A-F | GAGAGCAGAAGACCGAAAGGA |
| qPCR | GADD45A-R | CACAACACCACGTTATCGGG |
| qPCR | GADD45B-F | TACGAGTCGGCCAAGTTGATG |
| qPCR | GADD45B-R | GGATGAGCGTGAAGTGGATTT |
| qPCR | MCM3-F | GCGCAGGAAAAACGAGAAGAG |
| qPCR | MCM3-R | AATGGAGGCCACAAAATCCTTT |
| qPCR | MCM5-F | AGCATTCGTAGCCTGAAGTCG |
| qPCR | MCM5-R | CGGCACTGGATAGAGATGCG |
| qPCR | MCM7-F | GCCTGTGGGAAATATCCCTCG |
| qPCR | MCM7-R | GTACCACCTGTCGGAACCC |
The protein lysate was transferred to the polyvinylidene fluoride membrane by electrophoresis and membrane transfer. After closure, the primary antibody was incubated overnight, and the corresponding secondary antibody was incubated after washing and exposed by the molecular imager (Bio-Rad, United States). The antibodies used in western-blot were: BTNL9 (bs-8434R, Bioss); BTNL9 (TA338873, OriGene); CDC20 (10252-1-AP, Proteintech); Ki-67 (9027, Cell Signaling Tech), MCM3 (15597-1-AP, Proteintech); MCM5 (67049-1-Ig, Proteintech); PTTG1 (sc-56207, Santa Cruz Biotech); GADD45A (sc-377311, Santa Cruz Biotec), GAPDH (6004-1-Ig, Proteintech); anti-rabbit IgG (A0208, Beyotime Biotech), anti-mouse (A0216, Beyotime Biotech).
After the cells were transferred to a slide, 4% paraformaldehyde was fixed. After 0.5%Triton was used, the immunostaining blocking solution was added, the primary antibody was incubated overnight, the secondary antibody was incubated, and DAPI was added. The sections were observed by a confocal microscope (Leica, Germany) after the anti-quench agent was added. The fluorescence intensity was quantitatively analyzed using the Image J software (Version 1.8.0.112, National Institutes of Health, United States). CDC20 primary antibody (10252-1-AP, Proteintech) and ABflo 594-conjugated goat anti-rabbit IgG (AS039, ABclonal) were used to perform the immunofluorescence assays.
Four 4-week-old female BALB/c nude mice were purchased from Chengdu Yaokang Biotechnology. They were fed in a specific pathogen-free environment. The experiment was conducted in strict accordance with the requirements stipulated by the Experimental Animal Management and Use Committee of Chengdu Medical College. MIA PaCa-2 cells (3 × 106) overexpressing BTNL9 and the control group were suspended in 150 μL PBS and injected subcutaneously into the left and right lateral thighs of mice, respectively. The volume of xenograft tumor was calculated as follows: V = length × width2 × 0.52. The measurement results were recorded every 3 days.
The total RNA expression of BTNL9 overexpressed and MIA PaCa-2 cells in the control group were sequenced by Novogene (Beijing, China), which completed the entire detection through sample detection, library construction, library detection, computer sequencing, data quality control, and bioinformatic analysis. RNA samples and libraries were inspected with an Agilent 2100 bioanalyzer. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumia, United States) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Nova-seq platform, and 150 bp paired-end reads were generated.
The expression difference of BTNL9 was analyzed by GSE62165, GSE62452, GSE71729, GSE28735, and GSE15471 PC expression sets in the Gene Expression Omnibus database. The expression of BTNL9 in PC tissues and normal tissues in The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression Project databases was investigated and analyzed by Gene Expression Profiling Interactive Analysis. Survival analysis was performed by downloading RNA sequencing and survival data of PC from the TCGA database.
All data were statistically analyzed by R software (version 4.1.1) or GraphPad Prism software (version 8.0.2). The χ2 test was used for comparison of counting data, and measurement data was recorded as mean ± SD. The t-test or Wilcoxon test was used for comparison between two groups, one-way analysis of variance was used for comparison of multiple groups, and least significant difference t-test was used for pound-wise multiple comparisons. P < 0.05 was considered statistically significant.
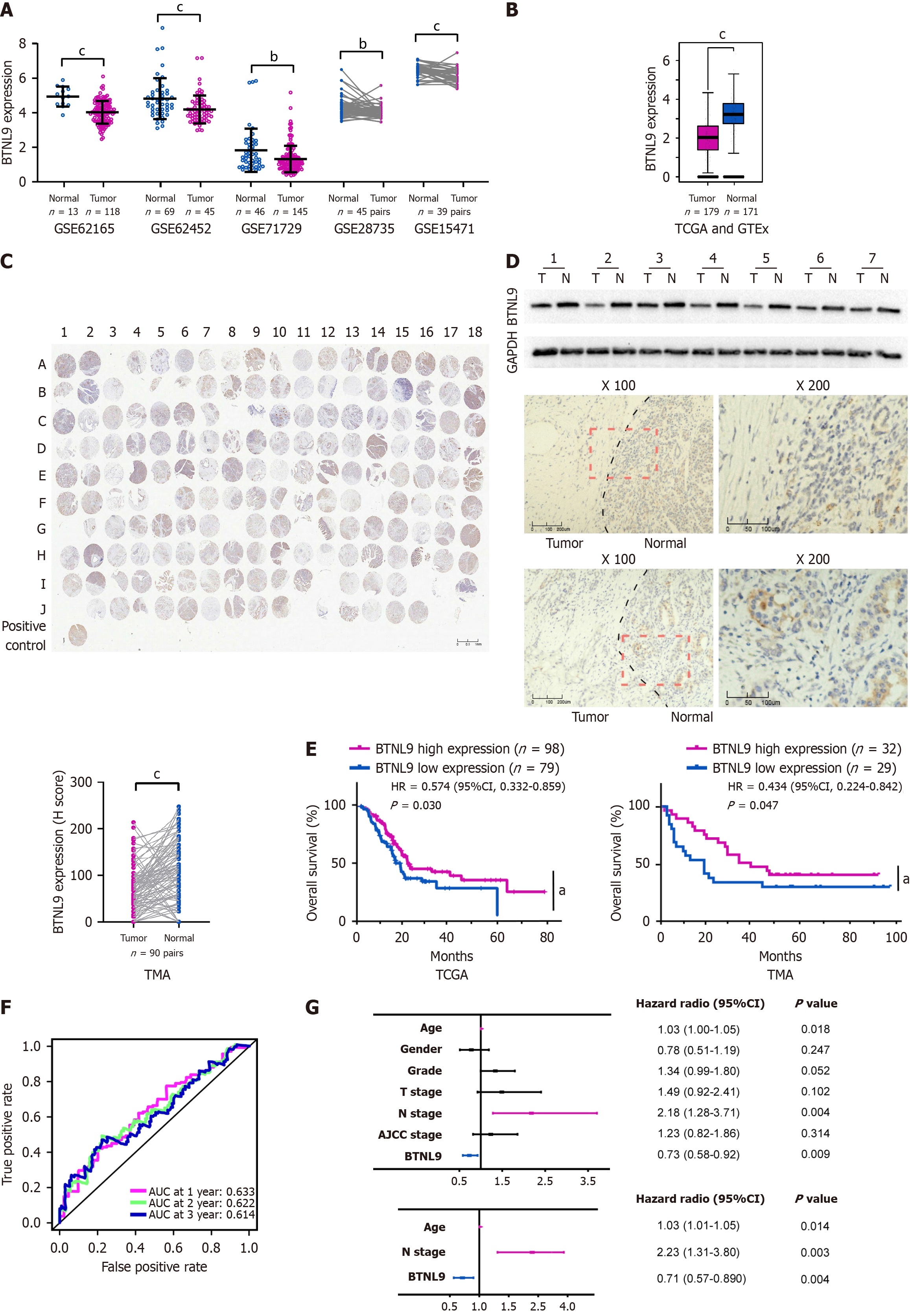
In five RNA expression profiling data sets (GSE62452, GSE62165, GSE71729, GSE28735, GSE15471), the expression level of BTNL9 in PC tissues was lower than that in adjacent normal tissues (Figure 1A). Similarly, it was also found that the expression of BTNL9 in PC tissues was downregulated compared with that in normal pancreatic tissues according to the mRNA sequencing data in TCGA and the Genotype-Tissue Expression Project databases (Figure 1B). In order to validate the expression level of the BTNL9 protein, IHC analysis was conducted on a TMA comprising 90 patients with PC. It showed that the expression of BTNL9 in PC tissues was significantly lower than that in adjacent normal tissues (Figure 1C). Simultaneously, we detected the expression level of BTNL9 in 7 cases of PC from our hospital and observed predominant localization of BTNL9 in the cell membrane and cytoplasm. Moreover, the expression level of BTNL9 was significantly elevated in adjacent normal tissues compared with PC tissues (Figure 1D).
According to the RNA sequencing data and survival data of PC in TCGA database, the OS of patients with high BTNL9 expression was longer than the patients with low expression. Similarly, for 61 patients with complete survival data and BTNL9 expression scores in TMA, OS was shortened in patients with low BTNL9 expression (Figure 1E). In addition, based on data from TCGA database, it could be found that the expression of BTNL9 has a good ability to predict survival, and the aera under the curve (AUC) values for predicting 1-year, 2-year and 3-year survival were 0.633, 0.622 and 0.614, respectively (Figure 1F). Multivariate analysis showed that the expression level of BTNL9 in tumor tissues was an independent factor affecting the prognosis of patients with PC, and its expression was associated with a good prognosis (Figure 1G).
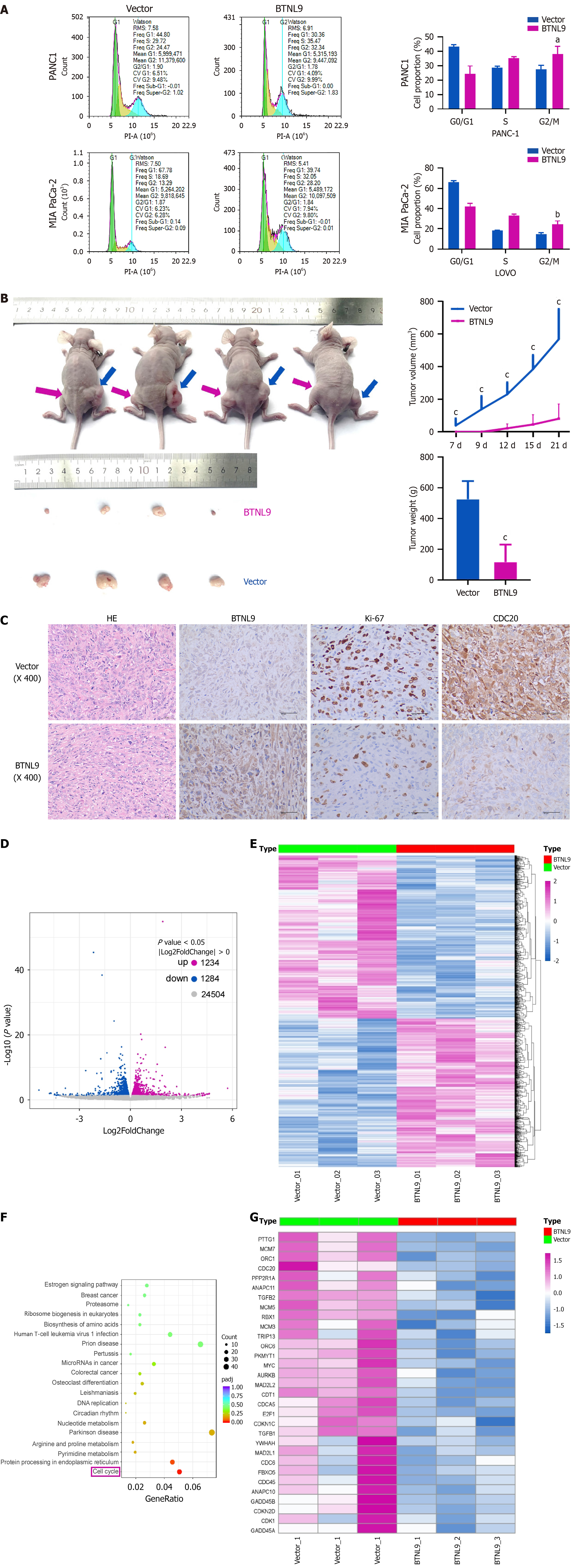
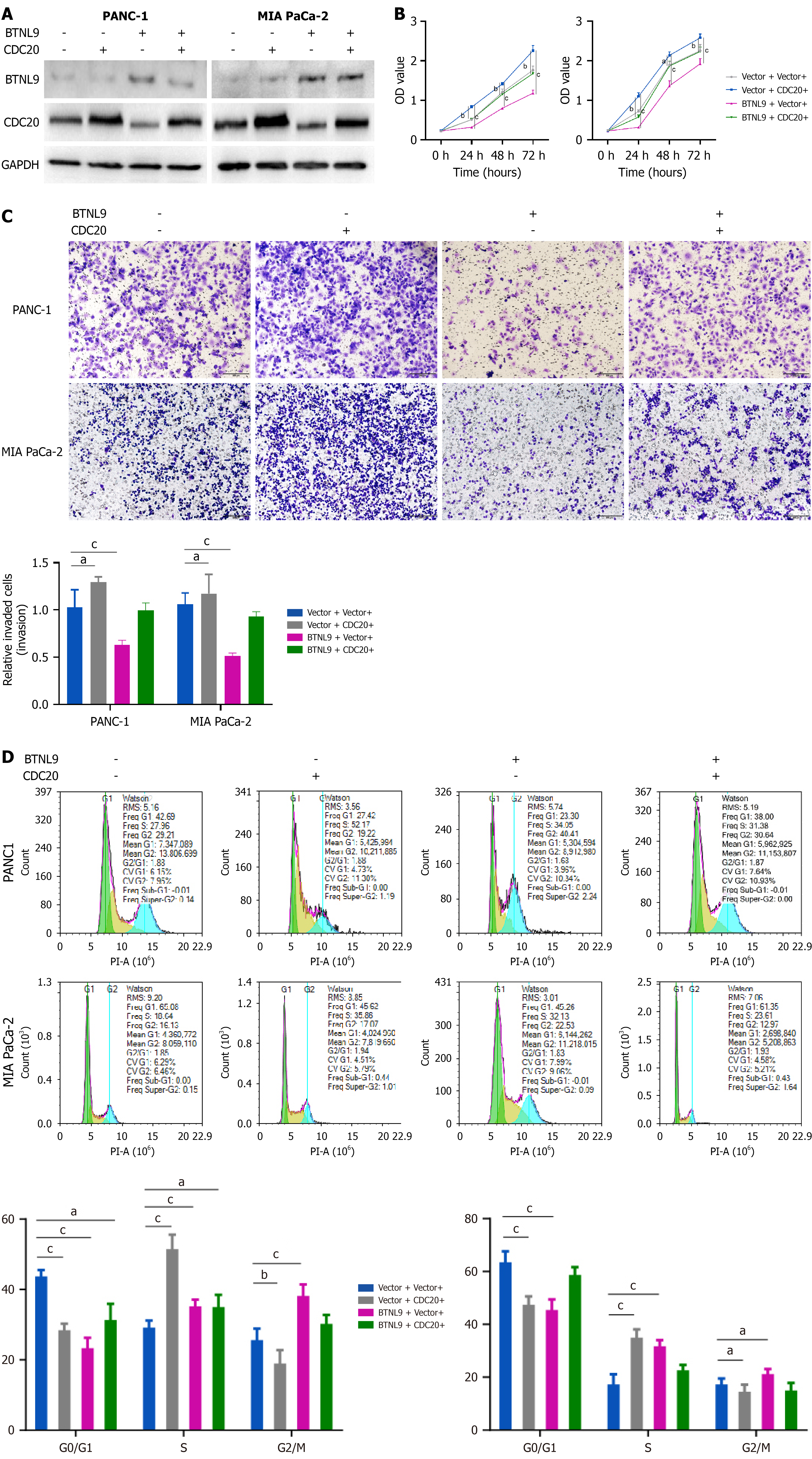
Through the investigation on the expression level of BTNL9 in common PC cell lines, low expression was found in PANC-1 and MIA PaCa-2 cells (Figure 2A). The cell lines were selected for the construction of the BTNL9 overexpression stable cellular model (Figure 2B). In vitro, the proliferation ability of overexpressed BTNL9 cells was significantly reduced compared with control cells (Figure 2C). The number of clones formed by BTNL9-overexpressing cells exhibited a notable decrease compared with control cells (Figure 2D). The proportion of EdU-positive staining in PC cells was found to be lower in BTNL9-overexpressing cells than in control cells (Figure 2E). Transwell assay results demonstrated that overexpression of BTNL9 led to impaired migration and invasion abilities (Figure 2F).
Flow analysis showed that PANC-1 and MIA PaCa-2 cells overexpressing BTNL9 were significantly arrested at the G2/M phase than control cells (Figure 3A). In vivo, the experiments have confirmed that the tumorigenic ability of subcutaneous graft tumors was reduced in MIA PaCa-2 cells overexpressing BTNL9 (Figure 3B and C). The RNA sequencing analysis was performed on MIA PaCa-2 cells overexpressing BTNL9 and control cells to determine their differential gene expression profiles. Overall, 1234 significantly upregulated genes and 1284 significantly downregulated genes were found by difference analysis (Figure 3D and E). In Kyoto Encyclopedia of Genes and Genomes enrichment analysis, low expression of BTNL9 was found to be significantly enriched in the cell cycle, protein processing in the endoplasmic reticulum, pyrimidine metabolism, and other signaling pathways (Figure 3F). In the cell cycle-related genes, PTTG1, MCM7, ORC1, and CDC20 were found significantly downregulated in BTNL9-overexpressed cells (Figure 3G).
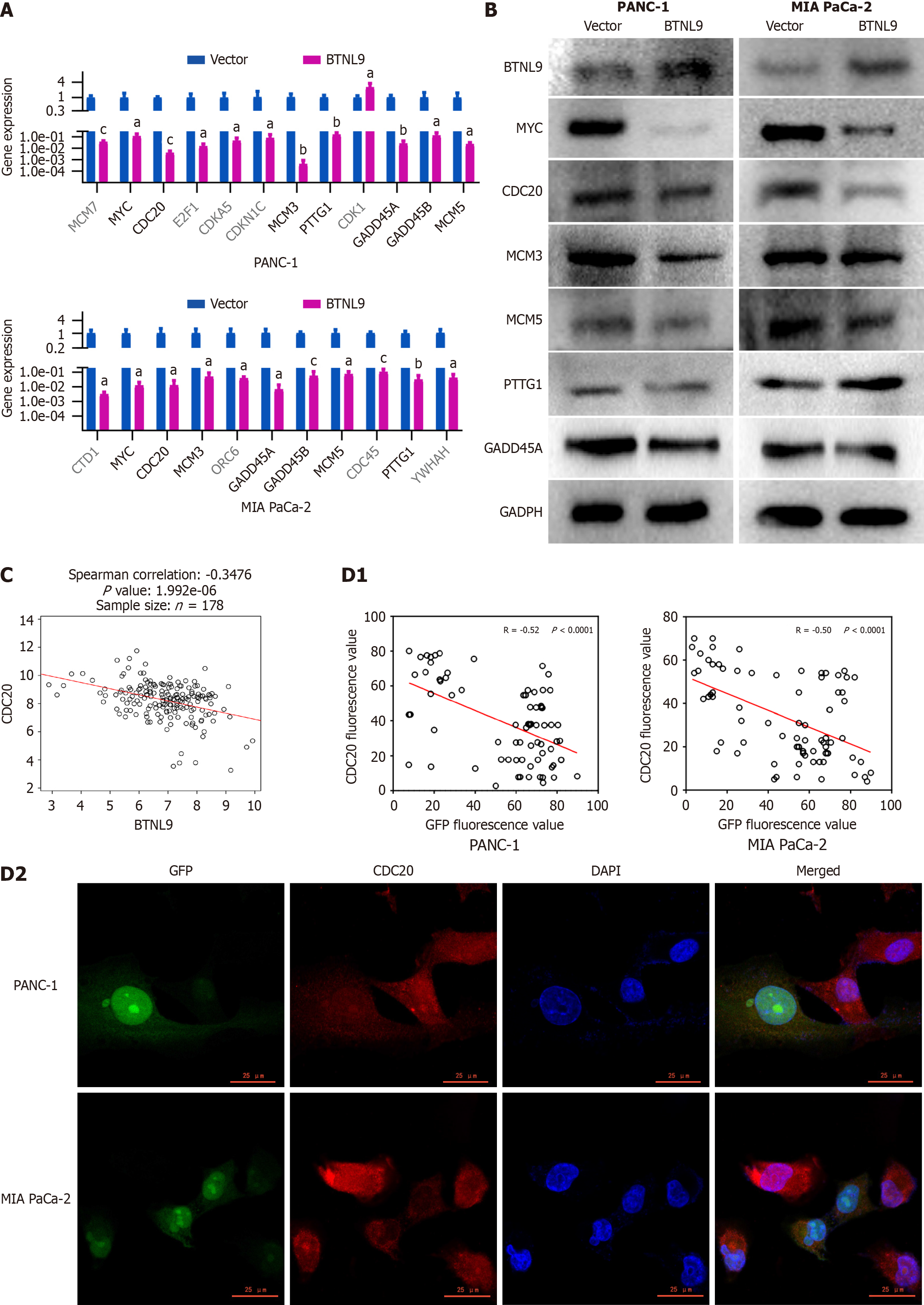
Through RT-qPCR validation, significant differential expression was observed in MYC, CDC20, MCM3, PTTG1, GADD83, GADD85, and MCM5 between BTNL9-overexpressing cells and control cells. Notably, all these genes were downregulated in the BTNL9-overexpressing cells (Figure 4A). Further detection of their protein expression showed that MYC and CDC20 were downregulated in BTNL9-overexpressed cells in PANC-1 and MIA PaCa-2 cells, while no significant differences were found in MCM3, MCM5, and GADD45A (Figure 4B). Applying TCGA RNA sequencing data of PC, it was found that CDC20 was significantly negatively correlated with BTNL9 expression (Figure 4C). Therefore, CDC20 was selected as a possible downstream molecule of BTNL9. It was observed that the expression levels of CDC20 and BTNL9 exhibited a significant negative correlation in the BTNL9-overexpressing cells by immunofluorescence detection (Figure 4D). CDC20 was mainly expressed in the nucleus and cytoplasm. The expression of CDC20 was also found to be downregulated in xenograft tumor tissues upon treatment with BTNL9-overexpressed cells (Figure 3C).
In order to further explore the influence of BTNL9/CDC20 on cell phenotype, rescue experiments were carried out (Figure 5A). In the CCK8 experiment, it was found BTNL9-overexpressed cells transfected with CDC20 overexpression could restore their cell proliferation ability, and the proliferation ability was close to that of the control cells (Figure 5B). In the migration experiment it was observed that transfection of CDC20 for overexpression restored the migratory capacity of BTNL9-overexpression cells (Figure 5C). Flow cytometry analysis revealed that CDC20 overexpression increased the proportion of cells in the S phase, thereby alleviating G2/M phase arrest in BTNL9-overexpressing cells (Figure 5D).
For an extensive period, the analysis of pivotal molecular entities in tumor development has served as the fundamental basis for advancing precision medicine in oncology. In the field of PC, some key molecules have been discovered, including KRAS, TP53, SMAD4, and CDKN2A[11]. In terms of clinical application, molecularly-matched targeted drugs have been developed for the KRAS G12C mutation, alterations of DNA damage response and repair pathways (BRCA1, BRCA2, PALB2, ATM), NTRK1/NTRK2/NTRK3/ROS1 fusions, BRAFV600E mutation, and FGFR2 fusion[12-18]. This brings new hope for the posterior-line treatment of patients with advanced or metastatic PC. Unfortunately, due to the small proportion of patients with the above molecular characteristics, only 10%-25% of patients can receive precision treatment with the above molecular matching[19-22].
To elucidate more key molecules of PC and enable more patients to receive tumor precision medicine is one of the favorable ways to improve the prognosis of patients in the future. This study explored the role of BTNL9 in the development of PC, excavated a novel gene related to the cell cycle of PC, and found that it was an upstream molecule of CDC20, an important regulator of G2/M phase in PC. Similarly, BTNL9 also plays a role in cell cycle regulation in breast cancer. Researchers have found that BTNL9 can affect the P53/CDC25C and P53/GADD45 signaling pathways of breast cancer cells[10]. On the other hand, previous studies have found that the increased expression of downstream molecule CDC20 was significantly correlated with the poor prognosis of PC and confirmed that downregulated CDC20 expression inhibited the proliferation and metastasis ability of PC cells[23,24]. As an upstream regulatory molecule, BTNL9 is speculated to play a more obvious role in G2/M phase regulation that provides a new clue for basic and translational research on specific cell cycle phases of PC in the future.
Due to the poor prognosis of PC, prognosis prediction may directly affect the decision-making of clinical treatment. Prognostic biomarkers of PC found in previous studies include CA19-9, glycans, microRNAs, thrombospondin-1, thrombospondin-2, protein metabolite panels, etc.[25-29]. In recent years although some progress has been made in enhancing diagnostic efficiency through the integration of multiple markers, there still exist challenges in establishing more precise prediction methods. In this study, we found that BTNL9 expression was associated with OS through analysis of TMA and TCGA database and found that BTNL9 may be a predictive marker in prognosis prediction. In terms of its predictive efficacy, the AUC values of 1-year, 2-year, and 3-year OS prediction calculated by using the expression of BTNL9 alone were all above 0.6, which was close to the efficacy of serum CA19-9 alone. BTNL9 demonstrated excellent predictive efficacy for OS. Its integration with other established biomarkers may enhance prognostic risk assessment for PC, thereby playing a more significant predictive role. This intriguing finding indicates future clinical potential.
PC is a complex and refractory disease, and the breakthrough point of the future is the development of comprehensive treatment. Chemotherapy, radiotherapy, targeted therapy, immunotherapy, and other therapeutic means should be more reasonable applications and more scientific combinations. Improving the efficacy of traditional treatment is also an important clinical issue. In this study BTNL9 was found to inhibit CDC20, leading to the arrest of tumor cells at G2/M. Previous studies have confirmed that G2/M phase is the most sensitive phase to radiotherapy. Therefore, it is imperative to investigate the impact of BTNL9 expression on the radiosensitivity of PC cells and its influence on the anti-tumor efficacy of phase-specific chemotherapy drugs such as gemcitabine, fluorouracil, paclitaxel, etc. Furthermore, considering the structural characteristics of the BTN/BTNL family and previous research findings, it is crucial to explore whether BTNL9 affects the tumor immune microenvironment. These are important directions to further explore the role of BTNL9 in PC.
This study had some limitations. This study found that BTNL9 exerted an anti-cancer effect by inhibiting CDC20-induced G2/M arrest in PC. The mechanism by which BTNL9 inhibits the expression of CDC20 needs further exploration. In addition, BTNL9 has also been found to have the ability to predict the prognosis of patients with PC. This finding still needs to be verified by a large sample cohort for further confirmation. For the methods of animal experiments, conducting subcutaneous tumorigenesis experiments independently in two groups can better eliminate the influence of local paracrine effects. Finally, the upstream regulatory mechanism of BTNL9 expression remains unclear and requires in-depth exploration.
The expression of BTNL9 was downregulated in PC, and it is a potential prognostic marker. BTNL9 played an anti-cancer role by inhibiting downstream CDC20 expression in PC.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12185] [Article Influence: 6092.5] [Reference Citation Analysis (6)] |
| 2. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5878] [Article Influence: 391.9] [Reference Citation Analysis (23)] |
| 3. | Peixoto RD, Ho M, Renouf DJ, Lim HJ, Gill S, Ruan JY, Cheung WY. Eligibility of Metastatic Pancreatic Cancer Patients for First-Line Palliative Intent nab-Paclitaxel Plus Gemcitabine Versus FOLFIRINOX. Am J Clin Oncol. 2017;40:507-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Abeler-Dörner L, Swamy M, Williams G, Hayday AC, Bas A. Butyrophilins: an emerging family of immune regulators. Trends Immunol. 2012;33:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O, Polyakova O, Roberts NA, Wesch D, Kabelitz D, Irving PM, John S, Mansour S, Bates PA, Vantourout P, Hayday AC. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol. 2018;19:1352-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 6. | Ho XD, Phung P, Q Le V, H Nguyen V, Reimann E, Prans E, Kõks G, Maasalu K, Le NT, H Trinh L, G Nguyen H, Märtson A, Kõks S. Whole transcriptome analysis identifies differentially regulated networks between osteosarcoma and normal bone samples. Exp Biol Med (Maywood). 2017;242:1802-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Hsu YL, Hung JY, Lee YL, Chen FW, Chang KF, Chang WA, Tsai YM, Chong IW, Kuo PL. Identification of novel gene expression signature in lung adenocarcinoma by using next-generation sequencing data and bioinformatics analysis. Oncotarget. 2017;8:104831-104854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Jiang Z, Liu F. Butyrophilin-Like 9 (BTNL9) Suppresses Invasion and Correlates with Favorable Prognosis of Uveal Melanoma. Med Sci Monit. 2019;25:3190-3198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Lebrero-Fernández C, Wenzel UA, Akeus P, Wang Y, Strid H, Simrén M, Gustavsson B, Börjesson LG, Cardell SL, Öhman L, Quiding-Järbrink M, Bas-Forsberg A. Altered expression of Butyrophilin (BTN) and BTN-like (BTNL) genes in intestinal inflammation and colon cancer. Immun Inflamm Dis. 2016;4:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Mo Q, Xu K, Luo C, Zhang Q, Wang L, Ren G. BTNL9 is frequently downregulated and inhibits proliferation and metastasis via the P53/CDC25C and P53/GADD45 pathways in breast cancer. Biochem Biophys Res Commun. 2021;553:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Lowery MA, Jordan EJ, Basturk O, Ptashkin RN, Zehir A, Berger MF, Leach T, Herbst B, Askan G, Maynard H, Glassman D, Covington C, Schultz N, Abou-Alfa GK, Harding JJ, Klimstra DS, Hechtman JF, Hyman DM, Allen PJ, Jarnagin WR, Balachandran VP, Varghese AM, Schattner MA, Yu KH, Saltz LB, Solit DB, Iacobuzio-Donahue CA, Leach SD, O'Reilly EM. Real-Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clin Cancer Res. 2017;23:6094-6100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 12. | Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020;383:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1295] [Article Influence: 215.8] [Reference Citation Analysis (0)] |
| 13. | Bekaii-Saab TS, Yaeger R, Spira AI, Pelster MS, Sabari JK, Hafez N, Barve M, Velastegui K, Yan X, Shetty A, Der-Torossian H, Pant S. Adagrasib in Advanced Solid Tumors Harboring a KRAS(G12C) Mutation. J Clin Oncol. 2023;41:4097-4106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 192] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 14. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1756] [Article Influence: 250.9] [Reference Citation Analysis (0)] |
| 15. | Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N, Mascarenhas L, Geoerger B, Dowlati A, Pappo AS, Bielack S, Doz F, McDermott R, Patel JD, Schilder RJ, Tahara M, Pfister SM, Witt O, Ladanyi M, Rudzinski ER, Nanda S, Childs BH, Laetsch TW, Hyman DM, Drilon A. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 750] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 16. | Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 1222] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 17. | Li HS, Yang K, Wang Y. Remarkable response of BRAF (V600E)-mutated metastatic pancreatic cancer to BRAF/MEK inhibition: a case report. Gastroenterol Rep (Oxf). 2022;10:goab031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Helal C, Valéry M, Ducreux M, Hollebecque A, Smolenschi C. FGFR2 fusion in metastatic pancreatic ductal adenocarcinoma: Is there hope? Eur J Cancer. 2022;176:168-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 19. | Massard C, Michiels S, Ferté C, Le Deley MC, Lacroix L, Hollebecque A, Verlingue L, Ileana E, Rosellini S, Ammari S, Ngo-Camus M, Bahleda R, Gazzah A, Varga A, Postel-Vinay S, Loriot Y, Even C, Breuskin I, Auger N, Job B, De Baere T, Deschamps F, Vielh P, Scoazec JY, Lazar V, Richon C, Ribrag V, Deutsch E, Angevin E, Vassal G, Eggermont A, André F, Soria JC. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017;7:586-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 20. | Le Tourneau C, Delord JP, Gonçalves A, Gavoille C, Dubot C, Isambert N, Campone M, Trédan O, Massiani MA, Mauborgne C, Armanet S, Servant N, Bièche I, Bernard V, Gentien D, Jezequel P, Attignon V, Boyault S, Vincent-Salomon A, Servois V, Sablin MP, Kamal M, Paoletti X; SHIVA investigators. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 830] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 21. | Trédan O, Wang Q, Pissaloux D, Cassier P, de la Fouchardière A, Fayette J, Desseigne F, Ray-Coquard I, de la Fouchardière C, Frappaz D, Heudel PE, Bonneville-Levard A, Fléchon A, Sarabi M, Guibert P, Bachelot T, Pérol M, You B, Bonnin N, Collard O, Leyronnas C, Attignon V, Baudet C, Sohier E, Villemin JP, Viari A, Boyault S, Lantuejoul S, Paindavoine S, Treillleux I, Rodriguez C, Agrapart V, Corset V, Garin G, Chabaud S, Pérol D, Blay JY; ProfiLER investigators. Molecular screening program to select molecular-based recommended therapies for metastatic cancer patients: analysis from the ProfiLER trial. Ann Oncol. 2019;30:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 22. | Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, Hellmann MD, Barron DA, Schram AM, Hameed M, Dogan S, Ross DS, Hechtman JF, DeLair DF, Yao J, Mandelker DL, Cheng DT, Chandramohan R, Mohanty AS, Ptashkin RN, Jayakumaran G, Prasad M, Syed MH, Rema AB, Liu ZY, Nafa K, Borsu L, Sadowska J, Casanova J, Bacares R, Kiecka IJ, Razumova A, Son JB, Stewart L, Baldi T, Mullaney KA, Al-Ahmadie H, Vakiani E, Abeshouse AA, Penson AV, Jonsson P, Camacho N, Chang MT, Won HH, Gross BE, Kundra R, Heins ZJ, Chen HW, Phillips S, Zhang H, Wang J, Ochoa A, Wills J, Eubank M, Thomas SB, Gardos SM, Reales DN, Galle J, Durany R, Cambria R, Abida W, Cercek A, Feldman DR, Gounder MM, Hakimi AA, Harding JJ, Iyer G, Janjigian YY, Jordan EJ, Kelly CM, Lowery MA, Morris LGT, Omuro AM, Raj N, Razavi P, Shoushtari AN, Shukla N, Soumerai TE, Varghese AM, Yaeger R, Coleman J, Bochner B, Riely GJ, Saltz LB, Scher HI, Sabbatini PJ, Robson ME, Klimstra DS, Taylor BS, Baselga J, Schultz N, Hyman DM, Arcila ME, Solit DB, Ladanyi M, Berger MF. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2768] [Cited by in RCA: 2609] [Article Influence: 289.9] [Reference Citation Analysis (0)] |
| 23. | Chang DZ, Ma Y, Ji B, Liu Y, Hwu P, Abbruzzese JL, Logsdon C, Wang H. Increased CDC20 expression is associated with pancreatic ductal adenocarcinoma differentiation and progression. J Hematol Oncol. 2012;5:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Guo W, Zhong K, Wei H, Nie C, Yuan Z. Long non-coding RNA SPRY4-IT1 promotes cell proliferation and invasion by regulation of Cdc20 in pancreatic cancer cells. PLoS One. 2018;13:e0193483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Le Large TYS, Meijer LL, Paleckyte R, Boyd LNC, Kok B, Wurdinger T, Schelfhorst T, Piersma SR, Pham TV, van Grieken NCT, Zonderhuis BM, Daams F, van Laarhoven HWM, Bijlsma MF, Jimenez CR, Giovannetti E, Kazemier G. Combined Expression of Plasma Thrombospondin-2 and CA19-9 for Diagnosis of Pancreatic Cancer and Distal Cholangiocarcinoma: A Proteome Approach. Oncologist. 2020;25:e634-e643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Kaur S, Smith LM, Patel A, Menning M, Watley DC, Malik SS, Krishn SR, Mallya K, Aithal A, Sasson AR, Johansson SL, Jain M, Singh S, Guha S, Are C, Raimondo M, Hollingsworth MA, Brand RE, Batra SK. A Combination of MUC5AC and CA19-9 Improves the Diagnosis of Pancreatic Cancer: A Multicenter Study. Am J Gastroenterol. 2017;112:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 27. | Jenkinson C, Elliott VL, Evans A, Oldfield L, Jenkins RE, O'Brien DP, Apostolidou S, Gentry-Maharaj A, Fourkala EO, Jacobs IJ, Menon U, Cox T, Campbell F, Pereira SP, Tuveson DA, Park BK, Greenhalf W, Sutton R, Timms JF, Neoptolemos JP, Costello E. Decreased Serum Thrombospondin-1 Levels in Pancreatic Cancer Patients Up to 24 Months Prior to Clinical Diagnosis: Association with Diabetes Mellitus. Clin Cancer Res. 2016;22:1734-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Liu J, Gao J, Du Y, Li Z, Ren Y, Gu J, Wang X, Gong Y, Wang W, Kong X. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int J Cancer. 2012;131:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 29. | Fahrmann JF, Bantis LE, Capello M, Scelo G, Dennison JB, Patel N, Murage E, Vykoukal J, Kundnani DL, Foretova L, Fabianova E, Holcatova I, Janout V, Feng Z, Yip-Schneider M, Zhang J, Brand R, Taguchi A, Maitra A, Brennan P, Max Schmidt C, Hanash S. A Plasma-Derived Protein-Metabolite Multiplexed Panel for Early-Stage Pancreatic Cancer. J Natl Cancer Inst. 2019;111:372-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |