Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.108258
Revised: April 25, 2025
Accepted: June 12, 2025
Published online: July 15, 2025
Processing time: 96 Days and 20.9 Hours
Crohn's disease (CD)-related small bowel adenocarcinoma (SBA) is a rare adeno
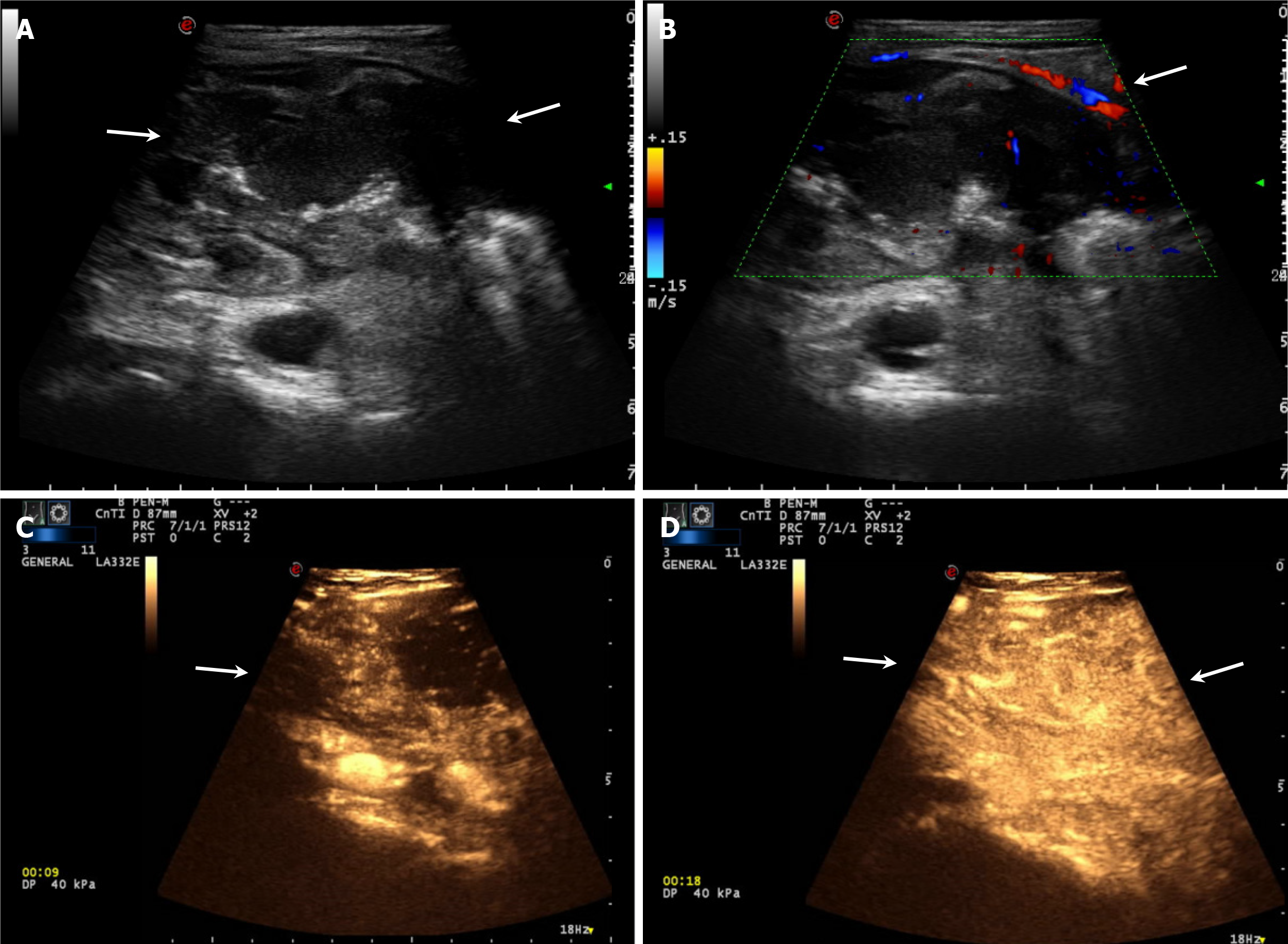
A 38-year-old male diagnosed with CD since 23 years of age was maintained in remission with mesalamine, although he did not take his medication regularly. The patient presented with recurrent dull abdominal pain, bloating, and a three-month history of diarrhea (3 times per day) with unformed stools. Abdominal examination revealed mildly diffuse tenderness. IUS revealed eccentric thickening (23 mm) in the terminal ileum. The hierarchical structure of the intestinal wall disappeared, revealing the “pseudo-kidney” sign. A stricture was identified in the terminal ileum with dilation of the proximal intestinal tract. Color Doppler flow imaging revealed linear blood flow. Contrast-enhanced ultrasound revealed highly heterogeneous enhancement with rapid washout in ileocecal junction, suggesting malignant transformation of CD with intestinal obstruction. Patho
Active surveillance for SBA using IUS is prudent, given its advantages of real-time dynamic imaging, high-detail resolution, and low cost.
Core Tip: Crohn’s disease (CD)-related small bowel adenocarcinoma (SBA) is a rare adenocarcinoma that is difficult to detect and diagnose in its early stages. Herein, selected features of plain and contrast-enhanced ultrasonography in a case of adenocarcinoma are described. This unusual case and review of the relevant literature aims to contribute to the current knowledge base to improve the diagnosis of CD-related SBA using imaging findings.
- Citation: Zhong MY, Jian GL, Ye JY, Chen KX, Huang WJ. Ultrasound diagnosis of small bowel adenocarcinoma in Crohn’s disease: A case report and review of literature. World J Gastrointest Oncol 2025; 17(7): 108258
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/108258.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.108258
Small-bowel tumors are rare, accounting for approximately 3%-5% of gastrointestinal tumors, among which small bowel adenocarcinoma (SBA) is the most common type, representing approximately 30%-40% of cases. SBA is most frequently observed in the duodenum (55%-82%), followed by the jejunum (11%-25%), and ileum (7%-17%)[1,2]. Crohn’s disease (CD)-related SBA is uncommon and has a poor prognosis and, moreover, is difficult to detect and diagnose in the early stages. CD is a risk factor for intestinal cancers. The risk for developing SBA in patients diagnosed with CD is 60 times higher than that in the general population, with an incidence rate of 3 per 10000[3,4]. Herein, we report a case of SBA in a patient with CD diagnosed using intestinal ultrasonography (IUS). We also review the relevant literature addressing imaging findings of CD-related SBA.
A 38-year-old male was admitted to the authors’ hospital with a 10-year history of borborygmi and abdominal bloating that had worsened over the previous week.
The patient was diagnosed with ileal CD at 23 years of age and was maintained in remission with mesalamine, although he did not take his medication regularly. He presented with recurrent dull abdominal pain, bloating, and a three-month history of diarrhea (3 times per day) with unformed stools. His Crohn’s Disease Activity Index score was 485.
The patient had a history of CD, but no history of hypertension, trauma, blood transfusion, or allergies to food or medications.
The patient denied any family history of malignant tumors.
Physical examination revealed mildly diffuse tenderness.
Laboratory investigations yielded the following findings: Hemoglobin, 92 g/L; high-sensitivity C-reactive protein, 41.05 mg/L; erythrocyte sedimentation rate, 26 mm/hour; platelet count, 406 × 109/L; and positivity for urine occult blood. Levels of tumor markers, including carbohydrate antigen 19-9, alpha-fetoprotein, and carcinoembryonic antigen, were within normal limits.
The patient underwent IUS examination using an ultrasound system (Mylab Twice, Esoate, Genoa, Italy) equipped with a 1.0-8.0 MHz low-frequency convex array probe and a 3.0-11.0 MHz intermediate frequency linear array probe. The IUS results revealed eccentric thickening (23 mm) and intestinal stenosis in the ileocecal junction of approximately 7.3 cm × 4.4 cm × 4.0 cm. The hierarchical structure of the intestinal wall disappeared, showing the pseudo-kidney sign. The proximal ileum was dilated (Figure 1A). Color Doppler flow imaging revealed linear blood flow (Figure 1B).
The patient underwent contrast-enhanced ultrasonography (CEUS) after providing consent for further diagnosis. A 2.4 mL ultrasound contrast agent (SonoVue, Bracco, Milan, Italy) suspension was injected through the left cubital vein, followed by a flush using saline (5 mL). CEUS revealed heterogeneous high enhancement with rapid washout in the ileocecal junction. The ultrasound diagnosis suggested ileocecal tumor and intestinal obstruction (Figure 1C and D).
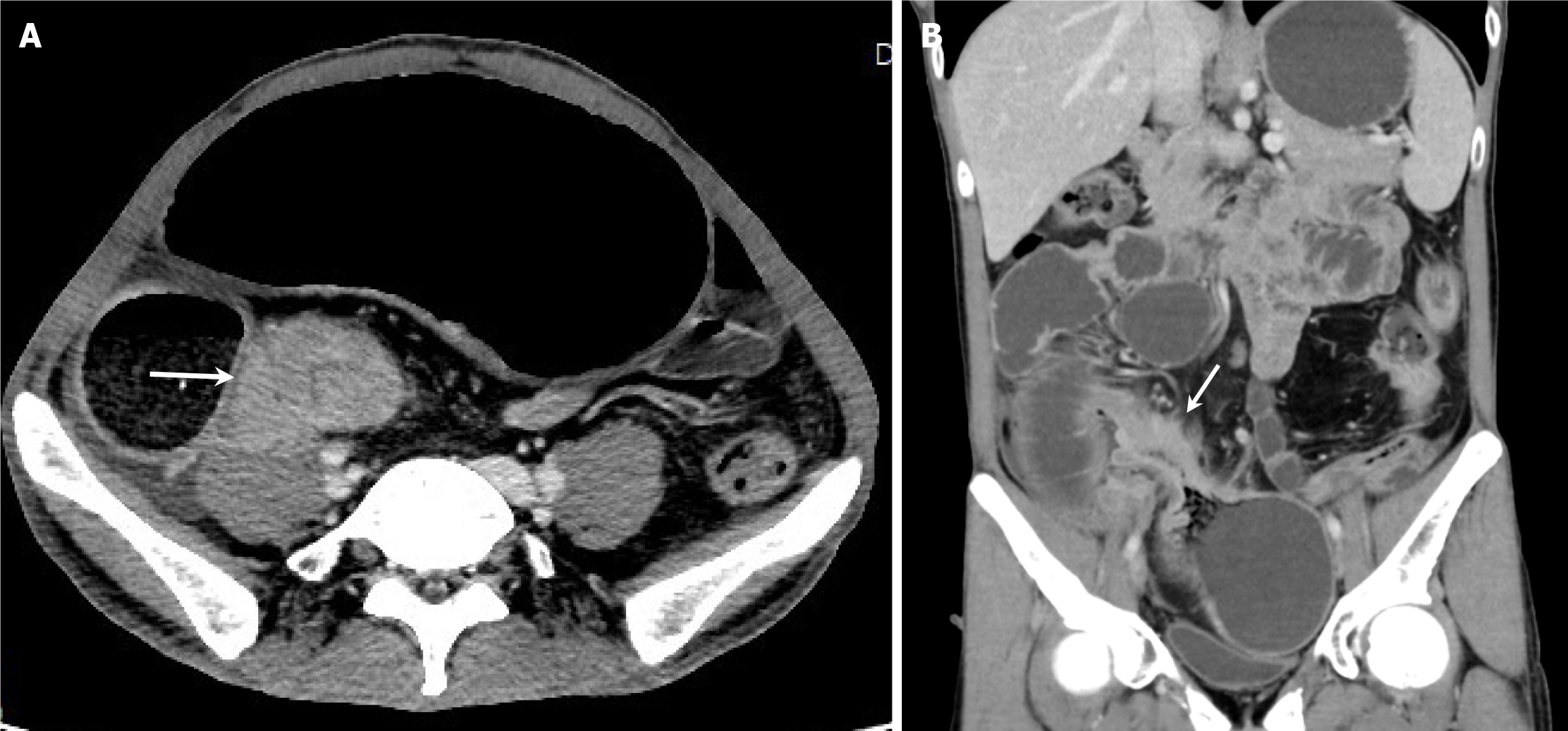
Computed tomography enterography (CTE) with contrast enhancement revealed terminal ileal thickening and a partial small bowel obstruction (Figure 2). Colonoscopy revealed longitudinal and irregular ulcers in the rectum and sigmoid colon. The ileocecal junction of the patient was not biopsied because the polypoid hyperplasia of the sigmoid colon caused intestinal stenosis.
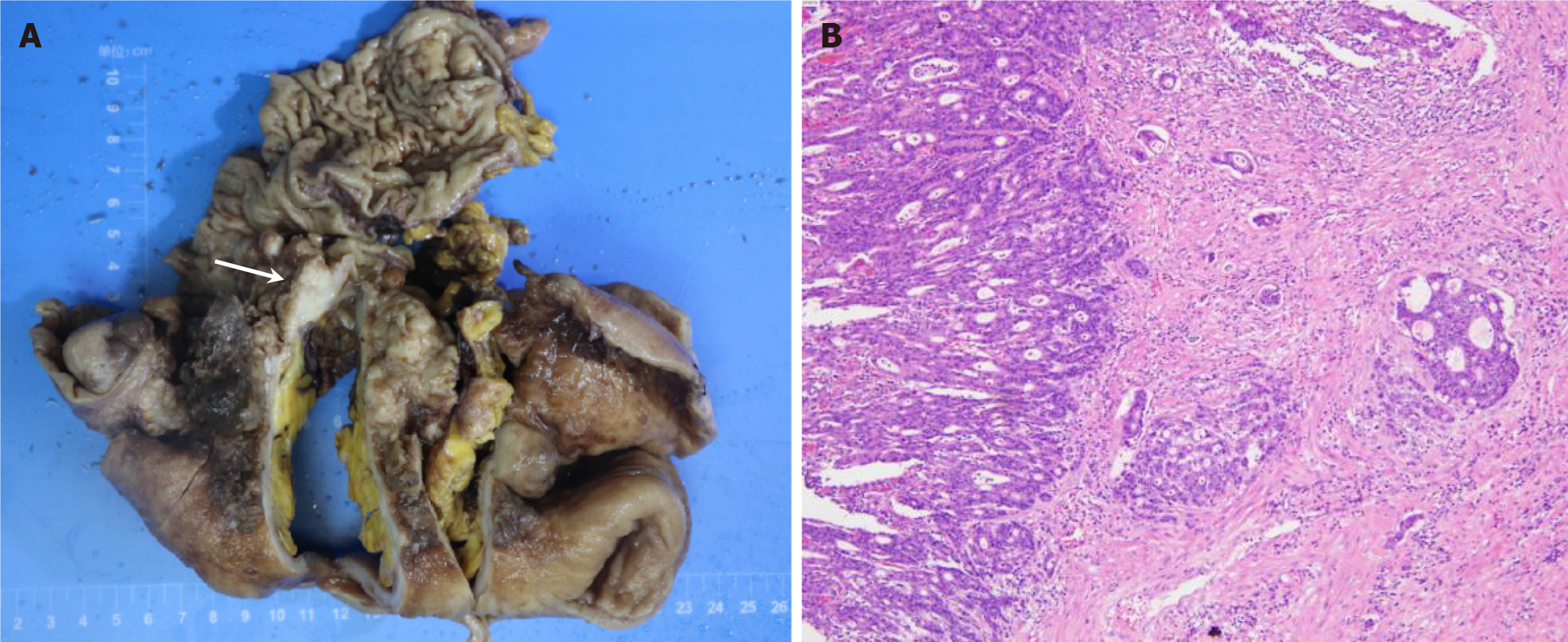
Exploratory laparotomy and right hemicolectomy were performed, and the affected small bowel, cecum, and ileocecal junction were resected. Intraoperatively, a tumor, measuring 6 cm in diameter, was found extending from the terminal ileum to the cecum, with proximal small intestinal dilation > 1 m in length (Figure 3A).
The results of pathological examination revealed a poorly differentiated adenocarcinoma in the ileocecal region, measuring approximately 8.0 cm × 7.0 cm × 4.5 cm, that extended through the bowel wall and invaded the serosa (Figure 3B). No cancer metastases were found in the lymph nodes, and the tumor was classified as T3N0M0 according to the TNM classification. Immunohistochemistry results were as follows: CK, positive (+); CK20 (+); CDX-2 (+); vimentin, negative (-); P40 (-); PMS2 (+); MLH1 (+); MSH2 (+); and MSH6 (+).
The patient underwent exploratory laparotomy and right hemicolectomy, and recovered uneventfully after surgery.
There were no obvious abnormalities on computed tomography (CT) or IUS for 6 months after the operation.
CD is a chronic inflammatory bowel disease of unknown etiology characterized by transmural inflammation, with a segmental distribution of lesions that can affect any part of the digestive tract, but is most commonly found in the terminal ileum and colon. Risk factors for CD-related SBA include a long disease course, lesions located in the jejunum or terminal ileum, stricturing or penetrating CD accompanied by fistula lesions[5], male sex, and occupational exposure to asbestos and other substances[4,6].
We reviewed the literature from 2005 to 2025 and found 15 publications addressing the imaging features of CD-related SBA in the bile ducts[7-11]. The demographic and clinical characteristics of patients described these 82 reported cases are summarized in Table 1[12-16]. SBA in patients with CD is more common in middle-age individuals who typically have a long history of inflammatory bowel disease[17-21]. Mean age at cancer diagnosis was 41.8 years of age while the mean duration of CD was 22.3 years, highlighting the difficulty in the correct and early diagnosis of this entity. There was a significant male predominance, with 57 males (69.5%) and 25 females (30.5%). These patients usually have a long history of CD treatment. Previous CD therapies included corticosteroids (58.8%), aminosalicylates (47.6%), immunomodulators (42.7%), and anti-tumor necrosis factor agents (25.6%)[22-24]. Clinical manifestations in these patients overlapped with those of fibrostenotic CD and active CD flares. As reported in the literature, the major symptoms in these patients included abdominal pain (88.9%), diarrhea (42.8%), vomiting (46.0%), weight loss (44.4%), anemia (33.3%), and hematochezia (15.9%). The present case report describes a 38-year-old male with a 13-year history of CD who was maintained in remission with mesalamine, although he did not take his medication regularly. He presented with generalized abdominal pain, bloating, diarrhea, and anemia consistent with previous reports.
| Characteristics | n = 82 | mean ± SD/percentage (%) | |
| Age(years) | 81 | 41.8 ± 13.2 | |
| ND | 1 | ||
| Duration of Crohn’s disease (years) | 72 | 19.3 ± 11.1 | |
| ND | 10 | ||
| Gender | Male | 57 | 69.51 |
| Female | 25 | 30.49 | |
| History of prior intestinal surgery | Yes | 39 | 47.56 |
| No | 43 | 52.44 | |
| History of prior IBD therapy | Corticosteroids | 45 | 58.44 |
| Aminosalicylate | 39 | 50.65 | |
| Immunomodulators | 35 | 45.45 | |
| Anti-TNF | 21 | 27.27 | |
| Antibiotics | 1 | 1.29 | |
| ND | 5 | ||
| Symptom | Abdominal pain | 56 | 88.89 |
| Diarrhea | 27 | 42.86 | |
| Nausea and vomiting | 29 | 46.03 | |
| Anemia | 21 | 33.33 | |
| Hematochezia | 10 | 15.87 | |
| Weight loss | 28 | 44.44 | |
| Fever | 2 | 3.17 | |
| Abdominal mass | 2 | 3.17 | |
| ND | 19 | ||
| Location | Terminal ileum | 34 | 44.74 |
| Ileum | 32 | 42.10 | |
| Jejunum | 7 | 9.21 | |
| Mid-small bowel | 3 | 3.95 | |
| ND | 6 | ||
| Colonoscopy | Indication of tumor | 5 | 38.46 |
| No indication of tumor | 8 | 61.54 | |
| ND | 69 | ||
| Tumor marker (CEA, CA19-9) | Elevated | 4 | 28.57 |
| Normal | 10 | 71.43 | |
| ND | 68 | ||
| Differentiation degree | Low differentiation | 11 | 45.83 |
| Moderate-low differentiation | 1 | 4.17 | |
| Moderate differentiation | 6 | 25 | |
| High differentiation | 6 | 25 | |
| ND | 58 | ||
| T classification | Tis | 1 | 2.33 |
| 1 | 1 | 2.33 | |
| 2 | 3 | 6.98 | |
| 3 | 15 | 34.88 | |
| 4 | 23 | 53.48 | |
| ND | 39 | ||
| N classification | Yes | 26 | 60.47 |
| No | 17 | 39.53 | |
| ND | 39 | ||
| M classification | Yes | 9 | 18.75 |
| No | 39 | 81.25 | |
| ND | 34 | ||
Imaging examinations for CD include CT, positron emission tomography (PET)/CT, abdominal radiography (i.e., X-ray), barium follow-through, CTE, MR enterography (MRE) and IUS. CT is an imaging examination widely used for generating detailed images of the internal structures of the body using X-rays and computer processing technology. CT is suitable for diagnosing a variety of diseases. PET/CT is an advanced imaging technique that combines PET with CT. PET/CT is not a routine method for examining CD but can be used for the early tumor diagnosis and staging. X-ray is a basic imaging examination method mainly used to observe the morphology and position of abdominal organs. However, the CD diagnostic capability of PET/CT is limited. Barium follow-through involves using oral- or enema-administered barium contrast to examine the gastrointestinal tract shape and function, which can serve as an initial screening tool for CD. CTE and MRE are used to visualize the intestinal lumen, mucosa, bowel wall, and extraintestinal tissue structures after orally administering contrast. MRE has become the preferred imaging modality for follow-up and re-examination in young patients with CD due to its lack of radiation exposure, high soft tissue contrast, and multiparametric and multisequence imaging abilities[25].
IUS provides real-time dynamic imaging and high-detail resolution, playing an important role in CD diagnosis, follow-up, and treatment assessment. The guidelines from several international societies now include IUS as the main diagnostic modality for inflammatory bowel disease[26-28]. The ultrasonography features for CD include increased bowel wall thickness (> 3 mm), increased bowel wall vascularity, abnormal changes in bowel wall stratification, and mesenteric fat proliferation[29]. In addition, IUS can be used diagnose CD-related complications, such as intestinal stenosis, fistulas, and intra-abdominal abscesses. The sensitivity and specificity of IUS in diagnosing intestinal stenosis ranges from 74% to 100% and 89% to 93%, respectively[30].
Currently, the ability to detect CD with SBA through imaging examination is limited[31]. The sensitivity of abdominal CT has been reported to be 11%[32]. If a predictive model could be developed to identify high-risk individuals with SBA among patients with CD, it would be meaningful for early diagnosis and improving patient prognosis[31]. Among the 82 patients with CD-related SBA, 61 underwent imaging examinations, including CT, CTE, PET/CT, MRE, abdominal radiography, and barium follow-through. The radiological features of these patients included bowel wall thickening (83.61%), focal loss of mural stratification (35.42%), adjacent fat stranding (33.33%), bowel obstruction (55.74%), and stricture disease (73.81%; Table 2). Radiological imaging indicated the presence of a tumor in only 17 (27.87%) cases and colonoscopy indicated tumors in 5 (38.46%). To date, no cases of SBA diagnosed in patients with CD using IUS have been reported. We are the first to report the detection of a small bowel tumor using IUS.
| Characteristics | n = 61 | Percentage (%) | |
| Bowel wall thickening | Yes | 51 | 83.61 |
| No | 10 | 16.39 | |
| Focal loss of mural stratification | Yes | 17 | 35.42 |
| No | 31 | 64.58 | |
| ND | 13 | ||
| Adjacent fat stranding | Yes | 14 | 33.33 |
| No | 28 | 66.67 | |
| ND | 19 | ||
| Bowel obstruction | Yes | 34 | 55.74 |
| No | 27 | 44.26 | |
| Stricturing disease | Yes | 31 | 73.81 |
| No | 11 | 26.19 | |
| ND | 19 | ||
| Lymphadenopathy | Yes | 28 | 45.90 |
| No | 33 | 54.10 | |
| Other evidence of distant spread (liver metastases, peritoneal metastasis, etc.) | Yes | 9 | 14.75 |
| No | 52 | 85.25 | |
| Penetrating disease (including fistula, abscess and perforation) | Yes | 18 | 29.51 |
| No | 43 | 70.49 | |
| Malignancy suggesting | Yes | 17 | 27.87 |
| No | 44 | 72.13 | |
In our case, SBA could not be diagnosed due to the narrow lumen of the sigmoid colon and the inability of the colonoscope to reach the tumor in the ileocecal junction for biopsy. CTE revealed thickening of the bowel wall in the terminal ileum and small bowel obstruction without suggesting the presence of a tumor. IUS was the first imaging modality used to indicate the presence of tumors. Based on this case, ultrasound features indicating tumors are summarized as follows: Asymmetric thickening of the intestinal wall; disappearance and stiffening of the hierarchical structure of the intestinal wall; presence of the pseudo-kidney sign; and significant―but rapidly subsiding―enhancement on CEUS.
Patients with CD should be thoroughly assessed using multimodal imaging methods, such as CTE, MR, and IUS. IUS can serve as a tool for long-term follow-up and assessing therapeutic efficacy. CTE or MRE can be used for further evaluation and CEUS for observing the enhancement pattern of the lesion when IUS detects radiological features suggestive of malignancy (such as the pseudo-kidney sign). In addition, PET/CT can be used for tumor staging.
The patient ultimately underwent right hemicolectomy, and the affected small bowel, cecum, and ileocecal junction were resected. The tumor was a poorly differentiated adenocarcinoma, staged as T3N0M0 according to the TNM classification. Based on the previous literature, TNM staging results among 43 patients were distributed as follows: Tis (in situ), n = 1 (2.33%); T1, n = 1 (2.33%); T2, n = 3 (6.98%); T3, n = 15 (34.88%); and T4, n = 23 (53.49%). Lymph node metastasis was present in 26 (60.47%) cases, and distant metastasis was observed in 4 (9.30%). This indicates that the majority of patients with CD, who undergo surgery and are diagnosed with SBA, are already at an advanced stage of disease. This should heighten awareness among clinicians that the early detection of SBA through imaging and endoscopic examinations, which enables early surgery and postoperative chemotherapy, has extremely important clinical significance.
CD-related SBA should be distinguished from sporadic SBA. CD-related SBA typically presents at a younger age, is predominantly located in the ileum, is often poorly differentiated, and is associated with a history of CD. Conversely, sporadic SBA usually occurs at an older age and is more frequently found in the jejunum and duodenum[30].
Regarding prognosis, the 5-year overall survival rate for patients with CD-related SBA is 29% (95%CI: 0.18-0.41)[33]. Factors such as gender, age, whether postoperative chemotherapy was administered, and the tumor's location have minimal impact on prognosis. However, patients who undergo radical surgery, have tumors with invasion limited to the mucosa or submucosa, have well-differentiated pathological types, and have no lymph node or distant metastasis tend to have a better prognosis.
Two limitations of this study must be acknowledged. First, IUS is highly operator-dependent, with imaging quality being easily affected by patient body habitus, intestinal gas, and the adequacy of bowel preparation. Second, we only report a single case. Further studies are needed to confirm the clinical value of IUS and CEUS in SBA in patients with CD.
In summary, he possibility of CD complications, including SBA, should be considered in patients with a long history of CD who present with abdominal pain, weight loss, and symptoms of intestinal obstruction, as the clinical manifestations of SBA are similar to those of CD exacerbation[34]. The pseudo-kidney sign observed with IUS and the substantial but rapidly subsiding enhancement observed with CEUS are helpful in identifying adenocarcinoma.
Diagnosing SBA in patients with CD using imaging examinations is challenging. IUS and CEUS may provide information for identifying malignant lesions, such as the pseudo-kidney sign and the substantial but rapidly subsiding enhancement. Therefore, we suggest that any suspicious radiological and/or endoscopic findings be followed-up with a confirmatory pathological diagnosis whenever possible. We report a case of a 38-year-old man with CD who was diagnosed with SBA using IUS. The patient experienced a favorable postoperative course and subsequently received capecitabine and oxaliplatin chemotherapy. IUS is expected to become an important imaging modality for early CD diagnosis, therapeutic assessment, and disease monitoring with the increasing incidence of CD.
| 1. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming DA, Garrido-Laguna I, Grem JL, Hoffe SE, Hubbard J, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen KS, Saltz LB, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gregory KM, Gurski LA. Small Bowel Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1109-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 2. | Locher C, Batumona B, Afchain P, Carrère N, Samalin E, Cellier C, Aparicio T, Becouarn Y, Bedenne L, Michel P, Parc Y, Pocard M, Chibaudel B, Bouché O; Thésaurus National de Cancérologie Digestive (TNCD). Small bowel adenocarcinoma: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis. 2018;50:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Yu J, Refsum E, Perrin V, Helsingen LM, Wieszczy P, Løberg M, Bretthauer M, Adami HO, Ye W, Blom J, Kalager M. Inflammatory bowel disease and risk of adenocarcinoma and neuroendocrine tumors in the small bowel. Ann Oncol. 2022;33:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Cahill C, Gordon PH, Petrucci A, Boutros M. Small bowel adenocarcinoma and Crohn's disease: any further ahead than 50 years ago? World J Gastroenterol. 2014;20:11486-11495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Liao X, Li G, McBride R, Houldsworth J, Harpaz N, Polydorides AD. Clinicopathological and Molecular Characterisation of Crohn's Disease-associated Small Bowel Adenocarcinomas. J Crohns Colitis. 2020;14:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Bojesen RD, Riis LB, Høgdall E, Nielsen OH, Jess T. Inflammatory Bowel Disease and Small Bowel Cancer Risk, Clinical Characteristics, and Histopathology: A Population-Based Study. Clin Gastroenterol Hepatol. 2017;15:1900-1907.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (2)] |
| 7. | Kaazan P, Slack T, Cooper C, Saad N, Martin N. Occult small bowel adenocarcinoma in a Crohn disease stricture. Intern Med J. 2022;52:1093-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Weber NK, Fletcher JG, Fidler JL, Barlow JM, Pruthi S, Loftus EV Jr, Pardi DS, Smyrk TC, Becker BD, Pasha SF, Bruining DH. Clinical characteristics and imaging features of small bowel adenocarcinomas in Crohn's disease. Abdom Imaging. 2015;40:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Korekawa K, Naito T, Fujishima F, Nagai H, Shimoyama Y, Moroi R, Shiga H, Kakuta Y, Masamune A. Small bowel cancer in a patient with Crohn's disease diagnosed preoperatively by double-balloon enteroscopy. Clin J Gastroenterol. 2023;16:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | de la Cruz Cuadrado C, López-Fernández J, García Plaza G, Montecino Romanini C, Hernández Hernández JR. Adenocarcinoma of the small intestine in Crohn's disease. Rev Esp Enferm Dig. 2021;113:798-799. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Pitra M, Karnos V. Small intestine adenocarcinoma associated with Crohn´s disease. Rozhl Chir. 2023;102:85-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Bryant RV, Prowse S, Nguyen-Hoang A, Lewis MC. Gastrointestinal: Adenocarcinoma in ileal Crohn's disease: A devil in disguise. J Gastroenterol Hepatol. 2017;32:1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Kodaira C, Osawa S, Mochizuki C, Sato Y, Nishino M, Yamada T, Takayanagi Y, Takagaki K, Sugimoto K, Kanaoka S, Furuta T, Ikuma M. A case of small bowel adenocarcinoma in a patient with Crohn's disease detected by PET/CT and double-balloon enteroscopy. World J Gastroenterol. 2009;15:1774-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Tirkes AT, Duerinckx AJ. Adenocarcinoma of the ileum in Crohn disease. Abdom Imaging. 2005;30:671-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Paparo F, Piccardo A, Clavarezza M, Piccazzo R, Bacigalupo L, Cevasco L, Marinaro E, Rollandi GA. Computed tomography enterography and 18F-FDG PET/CT features of primary signet ring cell carcinoma of the small bowel in a patient with Crohn's disease. Clin Imaging. 2013;37:794-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Fedele S, Delvecchio A, De Giorgi C, Duda L, Guglielmi A, Martines G. Small bowel adenocarcinoma in a patient with a 5-year history of untreated Crohn's disease: a case report. G Chir. 2018;39:383-387. [PubMed] |
| 17. | Tirath A, Christophi GP, Fraum TJ, Chatterjee D, Deepak P. Mucinous Small Bowel Adenocarcinoma Mimicking Change to Internal Penetrating Phenotype in Well-controlled Crohn's Disease. Am J Gastroenterol. 2018;113:1732-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Dsouza R, Varghese G, Korula DR, Dutta AK. Crohn's disease associated adenocarcinoma of ileocaecal region: a miscalculated approach. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Placé V, Hristova L, Dray X, Lavergne-Slove A, Boudiaf M, Soyer P. Ileal adenocarcinoma in Crohn's disease: magnetic resonance enterography features. Clin Imaging. 2012;36:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kronberger IE, Graziadei IW, Vogel W. Small bowel adenocarcinoma in Crohn's disease: a case report and review of literature. World J Gastroenterol. 2006;12:1317-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Tougeron D, Lefebure B, Savoye G, Tuech JJ, di Fiore F, Michel P. Small-bowel adenocarcinoma in patient with Crohn's disease: report of a series of three cases. Scand J Gastroenterol. 2008;43:1397-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Obara N, Koganei K, Tatsumi K, Futatsuki R, Kuroki H, Yamada K, Arai K, Sugita A, Hayashi H, Fukushima T. [A case of Crohn's disease complicated with simultaneous double cancers of the small bowel]. Nihon Shokakibyo Gakkai Zasshi. 2016;113:1901-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Nakao E, Tatsumi K, Kuroki H, Futatsuki R, Koganei K, Sugita A, Hayashi H, Yokoyama K. [Preoperative diagnosis of small intestinal cancer associated with Crohn's disease: a case report]. Nihon Shokakibyo Gakkai Zasshi. 2021;118:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Uzzan M, Assouline V, Chambenois E, Djabbari M, Arrive L, Charpy C, Luciani A, Sobhani I, Becq A, Beaugerie L, Svrcek M, Kirchgesner J. Focal loss of mural stratification as a radiological predictor for small bowel adenocarcinoma in Crohn's disease. Clin Res Hepatol Gastroenterol. 2023;47:102246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Li XH, Feng ST, Huang L, Zhoug J, Huang SY, Mao R, He Y, Liu Y, Xue HD, Zhao XS, Yan FH, Deng LP, Chen MH, Li ZP. [Expert Consensus on Imaging Examination and Reporting Standards for Inflammatory Bowel Disease in China]. Zhongguo Yanxing Changbing Zazhi. 2021;05:109-113. [DOI] [Full Text] |
| 26. | Maconi G, Nylund K, Ripolles T, Calabrese E, Dirks K, Dietrich CF, Hollerweger A, Sporea I, Saftoiu A, Maaser C, Hausken T, Higginson AP, Nürnberg D, Pallotta N, Romanini L, Serra C, Gilja OH. EFSUMB Recommendations and Clinical Guidelines for Intestinal Ultrasound (GIUS) in Inflammatory Bowel Diseases. Ultraschall Med. 2018;39:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 27. | Kucharzik T, Tielbeek J, Carter D, Taylor SA, Tolan D, Wilkens R, Bryant RV, Hoeffel C, De Kock I, Maaser C, Maconi G, Novak K, Rafaelsen SR, Scharitzer M, Spinelli A, Rimola J. ECCO-ESGAR Topical Review on Optimizing Reporting for Cross-Sectional Imaging in Inflammatory Bowel Disease. J Crohns Colitis. 2022;16:523-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 28. | Atkinson NS, Bryant RV, Dong Y, Maaser C, Kucharzik T, Maconi G, Asthana AK, Blaivas M, Goudie A, Gilja OH, Nolsøe C, Nürnberg D, Dietrich CF. WFUMB Position Paper. Learning Gastrointestinal Ultrasound: Theory and Practice. Ultrasound Med Biol. 2016;42:2732-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Bettenworth D, Bokemeyer A, Baker M, Mao R, Parker CE, Nguyen T, Ma C, Panés J, Rimola J, Fletcher JG, Jairath V, Feagan BG, Rieder F; Stenosis Therapy and Anti-Fibrotic Research (STAR) Consortium. Assessment of Crohn's disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019;68:1115-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 30. | Fields AC, Hu FY, Lu P, Irani J, Bleday R, Goldberg JE, Melnitchouk N. Small Bowel Adenocarcinoma: Is There a Difference in Survival for Crohn's Versus Sporadic Cases? J Crohns Colitis. 2020;14:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Shang LL, Tian CW, Yao LY, Cao Q. [Analysis of clinical characteristics of Crohn′s disease complicated with small intestinal cancer and literature review]. Zhonghua Yanxing Changbing Zazhi (Dianziban). 2024;08:77-81. |
| 32. | Grolleau C, Pote NM, Guedj NS, Zappa M, Theou-Anton N, Bouhnik Y, Panis Y, Cazals-Hatem DL. Small bowel adenocarcinoma complicating Crohn's disease: a single-centre experience emphasizing the importance of screening for dysplasia. Virchows Arch. 2017;471:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Argollo M, Gilardi D, Peyrin-Biroulet C, Chabot JF, Peyrin-Biroulet L, Danese S. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol Hepatol. 2019;4:643-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 34. | Wu MY, Niu WW, Zhang XL. [Research on Intestinal and Extraintestinal Tumors Associated with Inflammatory Bowel Disease]. Weichangbingxue He Ganbingxue Zazhi. 2021;30:259-263. |