Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.108205
Revised: May 12, 2025
Accepted: May 29, 2025
Published online: July 15, 2025
Processing time: 90 Days and 3.8 Hours
Definitive chemoradiotherapy is the standard treatment for unresectable, locally advanced esophageal cancer. However, radiotherapy (RT) often affects the immune system of patients. One of the possible mechanisms of lymphopenia after RT is that a large number of circulating lymphocytes in the systemic and pul
To determine the relationship between RT parameters, lymphocyte count and survival prognosis of esophageal cancer patients.
The clinical data of 112 patients with stage I-III ESCC who received definitive RT were analyzed retrospectively. The ALC values before RT, weekly during RT, and within 1 month after RT were determined. Logistic regression was used to evaluate the correlation between the parameters of radiation OARs and the lowest point of the ALC. Kaplan-Meier and Cox regression analyses were used to evaluate the relationship between the lowest point of the ALC and patient survival during RT.
The median value of the ALC before treatment was 1.57 × 109 cells/L, and 32 patients (28.6%) showed grade 4 ALC reduction during RT. The reduction in G4 ALC during RT was significantly associated with poor overall survival (OS) and progression-free survival. Multivariate analysis showed that stage III tumors (P = 0.003), high heart V10 (P = 0.046), high lung V5 (P = 0.048), and high lung V20 (P = 0.031) were associated with G4 ALC reduction during RT.
The reduction in G4 ALC is related to OS. Joint evaluation of the tumor stage and dose volume parameters has predictive value for G4 ALC reduction and OS.
Core Tip: Definitive chemoradiotherapy is the standard treatment for locally advanced esophageal cancer. However, radiotherapy (RT) often affects the immune system of patients. One of the possible mechanisms of lymphopenia after RT is that a large number of circulating lymphocytes in the systemic and pulmonary circulation will be killed by more sessions of low-dose radiation. This study showed that there was a relationship between radiation-induced lymphopenia (RIL) and the prognosis of esophageal squamous cell carcinoma patients, and identified several possible mechanisms of RT parameters in regulating the lymphocyte count during RT. The combination of tumor clinical stage and RT parameters has a certain predictive value for RIL and patient prognosis.
- Citation: Li SG, Liu Y, Zhang XY, Li YM, Shen WB, Zhu SC. Effects of radiotherapy on lymphocytes in patients with middle and lower esophageal cancer and its relationship with prognosis. World J Gastrointest Oncol 2025; 17(7): 108205
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/108205.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.108205
Esophageal cancer is the seventh most common malignant tumor worldwide, and is highly invasive[1]. The pathological type of esophageal cancer in China is mainly squamous cell carcinoma, accounting for more than 90% of the total number of cases[2]. Definitive chemoradiotherapy (CRT) is the standard treatment for unresectable, locally advanced esophageal cancer[3]. However, radiotherapy (RT) often affects the immune system of patients, especially lymphocytes which are very sensitive to radiation, and an extremely low radiation dose can lead to lymphocyte depletion. Therefore, radiation-induced lymphopenia (RIL) is a common adverse effect of RT[4]. As lymphocytes play a major role in anti-tumor immunity, many clinical studies have shown that there is a correlation between the absolute lymphocyte count (ALC) and the survival rate of cancer patients[5-7]. RIL has been reported to be negatively correlated with overall survival (OS) and progression-free survival (PFS) in a variety of cancers[8-10]. One of the possible mechanisms of lymphopenia after RT is that a large number of circulating lymphocytes in the systemic and pulmonary circulation will be killed by more sessions of low-dose radiation. The esophagus is an organ mainly located in the center of the chest. During the treatment of esophageal cancer with three-dimensional conformal RT or intensity-modulated RT (IMRT), most of the beams should pass through the lung tissue and heart; when middle and lower esophageal cancer is treated with RT, the heart and lung are exposed more. The primary objective of this study was to determine the relationship between reduction in the ALC during RT and survival prognosis of patients with middle and lower esophageal squamous cell carcinoma (ESCC), and to evaluate the impact of dose volume parameters of organs at risk (OARs) on the ALC.
Institutional review board approval was obtained before initiation of this study. We retrospectively analyzed the clinical data of ESCC patients admitted to the Fourth Hospital of Hebei Medical University from February 2016 to October 2019. All patients provided written informed consent to participate in the study before RT. The clinical TNM staging of all patients was carried out according to the 8th edition of the guidelines of the Union for International Cancer Control.
The eligibility criteria for this study were as follows: (1) ESCC confirmed by histopathology; (2) Stage I-III lesions determined by contrast-enhanced computed tomography (CT), endoscopic ultrasonography, or positron emission tomography-CT, and the lesions were located in the middle and lower segments of the esophagus (about 24-40 cm from the incisor); (3) Eastern Cooperative Oncology Group status score 0-2; and (4) patients who had completed the RT plan and had complete hematological data.
Exclusion criteria were: (1) Patients who had previously received radiation therapy; (2) Those suffering from a second primary tumor or hematological malignancy; and (3) Patients with serious infection or autoimmune disease.
All patients received IMRT and radiation was delivered by a 6MV high-energy linear accelerator. The involved-field irradiation (IFI) target volume, and gross tumor volume (GTV) which was the sum of the primary lesion (GTVp) and metastatic lymph node volume (GTVn) were determined. The lymph node clinical target volume (CTVn) included the GTVn with additional 5-mm expansion. The clinical target volume (CTV) was defined as the sum of CTVn and GTVp plus a longitudinal 3-cm margin along the esophagus and a 5-mm radial margin. The planning target volume (PTV) was defined as the CTV with 5-10 mm expansion. For elective nodal irradiation (ENI), the CTVn included both the clinically affected and uninvolved lymph node areas or stations (2/4/5/7/8/9 and 4/5/7/8/9/16/17, respectively) for middle and lower thoracic ESCC. The total dose was 54 to 64.8 Gy/27 to 32 fractions, 5 times/week.
Based on the CRT combined regimen, the patients were divided into the following subgroups: Simple RT, concurrent CRT and sequential CRT (did not receive concurrent RT but received induction chemotherapy or adjuvant chemotherapy). Among the patients receiving concurrent CRT, paclitaxel 50 mg/m2 and carboplatin (area under curve) area under the ROC curve = 2 was administered on the first day of each week for 4-6 consecutive weeks. For patients receiving sequential CRT, paclitaxel (175 mg/m2) was administered on the first day and cisplatin (25 mg/m2) on days 1-3, repeated every 4 weeks for a total of 4-6 cycles.
The ALC values were obtained within 1 week before treatment, weekly during RT, and 1 month after RT. In patients who lost the ALC data at the desired time point, the value closest to that date was used and only one patient had missing routine blood data during the first week of RT. Lymphopenia was defined as ALC < 1.0 × 109 cells/L. The degree of lymphopenia was graded according to the Common Terminology Criteria for Adverse Events Version 5.0. We collected the parameters for a dose volume histogram (DVH) of the lung, heart, aorta, sternum, and thoracic vertebra during RT. CT and esophagography were conducted to evaluate treatment response of the primary tumor following 4-6 weeks of treatment. Treatment response was defined according to the Response Evaluation Criteria in Solid Tumors Version 1.1.
Using our computer database, clinical data were recorded and the date of death due to any cause was calculated for each patient. Patients who survived were included in our survival analyses. Medical history, physical examination, laboratory examination, electrocardiography, abdominal ultrasonography, esophageal barium meal, and chest CT were performed every 3 months in the first 2 years and then every 6 months. Patients who were lost to follow-up were cut off at the last follow-up. The follow-up extended until the end of October 2021, with a median follow-up time of 42.6 months.
The Statistical Product and Service Solutions version 26.0 (SPSS 26.0, Chicago, IL, United States) software package was used for statistical analysis. Descriptive statistics summarized the baseline characteristics of patients. Dose volume parameters were dichotomized using the median. The primary end points of the study were OS and PFS, which were defined as the period from the beginning of treatment to the occurrence of corresponding events or the last follow-up. The survival curve was drawn using the Kaplan-Meier method, and the survival results of different grades of ALC reduction during RT were compared using the Log-rank test. Logistic regression was used to analyze the related RT factors of G4 ALC reduction. The Cox proportional hazard model was used to conduct multiple factor analysis to determine the risk factors. P < 0.05 indicated that the difference was statistically significant.
Five patients were lost to follow-up in this study, with a follow-up rate of 95.5%. A total of 112 patients with middle and lower ESCC were included in the study. The median age was 67 years (range, 40-83 years), 83 patients were male (74.1%) and 63 patients (72%) were in stage II. The median primary tumor length was 5.5 cm (range, 2-11 cm). A total of 71 cases (63.4%) received RT combined with chemotherapy, including 44 cases (39.3%) treated with concurrent CRT and 27 cases (24.1%) treated with sequential CRT. All patients were treated with IMRT. The median follow-up time was 42.6 months. Table 1 shows patient baseline characteristics, tumor characteristics, and the treatment plan.
| Characteristics | All patients, n = 112 | G1-G3 ALC nadir, n = 80 | G4 ALC nadir, n = 32 | P value |
| Gender | 0.041 | |||
| Male | 83 (74.1) | 55 (68.8) | 28 (87.5) | |
| Female | 29 (25.9) | 25 (31.3) | 4 (12.5) | |
| Age, years | 0.064 | |||
| ≤ 67 | 58 (51.8) | 37 (46.3) | 21 (65.6) | |
| > 67 | 54 (48.2) | 43 (53.8) | 11 (34.4) | |
| BMI | 0.501 | |||
| < 24 kg/m2 | 68 (60.7) | 47 (58.8) | 21 (65.6) | |
| ≥ 24 | 44 (39.3) | 33 (41.3) | 11 (34.4) | |
| Tumor length (cm) | 0.509 | |||
| ≤ 5 | 51 (45.5) | 38 (47.5) | 13 (40.6) | |
| > 5 | 61 (54.5) | 42 (52.5) | 19 (59.4) | |
| T stage | 0.509 | |||
| T1 + T2 | 51 (45.5) | 38 (47.5) | 12 (37.5) | |
| T3 + T4 | 61 (54.5) | 42 (52.5) | 20 (62.5) | |
| N stage | 0.017 | |||
| N0 | 17 (15.2) | 14 (17.5) | 3 (9.4) | |
| N1 | 67 (59.8) | 52 (65.0) | 15 (46.9) | |
| N2 | 28 (25.0) | 14 (17.5) | 14 (43.8) | |
| Clinical stage | 0.003 | |||
| I + II | 73 (65.2) | 59 (73.8) | 14 (43.8) | |
| III | 39 (34.8) | 21 (26.3) | 18 (56.3) | |
| RT dose | 0.336 | |||
| ≤ 60 Gy | 62 (55.4) | 42 (52.5) | 20 (62.5) | |
| > 60 Gy | 50 (44.6) | 38 (47.5) | 12 (37.5) | |
| Treatment regimen | 0.244 | |||
| RT alone | 41 (36.6) | 33 (41.3) | 8 (25.0) | |
| Sequential CRT | 27 (24.1) | 17 (21.3) | 10 (31.3) | |
| Concurrent CRT | 44 (39.3) | 30 (37.5) | 14 (43.8) | |
| RT technique | 0.321 | |||
| ENI | 41 (36.6) | 27 (33.8) | 14 (43.8) | |
| IFI | 71 (63.4) | 53 (66.3) | 18 (56.3) |
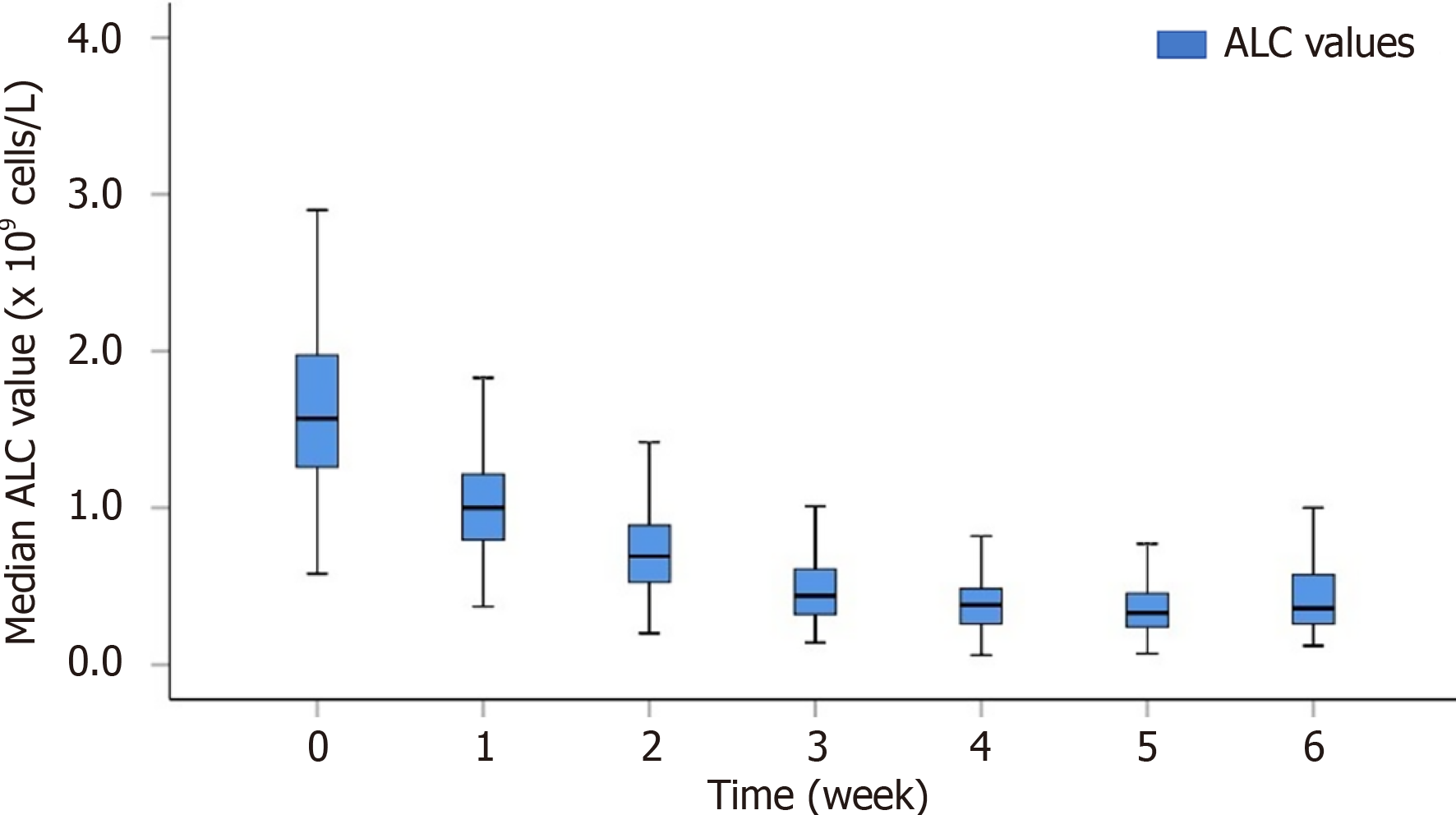
In order to understand the change in the ALC during RT more intuitively, we plotted the trend of the ALC value over time (Figure 1). The median ALC value before treatment was 1.57 × 109 cells/L. The ALC gradually decreased during RT, and most patients reached the plateau stage near the end of RT. The median ALC value during RT decreased to 1.00 × 109 cells/L, 0.69 × 109 cells/L, 0.44 × 109 cells/L, 0.38 × 109 cells/L, 0.33 × 109 cells/L, and 0.36 × 109 cells/L from the 1st week to the 6th week, respectively.
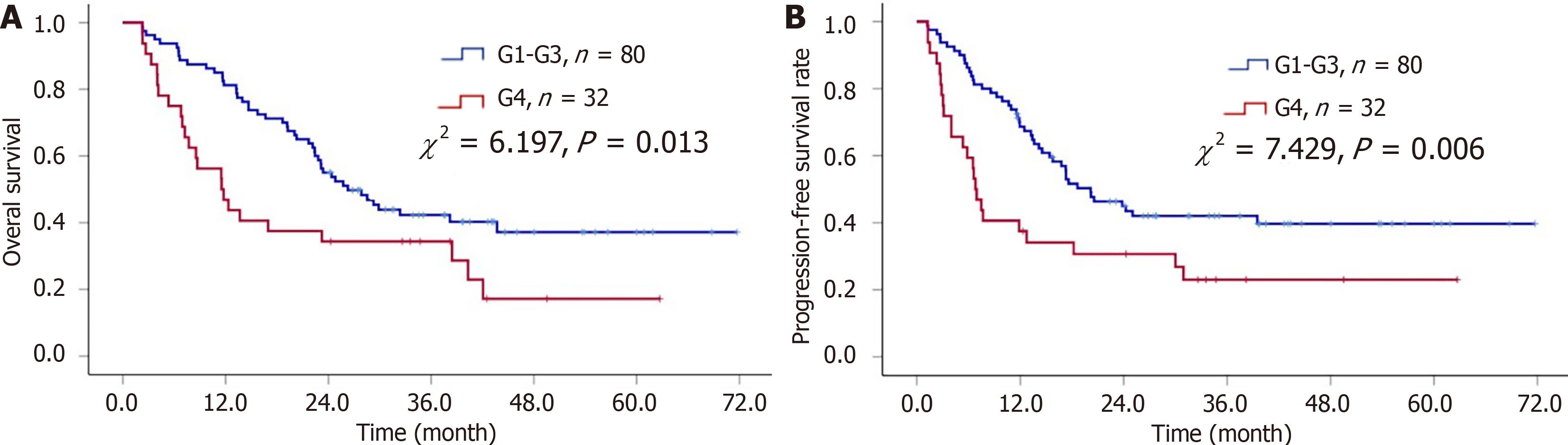
At a median follow-up of 42.6 months, 70 patients (62.5%) had died. G4 Lymphocytopenia was associated with a shorter median OS (11.5 months vs 26.3 months) (P = 0.013; Figure 2A). Multivariate analysis showed that the length of the primary esophageal tumor (HR, 1.88; 95%CI: 1.14-3.11; P= 0.015), T stage (HR, 1.88; 95%CI: 1.14-3.11; P = 0.015), and G4 ALC reduction during RT (HR, 1.97; 95%CI: 1.22-3.26; P = 0.008) were independent prognostic factors for poor OS.
During the follow-up period, a total of 70 patients (62.5%) had local recurrence or distant metastasis, with a median PFS of 17.3 months. Kaplan-Meier analysis showed that G4 Lymphocytopenia was associated with a shorter median PFS (6.8 months vs 20.2 months) (P = 0.006; Figure 2B).
Univariate analysis showed that the length of the primary esophageal tumor, T stage, N stage, clinical stage, treatment regimen, RT technique, and minimum ALC grade were significantly correlated with PFS (all P < 0.005, Table 2). Multivariate analysis showed that stage III tumors (HR, 2.24; 95%CI: 1.35-3.71; P = 0.002), ENI irradiation (HR, 2.34; 95%CI: 1.32-4.14; P = 0.004), and G4 ALC reduction during RT (HR, 1.87; 95%CI: 1.09-3.20; P = 0.024) were significantly associated with poor PFS (Table 2).
| Characteristics | OS | PFS | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| m-OS (95%CI) | P value | HR (95%CI) | P value | m-PFS (95%CI) | P value | HR (95%CI) | P value | |
| Gender | 0.810 | 0.907 | ||||||
| Male | 23.2 (14.8-31.7) | 18.2 (10.4-26.0) | ||||||
| Female | 23.4 (20.0-26.8) | 16.8 (11.9-21.7) | ||||||
| Age | 0.947 | 0.764 | ||||||
| ≤ 67 years | 23.2 (12.1-34.4) | 13.4 (6.4-20.4) | ||||||
| > 67 years | 23.1 (16.1-30.2) | 18.6 (12.8-24.5) | ||||||
| BMI | 0.649 | 0.742 | ||||||
| < 24 kg/m2 | 23.1 (17.0-29.2) | 15.8 (9.4-22.2) | ||||||
| ≥ 24 | 23.2 (15.7-30.7) | 17.3 (11.0-23.6) | ||||||
| Tumor length | 0.002 | 1.88 (1.14-3.12) | 0.014 | 0.012 | 1.08 (0.13-8.76) | 0.942 | ||
| ≤ 5 cm | 42.1 (27.3-56.8) | 25.0 (1.0-49.1) | ||||||
| > 5 cm | 17.0 (7.7-26.2) | 11.9 (9.0-14.8) | ||||||
| T stage | 0.002 | 1.88 (1.14-3.12) | 0.014 | 0.012 | 1.62 (0.19-13.84) | 0.66 | ||
| T1-T2 | 42.1 (27.3-56.8) | 25.0 (1.0-49.1) | ||||||
| T3-T4 | 17.0 (7.7-26.2) | 11.9 (9.0-14.8) | ||||||
| N stage | 0.000 | 0.55 (0.34-1.00) | 0.051 | 0.000 | 0.38 (0.15-1.01) | 0.053 | ||
| N0 | - | - | ||||||
| N1 | 24.4 (19.1-29.7) | 17.3 (10.9-23.7) | ||||||
| N2 | 12.3 (10.4-14.3) | 6.6 (4.6-8.7) | ||||||
| TNM stage | 0.000 | 0.44 (0.19-1.03) | 0.058 | 0.000 | 2.24 (1.35-3.71) | 0.002 | ||
| I + II | 32.4 (18.7-46.0) | 25.0 (1.2-48.9) | ||||||
| III | 11.8 (7.5-16.0) | 6.6 (5.6-7.6) | ||||||
| RT dose | 0.110 | 0.540 | ||||||
| ≤ 60 Gy | 22.8 (15.8-29.8) | 13.4 (8.4-18.4) | ||||||
| > 60 Gy | 29.3 (12.5-46.0) | 18.6 (14.1-23.1) | ||||||
| Treatment regimen | 0.042 | 1.59 (0.88-2.86) | 0.093 | 0.033 | 1.83 (0.19-1.03) | 0.058 | ||
| RT alone | 22.4 (17.5-27.3) | 14.2 (7.3-21.0) | ||||||
| Sequential CRT | 14.0 (9.7-18.2) | 9.9 (0.8-19.0) | ||||||
| Concurrent CRT | 38.4 (16.5-60.4) | - | ||||||
| RT technique | 0.028 | 0.77 (0.44-1.36) | 0.364 | 0.001 | 2.34 (1.32-4.14) | 0.004 | ||
| ENI | 42.1 (25.1-59.0) | - | ||||||
| IFI | 22.4 (16.5-28.3) | 12.7 (9.7-15.7) | ||||||
| ALC nadir | 0.013 | 1.98 (1.20-3.28) | 0.008 | 0.006 | 1.87 (1.09-3.20) | 0.023 | ||
| G1-G3 | 26.3 (19.8-32.7) | 20.2 (13.3-27.0) | ||||||
| G4 | 11.5 (6.5-16.6) | 6.8 (5.5-8.1) | ||||||
| ALC nadir time | 0.041 | 1.23 (0.73-2.06) | 0.443 | 0.360 | ||||
| 1-4 week | 16.7 (6.8-26.5) | 14.2 (6.8-21.5) | ||||||
| 5-6 week | 29.9 (14.5-45.2) | 17.3 (8.3-26.3) | ||||||
A total of 32 patients (28.6%) had G4 ALC reductions during RT in this study. We found that patients with G4 ALC reductions showed no significant differences in age, esophageal tumor length, treatment regimen, and RT technique. Multivariate analysis showed that male patients (P = 0.045) and patients with stage III tumors (P = 0.003), higher heart V10 (P = 0.046), and higher lung V5 (P = 0.048) and lung V20 (P = 0.031) were more likely to have G4 ALC reductions during RT (Table 3).
| Characteristics | Univariate | Multivariate | ||
| OR | P value | OR | P value | |
| Gender (male vs female) | 3.182 | 0.048 | 4.928 | 0.045 |
| Age (≤ 67 years vs > 67 years) | 0.451 | 0.067 | ||
| BMI (< 24 vs ≥ 24 kg/m2) | 0.746 | 0.502 | ||
| Tumor length (≤ 5 cm vs > 5 cm) | 1.322 | 0.510 | ||
| T stage (1+2 vs 3+4) | 1.322 | 0.510 | ||
| N stage (0 vs 1 vs 2) | 2.549 | 0.011 | 0.683 | 0.176 |
| TNM stage (I+II vs III) | 3.612 | 0.003 | 6.754 | 0.003 |
| Treatment regimen RT alone vs SCRT vs CCRT | 1.351 | 0.219 | ||
| RT technique ENI vs IFI | 0.655 | 0.322 | ||
| Dosimetric parameters of OARs | ||||
| Heart V5 (≤ 93% vs > 93%) | 3.458 | 0.006 | 0.518 | 0.228 |
| Heart V10 (≤ 86% vs > 86%) | 3.833 | 0.003 | 12.808 | 0.046 |
| Aorta V5 (≤ 85% vs > 85%) | 2.829 | 0.019 | 1.054 | 0.955 |
| Aorta V30 (≤ 78% vs > 78%) | 2.374 | 0.044 | 1.288 | 0.713 |
| Thoracic vertebrae Dmean (≤ 32 Gy vs > 32 Gy) | 4.259 | 0.001 | 0.470 | 0.339 |
| Thoracic vertebrae V5 (≤ 69% vs > 69%) | 3.300 | 0.007 | 0.803 | 0.822 |
| Thoracic vertebrae V10 (≤ 67% vs > 67%) | 3.133 | 0.010 | 1.938 | 0.462 |
| Lung Dmean (≤ 13 Gy vs >13 Gy) | 2.719 | 0.022 | 0.873 | 0.092 |
| Lung V5 (≤ 58% vs > 58%) | 3.300 | 0.007 | 5.324 | 0.048 |
| Lung V10 (≤ 42% vs > 42%) | 3.640 | 0.004 | 0.646 | 0.187 |
| Lung V20 (≤ 25% vs > 25%) | 2.864 | 0.016 | 7.631 | 0.031 |
Subgroup influencing factor analysis based on chemotherapy status showed that patients with higher heart V10 (P = 0.037) and lung V5 (P = 0.039) who received RT alone were more likely to experience G4 grade ALC reduction. Patients with higher levels of heart V5 (P = 0.039) and lung V5 (P = 0.045) who received sequential CRT were more likely to experience G4 grade ALC reduction (Table 4).
| Characteristics | RT alone | Sequential CRT | Concurrent CRT | |||
| Univariate P | Multivariate P | Univariate P | Multivariate P | Univariate P | Multivariate P | |
| Gender (male vs female) | 0.952 | 0.097 | 0.092 | |||
| Age (≤ 67 years vs > 67 years) | 0.816 | 0.590 | 0.226 | |||
| BMI (< 24 vs ≥ 24 kg/m2) | 0.292 | 0.384 | 0.541 | |||
| Tumor length (≤ 5 cm vs > 5 cm) | 0.800 | 0.081 | 0.679 | |||
| T stage (1+2 vs 3+4) | 0.800 | 0.081 | 0.976 | |||
| N stage (0 vs 1 vs 2) | 0.358 | 0.146 | 0.355 | |||
| TNM stage (I+II vs III) | 0.099 | 0.212 | 0.050 | |||
| RT technique ENI vs IFI | 0.964 | 0.656 | 0.533 | |||
| Dosimetric parameters of OARs | ||||||
| Heart V5 (≤ 93% vs > 93%) | 0.037 | 0.051 | 0.013 | 0.039 | 0.015 | 0.048 |
| Heart V10 (≤ 86% vs > 86%) | 0.058 | 0.037 | 0.001 | 0.509 | 0.026 | 0.033 |
| Aorta V5 (≤ 85% vs > 85%) | 0.817 | 0.160 | 0.032 | 0.926 | ||
| Aorta V30 (≤ 78% vs > 78%) | 0.380 | 0.384 | 0.195 | |||
| Thoracic vertebrae Dmean (≤ 32 Gy vs > 32 Gy) | 0.129 | 0.093 | 0.032 | 0.612 | ||
| Thoracic vertebrae V5 (≤ 69% vs > 69%) | 0.380 | 0.401 | 0.018 | 0.485 | ||
| Thoracic vertebrae V10 (≤ 67% vs > 67%) | 0.380 | 0.401 | 0.018 | 0.895 | ||
| Lung Dmean (≤ 13 Gy vs > 13 Gy) | 0.022 | 0.483 | 0.160 | 0.276 | ||
| Lung V5 (≤ 58% vs > 58%) | 0.019 | 0.039 | 0. 016 | 0.045 | 0.013 | 0.043 |
| Lung V10 (≤ 42% vs > 42%) | 0.012 | 0.053 | 0.026 | 0.084 | 0.016 | 0.135 |
| Lung V20 (≤ 25% vs > 25%) | 0.029 | 0.064 | 0.039 | 0.054 | 0.033 | 0.012 |
In order to determine the effect of dosimetric parameters of OARs on the survival of patients, we evaluated the effect of 5 to 30 Gy volume percentage received by the lung, heart, aorta, sternum and thoracic vertebrae on the survival of patients with esophageal cancer (lung V5-V20, heart V5-V20, aorta V5-V30, sternum V5-V20, and thoracic vertebrae V5-V20). No statistically significant parameters were identified.
In order to identify the parameters that accurately predict the prognosis of ESCC patients, we combined tumor TNM staging with heart V10, lung V5, and lung V20. We defined new indicators coTNM-heart V10, coTNM-lung V5, and coTNM-lung V20, and we scored the patients for risk as follows: (1) Patients with stage I + II and lower heart V10, lung V5, and lung V20 were rated as having a score of 0 points; (2) Patients with stage I + II and higher heart V10, lung V5, and lung V20 or patients with stage III and lower heart V10, lung V5, and lung V20 were rated as having a score of 1 point; and (3) Patients with stage III and higher heart V10, lung V5, and lung V20 were rated as having a score of 2 points. The χ2 test was used to compare the proportion of G4 ALC reduction in patients with different scores in coTNM-heart V10, coTNM-lung V5, and coTNM-lung V20. The results showed that with an increased score, the proportion of patients with G4 ALC reduction increased correspondingly, and the differences between groups were statistically significant (Table 5). χ2 test results showed that the survival of patients in the group with score 2 of coTNM-lung V5 and coTNM-lung V20 was worse than that of patients in the groups with score 0 and 1, and the difference was statistically significant (Table 6).
| No. | G1-G3 80 (%) | G4 32 (%) | χ2 | P value | |
| CoTNM-heartV10 | 16.697 | 0.000 | |||
| 0 score | 40 | 37 (46.3) | 3 (9.4) | ||
| 1 score | 50 | 33 (41.3) | 17 (53.1) | ||
| 2 score | 22 | 10 (12.5) | 12 (27.5) | ||
| CoTNM-lungV5 | 10.997 | 0.004 | |||
| 0 score | 40 | 35 (43.8) | 5 (15.6) | ||
| 1 score | 33 | 24 (30.0) | 9 (28.1) | ||
| 2 score | 39 | 21 (26.3) | 18 (56.3) | ||
| CoTNM-lungV20 | 16.923 | 0.000 | |||
| 0 score | 42 | 35 (43.8) | 7 (21.9) | ||
| 1 score | 48 | 37 (46.3) | 11 (34.4) | ||
| 2 score | 22 | 8 (10.0) | 14 (43.8) |
| Characteristics | N | Median OS | OS (%) | χ2 | P value | Median PFS | PFS (%) | χ2 | P value | ||||
| 1 year | 2 years | 3 years | 1 year | 2 years | 3 years | ||||||||
| CoTNM- heartV10 | 5.446 | 0.066 | 5.418 | 0.067 | |||||||||
| 0 score | 40 | 26.3 | 87.5 | 52.5 | 44.4 | 20.2 | 70.0 | 46.6 | 43.7 | ||||
| 1 score | 50 | 24.8 | 68.0 | 54.0 | 42.5 | 17.3 | 61.7 | 40.5 | 37.8 | ||||
| 2 score | 22 | 11.8 | 50.0 | 31.8 | 26.5 | 6.6 | 36.4 | 31.2 | 20.8 | ||||
| CoTNM-lungV5 | 17.499 | 0.000 | 17.440 | 0.000 | |||||||||
| 0 score | 40 | - | 82.5 | 62.5 | 54.6 | 20.2 | 75.0 | 48.5 | 45.9 | ||||
| 1 score | 33 | 29.9 | 84.8 | 57.6 | 44.9 | - | 72.5 | 53.2 | 49.1 | ||||
| 2 score | 39 | 11.8 | 48.7 | 28.2 | 21.2 | 6.6 | 33.3 | 22.2 | 15.2 | ||||
| CoTNM-lungV20 | 14.068 | 0.001 | 11.122 | 0.004 | |||||||||
| 0 score | 42 | 28.5 | 85.7 | 57.1 | 49.0 | 17.6 | 69.0 | 47.6 | 45.0 | ||||
| 1 score | 48 | 24.4 | 72.9 | 52.1 | 40.8 | 20.2 | 64.3 | 43.9 | 39.1 | ||||
| 2 score | 22 | 7.7 | 40.9 | 27.3 | 21.8 | 5.8 | 31.8 | 21.2 | 14.1 | ||||
In this study, we observed that the lymphocytes in ESCC patients were significantly decreased after RT, and G4 Lymphocyte reduction during RT was associated with poor OS and PFS. After adjusting for confounding factors, G4 lymphopenia still had significant prognostic value during RT. This study found that G4 lymphopenia was related to tumor stage, heart V10, lung V5 and lung V20. These results were closely related to clinical treatment. With the development of modern RT equipment and technology, RT parameters are adjusted to minimize the immunosuppressive effect caused by RIL, and then the RT effect on ESCC and other malignant tumors is optimized.
This study showed that the ALC value is a prognostic factor for ESCC patients after definitive RT. Lymphocytes play a key role in promoting a systemic anti-tumor immune response and are the main effector cells. Lymphopenia is the main manifestation of immunosuppression. Patients who are immunosuppressed may be more prone to tumor recurrence or metastasis, leading to poorer survival outcomes. A number of retrospective studies have found that treatment-related lymphopenia is closely associated with adverse outcomes in a variety of cancers, including glioblastoma, lung cancer, pancreatic cancer, cervical cancer, and esophageal cancer[6,9-12]. Deng et al[13] conducted a retrospective analysis of 755 patients with stage I-III esophageal cancer who received concurrent CRT with or without surgery. They found that patients with the lowest value of lymphocytes at grade G4 accounted for 38.9% of the study population (294/755). The 5-year OS rate in patients with G4 lymphopenia was 35.4%, and the 5-year OS rate in patients with G0-3 Lymphopenia was 51.8% (HR: 1.46, P < 0.001). The 5-year PFS rate in patients with G4 lymphopenia was 30.1%, and the 5-year PFS rate in patients with G0-3 Lymphopenia was 40.7% (HR: 1.34, P = 0.002). Xu et al[14] retrospectively analyzed the clinical characteristics of 436 patients with ESCC who received concurrent CRT, and measured the ALC values before, during, and 1 month after CRT. Of these patients, 103 had G4 lymphopenia during CRT. At the same time, they found that patients with G4 lymphopenia had a poor prognosis. The data obtained in this study showed that the OS and PFS of patients with G1-G3 Lymphopenia during RT were better than those of patients with G4 lymphopenia. This is consistent with the previously reported results. Given the radiosensitivity of lymphocytes, changes in the lymphocyte count may be a prognostic factor worthy of consideration.
Through multivariate analysis, we determined several parameters that can significantly predict G4 lymphopenia during RT. Lymphocytes are extremely sensitive to radiation and are often depleted by RT, with 50% of the lethal dose at only 1-2 Gy[15]. However, the mechanism of RIL is still unclear. Radiation of lymphoid organs, including spleen and lymph nodes may cause RIL. This seems to be due to greater exposure of lymphocytes at disease sites and larger radiation portals. Spleen V5 and mean spleen dose during RT for hepatocellular carcinoma or pancreatic cancer were associated with the lowest value of the ALC[16,17]. Xu et al[14] analyzed the relationship between DVH parameters and lymphocyte nadir in 436 ESCC patients, and observed that G4 lymphopenia was associated with a large PTV, and higher lung volume and heart volume. Xie et al[18] analyzed the influencing factors leading to severe RIL in 178 patients with non-small cell lung cancer during RT, and found that higher lung V5 and average lung dose were also independently associated with the incidence of severe RIL. In addition, the results of the RTOG0617 study showed that a higher cardiac dose and larger PTV were important predictors of poor OS[19]. As the anatomical position of the esophagus is close to the heart, lungs, and large blood vessels, lymphocytes may receive more radiation when passing through these organs in the irradiation field, which may aggravate the lymphopenia caused by RT. The results of this study support the notion that heart V10, lung V5 and lung V20 are important prognostic factors for G4 lymphopenia. This shows that RIL is mainly the result of frequent and low-dose radiation-induced damage to the circulating lymphocytes. In addition, we found that chemotherapy combined with RT had no significant effect on lymphocytes.
This study combined the traditional prognostic factors of esophageal cancer, tumor TNM stage, with the RT parameters that had a significant impact on the prognosis, heart V10, lung V5, and lung V20, and screened out new sensitive indicators, which can not only accurately predict the occurrence of G4 lymphopenia in patients with esophageal cancer during RT, but also have a certain predictive value for the survival and prognosis of patients with esophageal cancer. In future RT planning for esophageal cancer, especially for patients with late-stage middle and lower esophageal cancer, an attempt should be made to reduce the low-dose radiation volume to the heart and lung and reduce the risk of RIL, to improve survival benefits for esophageal cancer patients.
In addition to inducing lymphocytopenia to produce immunosuppressive effects, RT can also remodel the tumor microenvironment, thereby promoting the recruitment of immune cells in tumor tissues and enhancing the recognition and elimination of tumor cells by immune cells[20]. Studies have shown that programmed cell death ligand 1 expression is upregulated in the tumor microenvironment after RT; thus, it is essential to eliminate the immunosuppressive effect of RT to achieve the optimal antitumor effect[21]. However, when developing RT plans in clinical practice, we often ignore the influence of RT parameters on lymphocytes. The range of RT target areas for esophageal cancer is controversial; i.e., ENI and IFI. The ENI target range includes primary tumors and clinically unaffected regional lymph nodes with potential metastatic risk of cancer cells[22,23]. The results of this study showed that there was no significant difference in the lymphocyte count between the ENI and IFI groups, which may be related to the small number of cases. More cases are needed to further verify whether that IFI reduces the risk of RIL by reducing the target irradiation volume.
Some other groups have proposed special radiation techniques to preserve lymphocytes in other solid tumors. These techniques involve the use of stereotactic body RT to minimize the number of fractions and the PTV, to reduce the low-dose bath effect to circulating lymphocytes[24]. The use of proton therapy instead of photons has also been suggested[25]. As there is minimal exit dose beyond the Bragg peak, the total integral dose is reduced in proton therapy. This has been shown in the MD Anderson patient cohort, in which proton therapy was a predictor of higher lymphocyte nadir (compared with IMRT, OR = 4.18; P < 0.001). However, neither of these was standard for RT in esophageal cancer. Drug therapy in preserving circulating lymphocytes is also an attractive option. However, there is no well- established drug therapy at the time of this writing.
In this study, 23 (20.5%) and 12 (10.7%) patients had grade 2 and grade 3 acute radiation-induced esophagitis, respectively. In addition, 13 (11.6%) and 3 (2.7%) cases had grade 2 and grade 3 acute radiation pneumonitis, respectively. None of the patients experienced severe esophageal bleeding or perforation.
This study has some limitations. This was a single institution retrospective study, and the number of cases was relatively small; thus, there may be bias in case selection and information collection. Although one patient's complete blood count data were unavailable during week 1 of RT, this had no substantial impact on the aggregated data analysis. Adjuvant drugs or infection during treatment may have affected the lymphocyte count and could be a potential confounding variable. Therefore, subsequent studies should expand the sample size through multicenter investigations and extend the inclusion period. Future research should focus on exploring the molecular mechanisms underlying RIL, while also comparing the differential impacts of various RT techniques (photon vs proton/heavy ion) on lymphocyte depletion.
Despite these limitations, this study showed that there was a close relationship between RIL and the prognosis of ESCC patients, and identified several possible mechanisms of RT parameters in regulating the lymphocyte count during RT. The combination of tumor clinical stage and RT parameters has a certain predictive value for RIL and patient prognosis. It can provide a reference for patients with subsequent ESCC to optimize the RT plan, maintain the integrity of the immune system, and improve the prognosis.
This study identified a close relationship between RIL and the prognosis of ESCC patients, and determined possible mechanisms of RT parameters in regulating the lymphocyte count during RT. The combination of tumor clinical stage and RT parameters has a certain predictive value for RIL and patient prognosis. It can provide a reference for patients with subsequent ESCC to optimize the RT plan, maintain the integrity of the immune system, and improve the prognosis.
| 1. | Global Burden of Disease 2019 Cancer Collaboration, Kocarnik JM, Compton K, Dean FE, Fu W, Gaw BL, Harvey JD, Henrikson HJ, Lu D, Pennini A, Xu R, Ababneh E, Abbasi-Kangevari M, Abbastabar H, Abd-Elsalam SM, Abdoli A, Abedi A, Abidi H, Abolhassani H, Adedeji IA, Adnani QES, Advani SM, Afzal MS, Aghaali M, Ahinkorah BO, Ahmad S, Ahmad T, Ahmadi A, Ahmadi S, Ahmed Rashid T, Ahmed Salih Y, Akalu GT, Aklilu A, Akram T, Akunna CJ, Al Hamad H, Alahdab F, Al-Aly Z, Ali S, Alimohamadi Y, Alipour V, Aljunid SM, Alkhayyat M, Almasi-Hashiani A, Almasri NA, Al-Maweri SAA, Almustanyir S, Alonso N, Alvis-Guzman N, Amu H, Anbesu EW, Ancuceanu R, Ansari F, Ansari-Moghaddam A, Antwi MH, Anvari D, Anyasodor AE, Aqeel M, Arabloo J, Arab-Zozani M, Aremu O, Ariffin H, Aripov T, Arshad M, Artaman A, Arulappan J, Asemi Z, Asghari Jafarabadi M, Ashraf T, Atorkey P, Aujayeb A, Ausloos M, Awedew AF, Ayala Quintanilla BP, Ayenew T, Azab MA, Azadnajafabad S, Azari Jafari A, Azarian G, Azzam AY, Badiye AD, Bahadory S, Baig AA, Baker JL, Balakrishnan S, Banach M, Bärnighausen TW, Barone-Adesi F, Barra F, Barrow A, Behzadifar M, Belgaumi UI, Bezabhe WMM, Bezabih YM, Bhagat DS, Bhagavathula AS, Bhardwaj N, Bhardwaj P, Bhaskar S, Bhattacharyya K, Bhojaraja VS, Bibi S, Bijani A, Biondi A, Bisignano C, Bjørge T, Bleyer A, Blyuss O, Bolarinwa OA, Bolla SR, Braithwaite D, Brar A, Brenner H, Bustamante-Teixeira MT, Butt NS, Butt ZA, Caetano Dos Santos FL, Cao Y, Carreras G, Catalá-López F, Cembranel F, Cerin E, Cernigliaro A, Chakinala RC, Chattu SK, Chattu VK, Chaturvedi P, Chimed-Ochir O, Cho DY, Christopher DJ, Chu DT, Chung MT, Conde J, Cortés S, Cortesi PA, Costa VM, Cunha AR, Dadras O, Dagnew AB, Dahlawi SMA, Dai X, Dandona L, Dandona R, Darwesh AM, das Neves J, De la Hoz FP, Demis AB, Denova-Gutiérrez E, Dhamnetiya D, Dhimal ML, Dhimal M, Dianatinasab M, Diaz D, Djalalinia S, Do HP, Doaei S, Dorostkar F, Dos Santos Figueiredo FW, Driscoll TR, Ebrahimi H, Eftekharzadeh S, El Tantawi M, El-Abid H, Elbarazi I, Elhabashy HR, Elhadi M, El-Jaafary SI, Eshrati B, Eskandarieh S, Esmaeilzadeh F, Etemadi A, Ezzikouri S, Faisaluddin M, Faraon EJA, Fares J, Farzadfar F, Feroze AH, Ferrero S, Ferro Desideri L, Filip I, Fischer F, Fisher JL, Foroutan M, Fukumoto T, Gaal PA, Gad MM, Gadanya MA, Gallus S, Gaspar Fonseca M, Getachew Obsa A, Ghafourifard M, Ghashghaee A, Ghith N, Gholamalizadeh M, Gilani SA, Ginindza TG, Gizaw ATT, Glasbey JC, Golechha M, Goleij P, Gomez RS, Gopalani SV, Gorini G, Goudarzi H, Grosso G, Gubari MIM, Guerra MR, Guha A, Gunasekera DS, Gupta B, Gupta VB, Gupta VK, Gutiérrez RA, Hafezi-Nejad N, Haider MR, Haj-Mirzaian A, Halwani R, Hamadeh RR, Hameed S, Hamidi S, Hanif A, Haque S, Harlianto NI, Haro JM, Hasaballah AI, Hassanipour S, Hay RJ, Hay SI, Hayat K, Heidari G, Heidari M, Herrera-Serna BY, Herteliu C, Hezam K, Holla R, Hossain MM, Hossain MBH, Hosseini MS, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hsairi M, Huang J, Hugo FN, Hussain R, Hussein NR, Hwang BF, Iavicoli I, Ibitoye SE, Ida F, Ikuta KS, Ilesanmi OS, Ilic IM, Ilic MD, Irham LM, Islam JY, Islam RM, Islam SMS, Ismail NE, Isola G, Iwagami M, Jacob L, Jain V, Jakovljevic MB, Javaheri T, Jayaram S, Jazayeri SB, Jha RP, Jonas JB, Joo T, Joseph N, Joukar F, Jürisson M, Kabir A, Kahrizi D, Kalankesh LR, Kalhor R, Kaliyadan F, Kalkonde Y, Kamath A, Kameran Al-Salihi N, Kandel H, Kapoor N, Karch A, Kasa AS, Katikireddi SV, Kauppila JH, Kavetskyy T, Kebede SA, Keshavarz P, Keykhaei M, Khader YS, Khalilov R, Khan G, Khan M, Khan MN, Khan MAB, Khang YH, Khater AM, Khayamzadeh M, Kim GR, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Kopec JA, Koteeswaran R, Koul PA, Koulmane Laxminarayana SL, Koyanagi A, Kucuk Bicer B, Kugbey N, Kumar GA, Kumar N, Kumar N, Kurmi OP, Kutluk T, La Vecchia C, Lami FH, Landires I, Lauriola P, Lee SW, Lee SWH, Lee WC, Lee YH, Leigh J, Leong E, Li J, Li MC, Liu X, Loureiro JA, Lunevicius R, Magdy Abd El Razek M, Majeed A, Makki A, Male S, Malik AA, Mansournia MA, Martini S, Masoumi SZ, Mathur P, McKee M, Mehrotra R, Mendoza W, Menezes RG, Mengesha EW, Mesregah MK, Mestrovic T, Miao Jonasson J, Miazgowski B, Miazgowski T, Michalek IM, Miller TR, Mirzaei H, Mirzaei HR, Misra S, Mithra P, Moghadaszadeh M, Mohammad KA, Mohammad Y, Mohammadi M, Mohammadi SM, Mohammadian-Hafshejani A, Mohammed S, Moka N, Mokdad AH, Molokhia M, Monasta L, Moni MA, Moosavi MA, Moradi Y, Moraga P, Morgado-da-Costa J, Morrison SD, Mosapour A, Mubarik S, Mwanri L, Nagarajan AJ, Nagaraju SP, Nagata C, Naimzada MD, Nangia V, Naqvi AA, Narasimha Swamy S, Ndejjo R, Nduaguba SO, Negoi I, Negru SM, Neupane Kandel S, Nguyen CT, Nguyen HLT, Niazi RK, Nnaji CA, Noor NM, Nuñez-Samudio V, Nzoputam CI, Oancea B, Ochir C, Odukoya OO, Ogbo FA, Olagunju AT, Olakunde BO, Omar E, Omar Bali A, Omonisi AEE, Ong S, Onwujekwe OE, Orru H, Ortega-Altamirano DV, Otstavnov N, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakshir K, Pana A, Panagiotakos D, Panda-Jonas S, Pardhan S, Park EC, Park EK, Pashazadeh Kan F, Patel HK, Patel JR, Pati S, Pattanshetty SM, Paudel U, Pereira DM, Pereira RB, Perianayagam A, Pillay JD, Pirouzpanah S, Pishgar F, Podder I, Postma MJ, Pourjafar H, Prashant A, Preotescu L, Rabiee M, Rabiee N, Radfar A, Radhakrishnan RA, Radhakrishnan V, Rafiee A, Rahim F, Rahimzadeh S, Rahman M, Rahman MA, Rahmani AM, Rajai N, Rajesh A, Rakovac I, Ram P, Ramezanzadeh K, Ranabhat K, Ranasinghe P, Rao CR, Rao SJ, Rawassizadeh R, Razeghinia MS, Renzaho AMN, Rezaei N, Rezaei N, Rezapour A, Roberts TJ, Rodriguez JAB, Rohloff P, Romoli M, Ronfani L, Roshandel G, Rwegerera GM, S M, Sabour S, Saddik B, Saeed U, Sahebkar A, Sahoo H, Salehi S, Salem MR, Salimzadeh H, Samaei M, Samy AM, Sanabria J, Sankararaman S, Santric-Milicevic MM, Sardiwalla Y, Sarveazad A, Sathian B, Sawhney M, Saylan M, Schneider IJC, Sekerija M, Seylani A, Shafaat O, Shaghaghi Z, Shaikh MA, Shamsoddin E, Shannawaz M, Sharma R, Sheikh A, Sheikhbahaei S, Shetty A, Shetty JK, Shetty PH, Shibuya K, Shirkoohi R, Shivakumar KM, Shivarov V, Siabani S, Siddappa Malleshappa SK, Silva DAS, Singh JA, Sintayehu Y, Skryabin VY, Skryabina AA, Soeberg MJ, Sofi-Mahmudi A, Sotoudeh H, Steiropoulos P, Straif K, Subedi R, Sufiyan MB, Sultan I, Sultana S, Sur D, Szerencsés V, Szócska M, Tabarés-Seisdedos R, Tabuchi T, Tadbiri H, Taherkhani A, Takahashi K, Talaat IM, Tan KK, Tat VY, Tedla BAA, Tefera YG, Tehrani-Banihashemi A, Temsah MH, Tesfay FH, Tessema GA, Thapar R, Thavamani A, Thoguluva Chandrasekar V, Thomas N, Tohidinik HR, Touvier M, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tran MTN, Tripathy JP, Tusa BS, Ullah I, Ullah S, Umapathi KK, Unnikrishnan B, Upadhyay E, Vacante M, Vaezi M, Valadan Tahbaz S, Velazquez DZ, Veroux M, Violante FS, Vlassov V, Vo B, Volovici V, Vu GT, Waheed Y, Wamai RG, Ward P, Wen YF, Westerman R, Winkler AS, Yadav L, Yahyazadeh Jabbari SH, Yang L, Yaya S, Yazie TSY, Yeshaw Y, Yonemoto N, Younis MZ, Yousefi Z, Yu C, Yuce D, Yunusa I, Zadnik V, Zare F, Zastrozhin MS, Zastrozhina A, Zhang J, Zhong C, Zhou L, Zhu C, Ziapour A, Zimmermann IR, Fitzmaurice C, Murray CJL, Force LM. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8:420-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1180] [Cited by in RCA: 1346] [Article Influence: 336.5] [Reference Citation Analysis (0)] |
| 2. | Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 489] [Article Influence: 81.5] [Reference Citation Analysis (1)] |
| 3. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1395] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 4. | Heylmann D, Rödel F, Kindler T, Kaina B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim Biophys Acta. 2014;1846:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Liu LT, Chen QY, Tang LQ, Guo SS, Guo L, Mo HY, Chen MY, Zhao C, Guo X, Qian CN, Zeng MS, Bei JX, Tan J, Chen S, Hong MH, Shao JY, Sun Y, Ma J, Mai HQ. The Prognostic Value of Treatment-Related Lymphopenia in Nasopharyngeal Carcinoma Patients. Cancer Res Treat. 2018;50:19-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Lee G, Kim DW, Muralidhar V, Mitra D, Horick NK, Eyler CE, Hong TS, Drapek LC, Allen JN, Blaszkowsky LS, Giantonio B, Parikh AR, Ryan DP, Clark JW, Wo JY. Chemoradiation-Related Lymphopenia and Its Association with Survival in Patients with Squamous Cell Carcinoma of the Anal Canal. Oncologist. 2020;25:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Heo J, Chun M, Noh OK, Oh YT, Suh KW, Park JE, Cho O. Sustaining Blood Lymphocyte Count during Preoperative Chemoradiotherapy as a Predictive Marker for Pathologic Complete Response in Locally Advanced Rectal Cancer. Cancer Res Treat. 2016;48:232-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Byun HK, Kim N, Park S, Seong J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol. 2019;195:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Ahn S, Park JS, Jang J, Ahn KJ, Hong YK, Yang SH, Jeun SS. The association between total lymphocyte count after concomitant chemoradiation and overall survival in patients with newly diagnosed glioblastoma. J Clin Neurosci. 2020;71:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Abravan A, Faivre-Finn C, Kennedy J, McWilliam A, van Herk M. Radiotherapy-Related Lymphopenia Affects Overall Survival in Patients With Lung Cancer. J Thorac Oncol. 2020;15:1624-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, Campian J, Laheru DA, Zheng L, Wolfgang CL, Tran PT, Grossman SA, Herman JM. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol. 2015;38:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Onal C, Yildirim BA, Guler OC, Mertsoylu H. The Utility of Pretreatment and Posttreatment Lymphopenia in Cervical Squamous Cell Carcinoma Patients Treated With Definitive Chemoradiotherapy. Int J Gynecol Cancer. 2018;28:1553-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Deng W, Xu C, Liu A, van Rossum PSN, Deng W, Liao Z, Koong AC, Mohan R, Lin SH. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol. 2019;133:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Xu H, Lin M, Hu Y, Zhang L, Li Q, Zhu J, Wang S, Xi M. Lymphopenia During Definitive Chemoradiotherapy in Esophageal Squamous Cell Carcinoma: Association with Dosimetric Parameters and Patient Outcomes. Oncologist. 2021;26:e425-e434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 Positive Human T-Lymphocytes by an in Vitro Colony Formation Assay. Radiat Res. 1990;123:224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Liu J, Zhao Q, Deng W, Lu J, Xu X, Wang R, Li X, Yue J. Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat Oncol. 2017;12:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Venkatesulu BP, Chan DP, Giridhar P, Upadhyay R, Sharma A, Elghazawy H, Elumalai T, V P, Mallick S, Hsieh CE. A systematic review and meta-analysis of the impact of radiation-related lymphopenia on outcomes in pancreatic cancer. Future Oncol. 2022;18:1885-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Xie X, Lin SH, Welsh JW, Wei X, Jin H, Mohan R, Liao Z, Xu T. Radiation-induced lymphopenia during chemoradiation therapy for non-small cell lung cancer is linked with age, lung V5, and XRCC1 rs25487 genotypes in lymphocytes. Radiother Oncol. 2021;154:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, Bogart JA, Forster KM, Magliocco AM, Kavadi VS, Narayan S, Iyengar P, Robinson CG, Wynn RB, Koprowski CD, Olson MR, Meng J, Paulus R, Curran WJ Jr, Choy H. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2020;38:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 425] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 20. | Liao XY, Liu CY, He JF, Wang LS, Zhang T. Combination of checkpoint inhibitors with radiotherapy in esophageal squamous cell carcinoma treatment: A novel strategy. Oncol Lett. 2019;18:5011-5021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Chen MF, Chen PT, Chen WC, Lu MS, Lin PY, Lee KD. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget. 2016;7:7913-7924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, Muroyama Y, Anders RA, Sharabi AB, Velarde E, Mao W, Chaudhary KR, Chaimowitz MG, Wong J, Selby MJ, Thudium KB, Korman AJ, Ulmert D, Thorek DLJ, DeWeese TL, Drake CG. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin Cancer Res. 2018;24:5058-5071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 23. | Lyu J, Yisikandaer A, Li T, Zhang X, Wang X, Tian Z, Chen L, Lu B, Chen H, Yang J, Wang Q, Zhang J, Ma Y, Liu R, Liu R, Hage A, Lang J. Comparison between the effects of elective nodal irradiation and involved-field irradiation on long-term survival in thoracic esophageal squamous cell carcinoma patients: A prospective, multicenter, randomized, controlled study in China. Cancer Med. 2020;9:7460-7468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Crocenzi T, Cottam B, Newell P, Wolf RF, Hansen PD, Hammill C, Solhjem MC, To YY, Greathouse A, Tormoen G, Jutric Z, Young K, Bahjat KS, Gough MJ, Crittenden MR. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer. 2016;4:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Fang P, Shiraishi Y, Verma V, Jiang W, Song J, Hobbs BP, Lin SH. Lymphocyte-Sparing Effect of Proton Therapy in Patients with Esophageal Cancer Treated with Definitive Chemoradiation. Int J Part Ther. 2018;4:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |