Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.107971
Revised: May 8, 2025
Accepted: June 19, 2025
Published online: July 15, 2025
Processing time: 104 Days and 3.3 Hours
Metabolic dysregulation is considered a significant hallmark of hepatocellular carcinoma (HCC). SAC3 domain containing 1 (SAC3D1) functions in the cell cycle, and its expression is upregulated in various cancers. It is known that metabolic changes occur at different stages of the cell cycle to maintain the biosynthesis and replication of both normal and cancer cells. Based on the role of SAC3D1 in mitosis, we hypothesize that abnormal expression of SAC3D1 may affect cellular metabolism. However, it remains unclear whether SAC3D1 mediates the progression of HCC by regulating metabolic reprogramming.
To comprehensively elucidate the impact and molecular mechanism of SAC3D1 on the progression of HCC by regulating the metabolic reprogramming.
The constructed SAC3D1 overexpression and knockdown HCC cell lines were used for detecting cell proliferation, migration capabilities, as well as glycolysis and adenosine triphosphate (ATP) production rate assays. They were also employed for examining molecular markers associated with cell migration and glycolysis. The transcriptome sequencing data of cells have revealed the pathways potentially influenced by SAC3D1.The tail vein metastasis model and xenograft tumor experiments were utilized to demonstrate SAC3D1’s tumor-promoting effects in vivo.
SAC3D1 expression was upregulated and associated with poor prognosis in HCC patients. SAC3D1 enhanced the proliferation and migration abilities and reduced the population dependence of HCC cells in vitro and in vivo. The upregulation of SAC3D1 enhanced cellular glycolysis and ATP production. The cell transcriptome sequencing data revealed that SAC3D1 activated Wnt signaling pathway. SAC3D1 did not modulate the transcription of β-Catenin, while might inhibit its degradation. Further investigations indicated that the increase of SAC3D1 leads to more β-Catenin accumulating in the nucleus, facilitating the expression of c-Myc, one of the upstream regulatory factors of glycolysis. The iCRT3, an antagonist of β-Catenin, could counteract the increase of c-Myc induced by SAC3D1, while also downregulating the expression of glycolysis-related proteins.
This study found that SAC3D1 enhances HCC cell glycolysis and ATP production via the β-Catenin/c-Myc signaling axis, thereby promoting the progression of HCC.
Core Tip: The study revealed that the expression of SAC3 domain containing 1 (SAC3D1) was elevated in hepatocellular carcinoma and closely related to a poor prognosis. SAC3D1 facilitated the proliferation and migration of hepatocellular carcinoma cells in vitro and in vivo. The increase of SAC3D1 Leads to more β-Catenin accumulating in the nucleus, facilitating the expression of c-Myc, one of the upstream regulatory factors of glycolysis. The iCRT3, an antagonist of β-Catenin, could counteract the increase of c-Myc induced by SAC3D1, while also downregulating the expression of glycolysis-related proteins. In conclusion, SAC3D1 augmented glycolysis and adenosine triphosphate generation via β-Catenin/c-Myc axis.
- Citation: Lin XJ, Tang EJ, Sun B, Wang AL, Chen Y, Chen L, Xue YY, Li AJ, Liu CY. SAC3 domain containing 1 intervention in energy metabolism reprogramming assists in the progression of hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(7): 107971
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/107971.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.107971
Liver cancer is a common malignant tumor. According to global cancer statistics for 2022, liver cancer ranks sixth in incidence and third in mortality among all cancers worldwide[1], with hepatocellular carcinoma (HCC) accounting for 80%-90% of primary liver cancers[2,3]. The disturbance of the equilibrium between tumor-suppressing genes and oncogenic genes, the aberrant triggering of molecular signaling cascades, the differentiation process of HCC cells, and the modulation of angiogenesis all stand as crucial elements influencing the progression of HCC[4]. Currently, systemic treatment is the primary approach for managing intermediate to advanced HCC, which includes receptor tyrosine kinase inhibitors and immune checkpoint inhibitors[5-8]. Despite significant advancements in the clinical treatment of most HCC patients, challenges such as treatment resistance and disease progression remain prevalent[9-11]. Therefore, identifying new therapeutic targets for HCC continues to be an urgent issue that needs to be addressed.
SAC3 domain containing 1 (SAC3D1) was identified during the search for actin-related genes. Research has shown that SAC3D1 is a protein-coding gene located on chromosome 11, widely expressed in the cytoplasm, cytoskeleton, microtubule organizing centers, centrioles, and spindles. It has been demonstrated to function in the cell cycle, centriole replication, and spindle formation[12,13]. Recently, the role of SAC3D1 in cancer has received increasing attention, with evidence of its high expression in various malignant tumors[14,15]. Single-cell sequencing analyses have found a correlation between SAC3D1 and T cell exhaustion[16]. SAC3D1 is highly expressed in gastric cancer, promoting disease progression and affecting prognosis[17]. Contemporary oncology research has established a compelling association between aberrant metabolic pathways and hepatocarcinogenesis. A growing body of evidence indicates that the metabolic landscape undergoes dynamic remodeling throughout the mitotic cycle, facilitating the biosynthetic requirements of cellular proliferation in both physiological and pathological contexts[18]. Based on the function of SAC3D1 in the cell cycle and mitosis, it is hypothesized that abnormal expression of SAC3D1 may affect cellular metabolism. However, it remains unclear whether the upregulation of SAC3D1 in HCC cells mediates its progression through the regulation of metabolic reprogramming[19,20]. This study elucidates the mechanism by which SAC3D1 functions in HCC, implicating that SAC3D1 may serve as a prospective target for HCC therapy.
UALCAN (http://ualcan.path.uab.edu) is a comprehensive interactive online database. It provides transcriptomic data from The Cancer Genome Atlas (TCGA) database, methylation predictions, clinical data for a variety of cancers, and external links to other databases[21]. Gene Expression Profiling Interactive Analysis (http://gepia2.cancer-pku.cn/) includes RNA sequencing data from TCGA and the Genotype-Tissue Expression Project, offering various gene expression analyses, patient survival analyses, and analyses using standard processing pipelines[22]. We utilized these two databases to analyze the expression of SAC3D1 in different cancers, as well as the prognostic implications associated with the expression level of SAC3D1, including overall survival and progression-free survival analyses.
Human HCC cell lines MHCC-97H and PLC/PRF/5 were provided by Chinese Academy of Sciences Stem Cell Bank (Shanghai, China), and the cells were cultured in Dulbecco’s modified Eagle medium complete medium at 37 °C in a 50 mL/L CO2 incubator.
The overexpression vector LV-EFS > Kozak-Human SAC3D1 CDS[NM_001367485.1]-CMV > EGFP/T2A/Puro and the control vector LV-CMV > EGFP/T2A/Puro were constructed. The upregulation of SAC3D1 expression in cells was achieved through lentiviral infection. Additionally, the knockdown expression vector LV-U6 > hSAC3D1[shRNA]-PGK > EGFP/T2A/Puro and the control vector LV-U6 > Scramble-shRNA-PGK > EGFP/T2A/Puro were constructed. The knockdown of SAC3D1 expression was also realized via lentiviral infection. All lentiviruses and vectors were sourced from Cyagen (Jiangsu Province, China). The shRNA sequences are listed in Supplementary Table 1. All transfection procedures were conducted according to the manufacturer’s instructions.
Total RNA was extracted with TRIzol reagent (Invitrogen, United States). The RNA was reverse transcribed into cDNA using the RT Master Mix synthesis system (LS2050; Promega, United States). Subsequently, real-time quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed (LS2062; Promega, United States). All primers were synthesized by Generay Biotechnology (Shanghai, China). The primer sequences of each gene are displayed in Supplementary Table 2.
Cells were lysed with radio immunoprecipitation assay lysis buffer and quantified by bicinchoninic acid assay. All samples were analyzed according to the standard western blotting procedure. Relevant antibody information is listed in Supplementary Table 3.
Total RNA was extracted from stable expressing exogenous SAC3D1 MHCC-97H HCC cells and control cells, followed by transcriptome sequencing. Subsequently, differential gene expression analysis, clustering and visualization analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis, and gene set enrichment analysis (GSEA) were performed.
HCC cells were evenly seeded into plates. Once the cells had fully adhered and reached 80% confluence, scratch injuries were made to the cell monolayer. The positions of the cells were recorded under a microscope at 0 hour and after 48 hours of culture, followed by calculation of the migration rate.
HCC cells were evenly seeded in a 6-well plate at 50% confluence. Following the instructions provided with the BeyoClick™ 5-ethynyl-2’-deoxyuridine (EdU)-488 Cell Proliferation Detection Kit (C0071S, Beyotime, Shanghai, China) and BeyoClick™ EdU-555 Cell Proliferation Detection Kit (C0075S, Beyotime, Shanghai, China), EdU labeling was performed. To assess the proportion of cell proliferation, nuclei were stained. Subsequently, the number of proliferating cells was quantified under a fluorescence microscope.
Theglycolytic rate and adenosine triphosphate (ATP) production rate of cells were detected through SeahorseXFe96 analysis (Agilent, United States). Cells were seeded in cell culture plates at a density of 10000 cells per well and incubated overnight in a 37 °C, 50 mL/L CO2 incubator. Subsequently, measurements were conducted according to the instructions provided in the real-time ATP rate assay kit (103592-100, Agilent, United States) and the cell glycolytic rate assay kit (103344-100, Agilent, United States).
Tail vein metastasis model: Eight 5-week-old male Balb/c nude mice were stochastically allocated into two groups. Cells from the MHCC-97H control (Ctrl) group and the MHCC-97H SAC3D1 over expression (OE) group were administered via the tail vein (5 × 105 cells in 100 μL per mouse). After 30 days, the mice were humanely sacrificed by inhaling CO2 and then dissected to examine the formation of tumors in their lungs, livers, and kidneys. Subsequently, hematoxylin and eosin staining was carried out.
Subcutaneous tumorigenesis model: A total of eight 5-week-old male Balb/c nude mice were stochastically partitioned into two groups. Cells of MHCC-97H Ctrl and MHCC-97H SAC3D1 OE were subcutaneously inoculated into these nude mice (7 × 106 cells in 150 μL per mouse). Thirty days elapsed, and then the mice were humanely put to death by CO2 inhalation. Subsequently, the tumors were excised, weighed, and photographed. The length and width of the transplanted tumors were recorded and tumor volume determined using the formula: Volume = (length × width²)/2[23].
Immunohistochemistry staining was performed using the following antibodies: SAC3D1, β-Catenin, c-Myc, glucose transporter 1 (GLUT1), hexokinase 2 (HK2), pyruvate kinase M2 (PKM2), and lactate dehydrogenase A (LDHA). The detailed information on all antibodies can be found in Supplementary Table 3. Antigen retrieval was performed using 0.01 M citrate buffer (pH = 6.0) with 10 minutes of microwave irradiation at high power. The staining intensity was quantified using ImageJ software, and the positive rates of various indicators were calculated. This research was evaluated and authorized by the Institutional Animal Care and Use Committee of Naval Medical University, PLA.
The dataset was statistically assessed with GraphPad Prism 10 and SPSS 19.0, represented as mean ± SD. Between-group comparisons were assessed with analysis of variance. Observed effects of a P-value below 0.05 was defined as statistically significant. The grayscale values for western blotting were analyzed with ImageJ. Statistical graphs and data organization were created with GraphPad Prism 10 and Adobe Illustrator CS5.
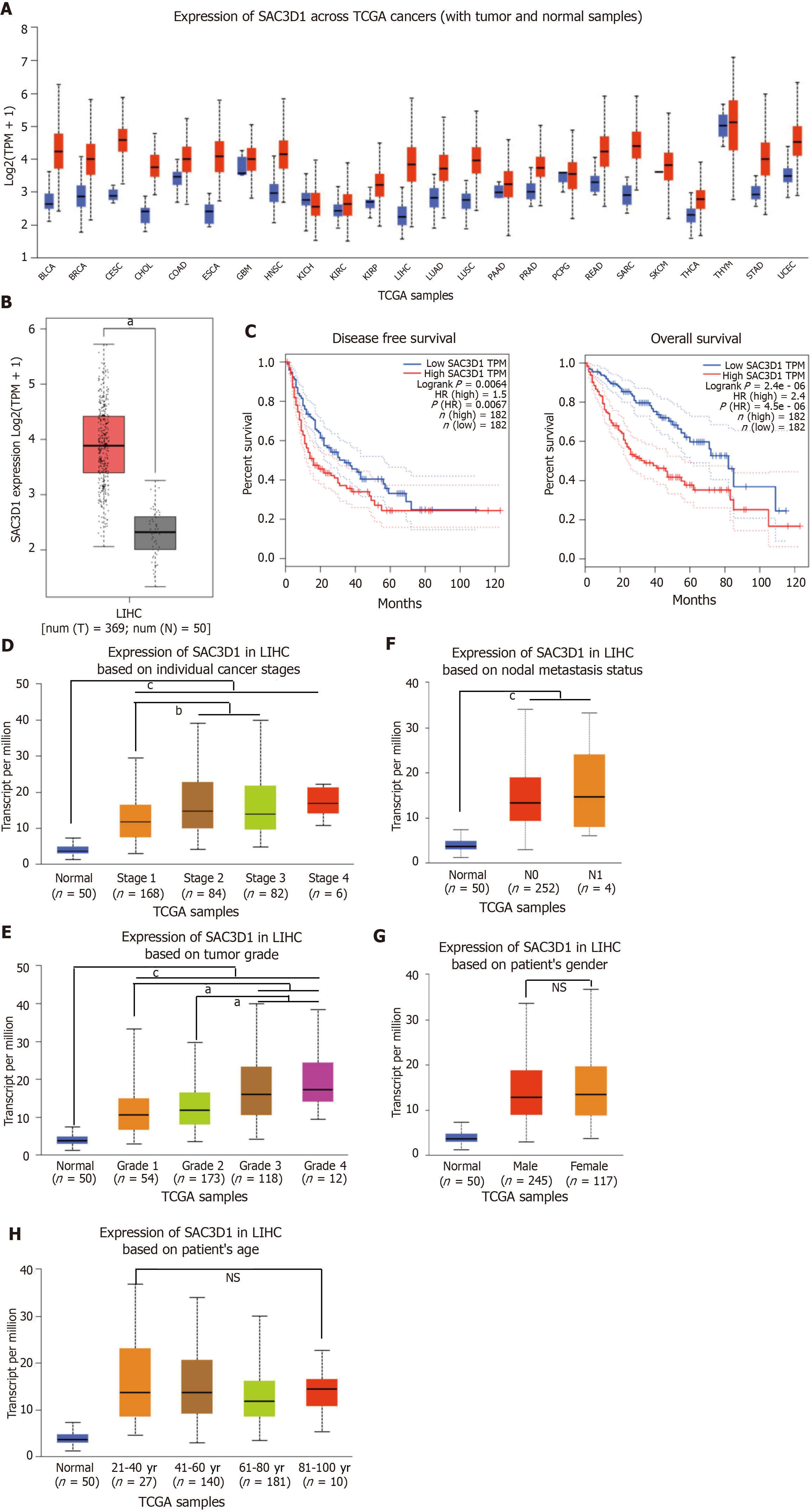
Online gene expression profiling tools UALCAN and Gene Expression Profiling Interactive Analysis were employed to analyze the expression of SAC3D1 across different cancers in TCGA database. Compared with adjacent non-cancerous tissues, SAC3D1 expression is significantly elevated in a variety of cancers, especially HCC (Figure 1A and B). Kaplan-Meier analysis showed that the HCC patients with high SAC3D1 expression levels had significantly lower disease-free survival and overall survival (Figure 1C, Supplementary Figure 1A and B). This indicated that the upregulation of SAC3D1 contributed to unsatisfactory prognosis in HCC patients. The results from UALCAN analysis demonstrated that SAC3D1 expression level correlated with tumor stages and grades in HCC patients, with higher transcription levels observed in high-grade HCC (Figure 1D and E). There was no statistical correlation found between SAC3D1 expression levels and lymph node metastasis, gender, or age (Figure 1F-H). Furthermore, Kaplan-Meier analysis showed that within different tumor grade groups of HCC patients, the overall survival rate in the group with high expression of SAC3D1 was lower than that in the group with low expression, with this difference being statistically significant (Supplementary Figure 1A). Western blotting was performed to assess the relative expression of SAC3D1 in HCC and adjacent non-tumor tissue samples. These findings are consistent with data from public databases (Supplementary Figure 1C), further supporting the relevance of SAC3D1 in HCC pathogenesis. In summary, the upregulation of SAC3D1 was associated with higher tumor grades and poor prognosis in HCC.
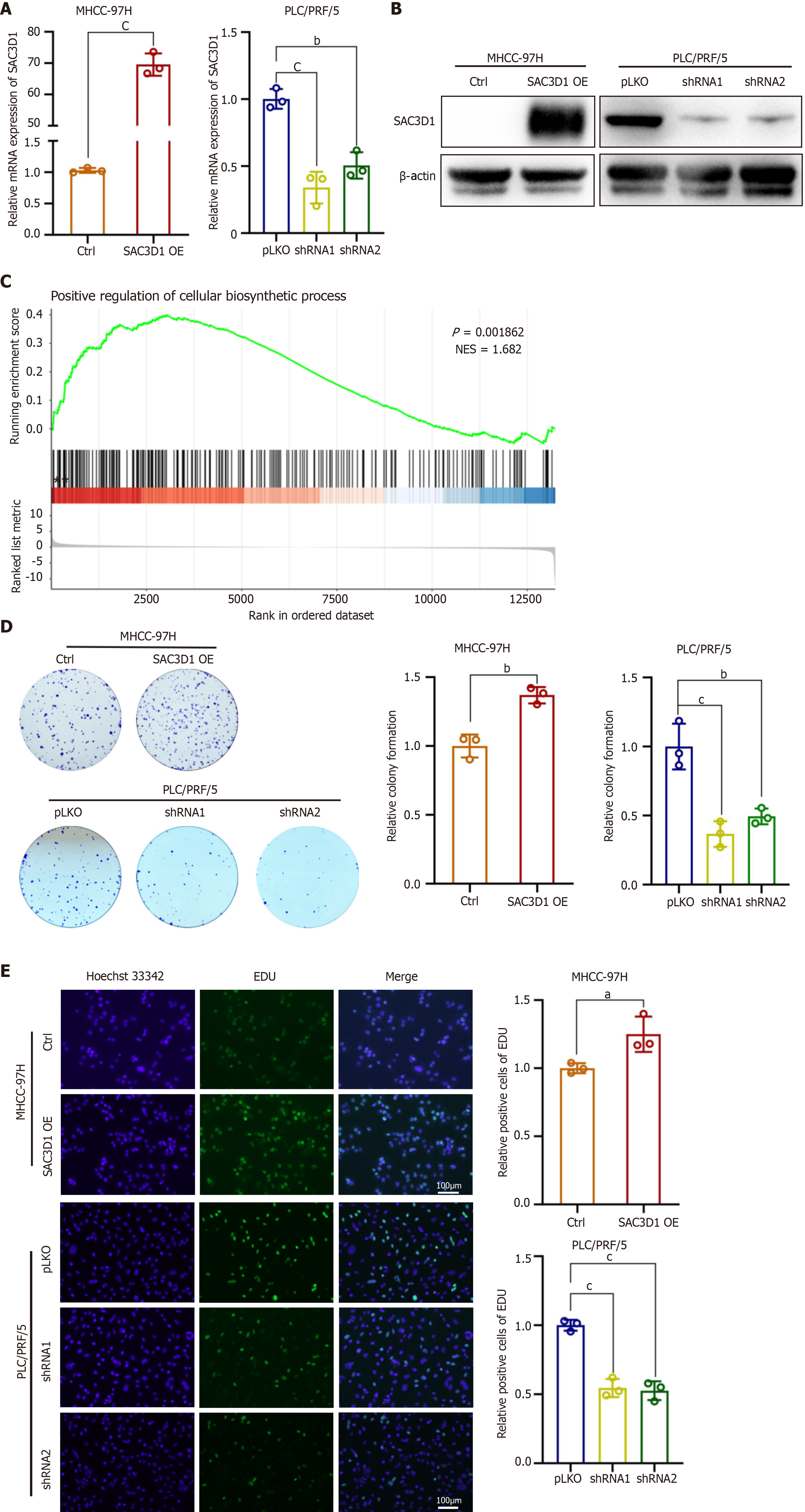
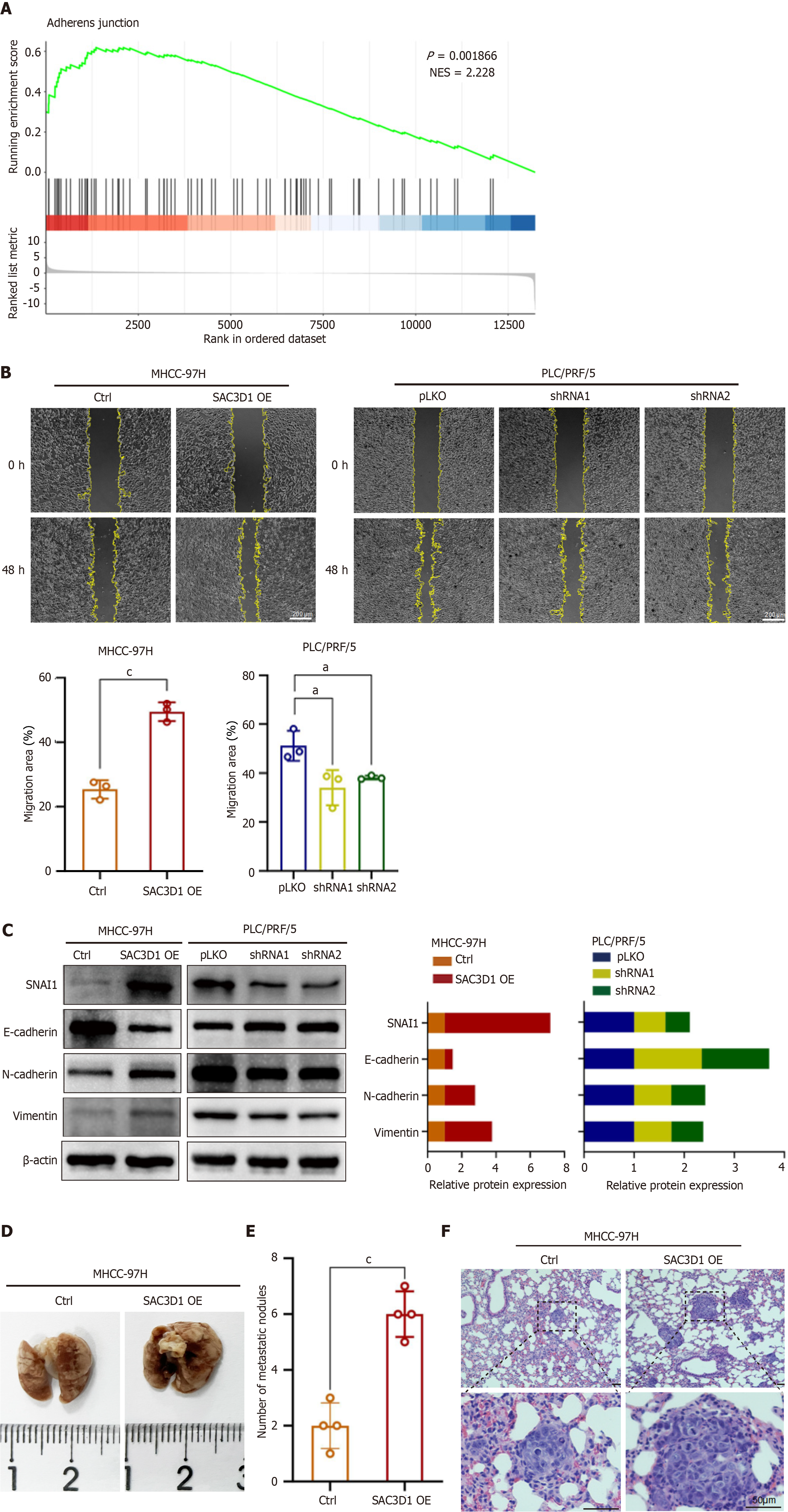
The expression of SAC3D1 in different HCC cell lines were evaluated by western blotting (Supplementary Figure 2A). Cell lines with either low (MHCC-97H) or high (PLC/PRF/5) levels of SAC3D1 expression were selected to establish SAC3D1 overexpression and knockdown cell lines through lentiviral infection. Western blotting and RT-qPCR experiments confirmed the effectiveness of the overexpression and knockdown (Figure 2A and B). To systematically elucidate the impact of SAC3D1 on HCC cells, whole transcriptome sequencing was performed on MHCC-97H SAC3D1 OE and Ctrl cells. Gene Ontology analysis of differentially expressed genes indicated that upregulation of SAC3D1 expression led to significant changes in genes associated with cell proliferation. Genes related to transcriptional regulation accounted for a substantial proportion of the differentially expressed genes (Supplementary Figure 2B). GSEA revealed that upregulation of SAC3D1 expression promoted processes such as biosynthesis and cell adhesion, and activation of cancer related signaling pathways in MHCC-97H cells (Figure 2C, Figure 3A, and Supplementary Figure 2C). EdU and colony formation assays demonstrated that SAC3D1 promoted the proliferation and clonogenicity of HCC cells, whereas knockdown of SAC3D1 expression exhibited a proliferation-inhibitory effect, corroborating the results of the GSEA analysis (Figure 2D and E). Additionally, wound healing assays indicated that SAC3D1 promoted the migration of HCC cells (Figure 3B). Western blotting results demonstrated that SAC3D1 modulated the expression of migration-related proteins in HCC cells (Figure 3C), confirming SAC3D1’s functional impact on cellular migration mechanisms. The tail vein injection metastasis model demonstrated that SAC3D1 facilitated the metastasis of HCC cells to the lungs in vivo (Figure 3D-F). No metastasis was found in liver and kidney tissues (Supplementary Figure 2D).
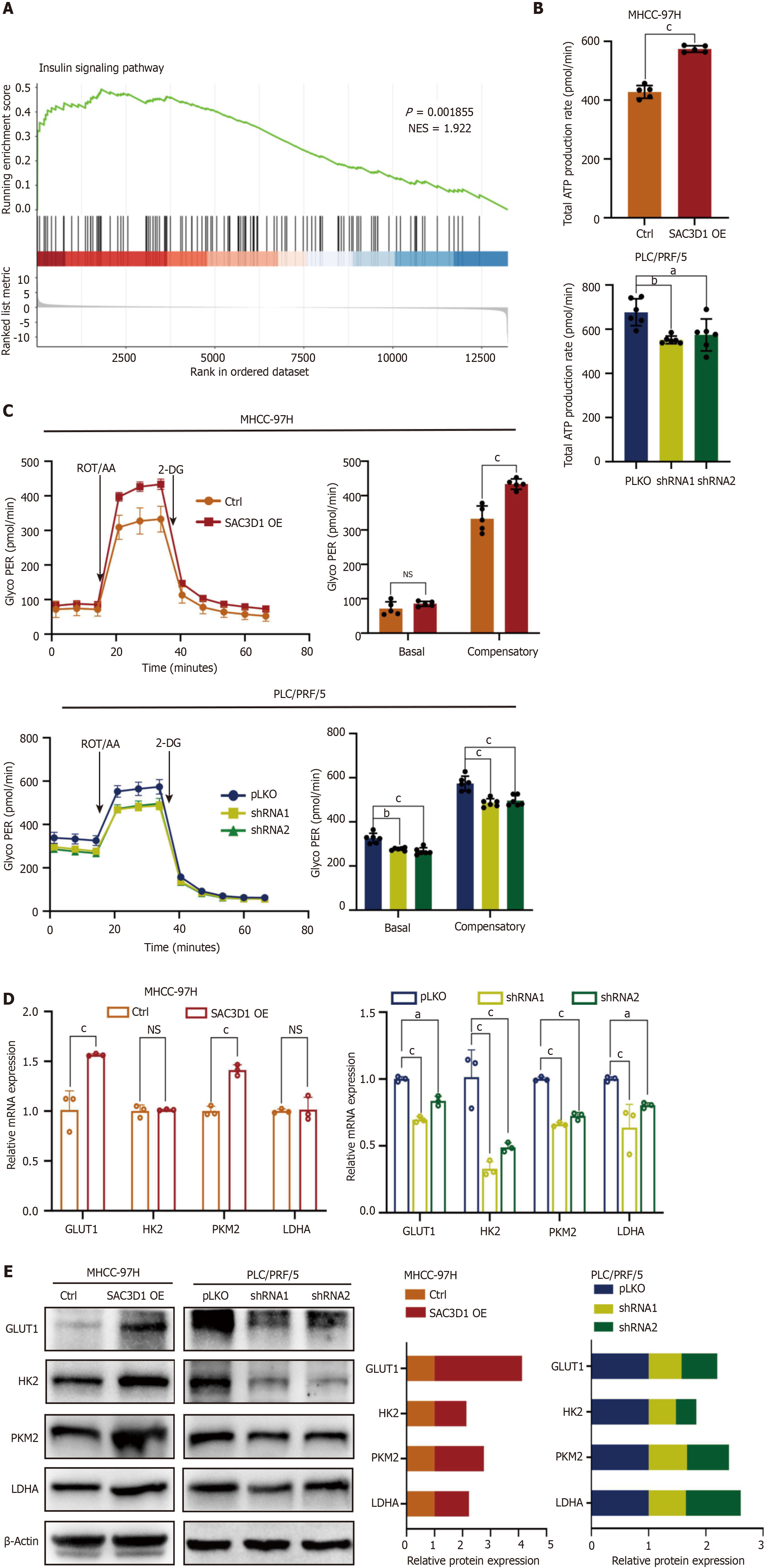
Metabolic reprogramming is a significant hallmark of malignant tumors, including HCC. This reprogramming is closely related to other characteristics of cancer, such as unlimited proliferative potential, immune evasion, and enhanced invasion and metastasis abilities[24-26]. According to the results of GSEA, the upregulation of SAC3D1 expression promoted activation of the insulin signaling pathway, which is closely associated with cellular energy metabolism (Figure 4A). It was thus hypothesized that SAC3D1 might participate in the regulation of energy metabolism in HCC cells. Real-time ATP rate analysis indicated that the upregulation of SAC3D1 expression significantly increased the total ATP production rate in HCC cells (Figure 4B). Glycolytic rate assessments revealed that the upregulation of SAC3D1 expression notably enhanced the compensatory glycolytic rate in HCC cells, suggesting that these cells possessed a higher glycolytic capacity. Conversely, the knockdown of SAC3D1 expression led to a significant reduction in glycolytic rates, including both basal and compensatory glycolytic rates (Figure 4C). Furthermore, SAC3D1 OE activated the expression of glycolysis-related genes such as GLUT1, HK2, and PKM2. In contrast, the knockdown of SAC3D1 inhibited the expression of these genes (Figure 4D and E). Collectively, these findings indicated that the upregulation of SAC3D1 expression enhanced the glycolytic capacity and total ATP yield in HCC cells to support their extensive biosynthesis and proliferation.
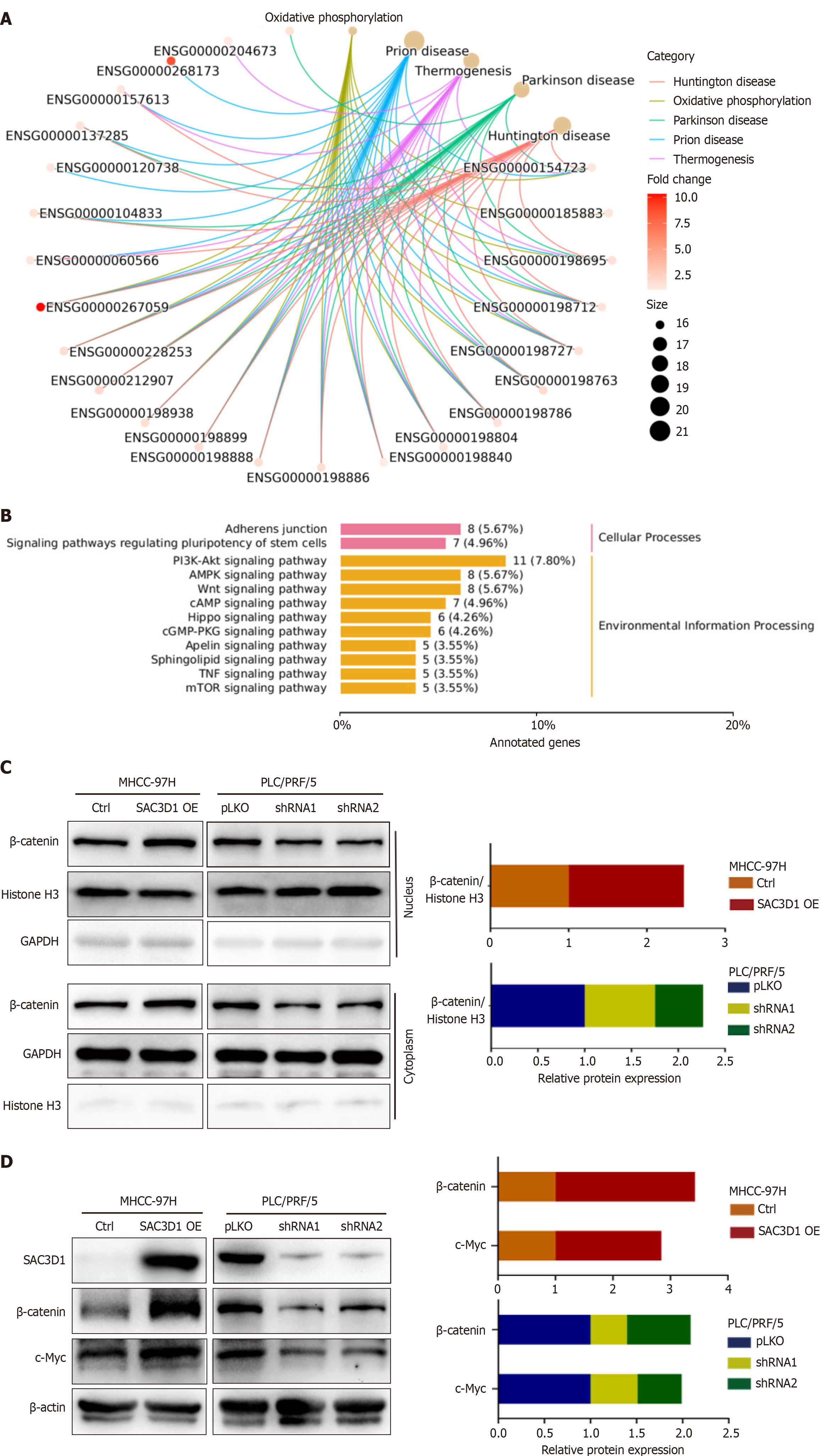
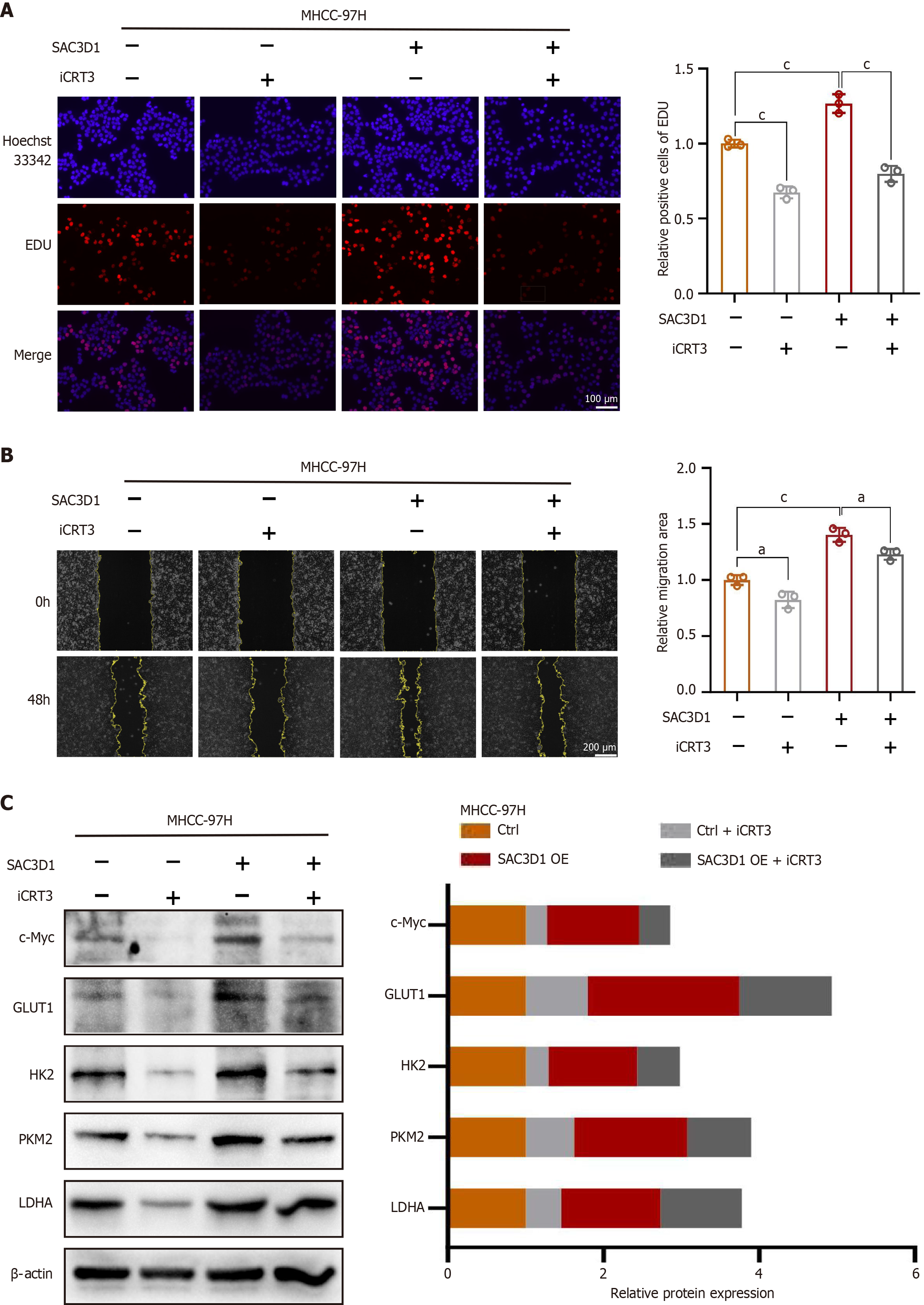
KEGG analysis of the cell transcriptome sequencing data revealed changes in signaling pathways induced by the upregulation of SAC3D1 expression (Figure 5A and B). The results indicated that SAC3D1 promoted activation of the Wnt signaling pathway, with β-Catenin being a key molecule in this pathway. Nuclear-cytoplasmic fractionation assay results demonstrated that OE of SAC3D1 enhanced nuclear translocation of β-Catenin, while knockdown of SAC3D1 reduced the extent of β-Catenin nuclear accumulation (Figure 5C). Western blotting results demonstrated that SAC3D1 enhanced the expression of β-Catenin (Figure 5D). However, RT-qPCR results indicated that SAC3D1 did not significantly regulate the transcriptional level of β-Catenin, suggesting that SAC3D1 might enhance its intracellular expression by inhibiting β-Catenin degradation (Supplementary Figure 2E). As is well-recognized, once β-Catenin translocates to the nucleus, it can bind to T-cell factor/lymphoid enhancer factor (TCF/LEF), facilitating the expression of several transcription factors, including c-Myc. Previous reports have shown that the oncogene c-Myc could regulate the transcription of genes such as GLUT1, ASCT2, HK2, PKM2, LDHA, and GLS1, thereby participating in the regulation of glycolysis and glutamine metabolism[27]. Western blotting results confirmed that the upregulation of SAC3D1 expression promoted the expression of c-Myc protein, while knockdown of SAC3D1 expression inhibited c-Myc expression (Figure 5D). The iCRT3, an antagonist of β-Catenin, can competitively inhibit the nuclear translocation of β-Catenin. In vitro, iCRT3 could block SAC3D1’s regulatory effects on HCC cell functions (Figure 6). Experimental evidence indicated that iCRT3 could counteract the increase in c-Myc induced by SAC3D1, while also downregulating the expression of GLUT1, HK2, and PKM2 proteins (Figure 6C). These findings suggested that SAC3D1 regulated the energy metabolism reprogramming of HCC cells through the β-Catenin/c-Myc signaling axis.
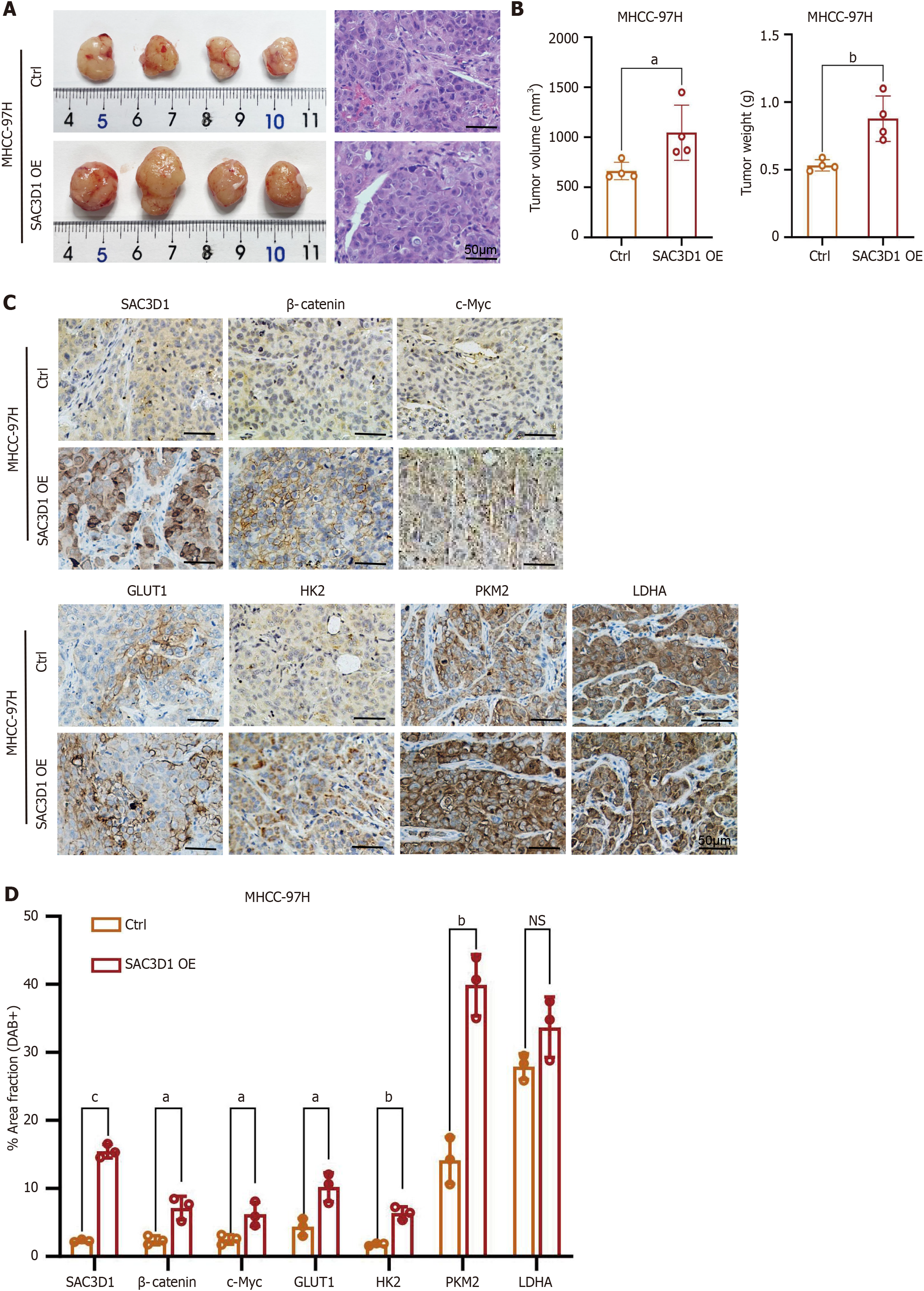
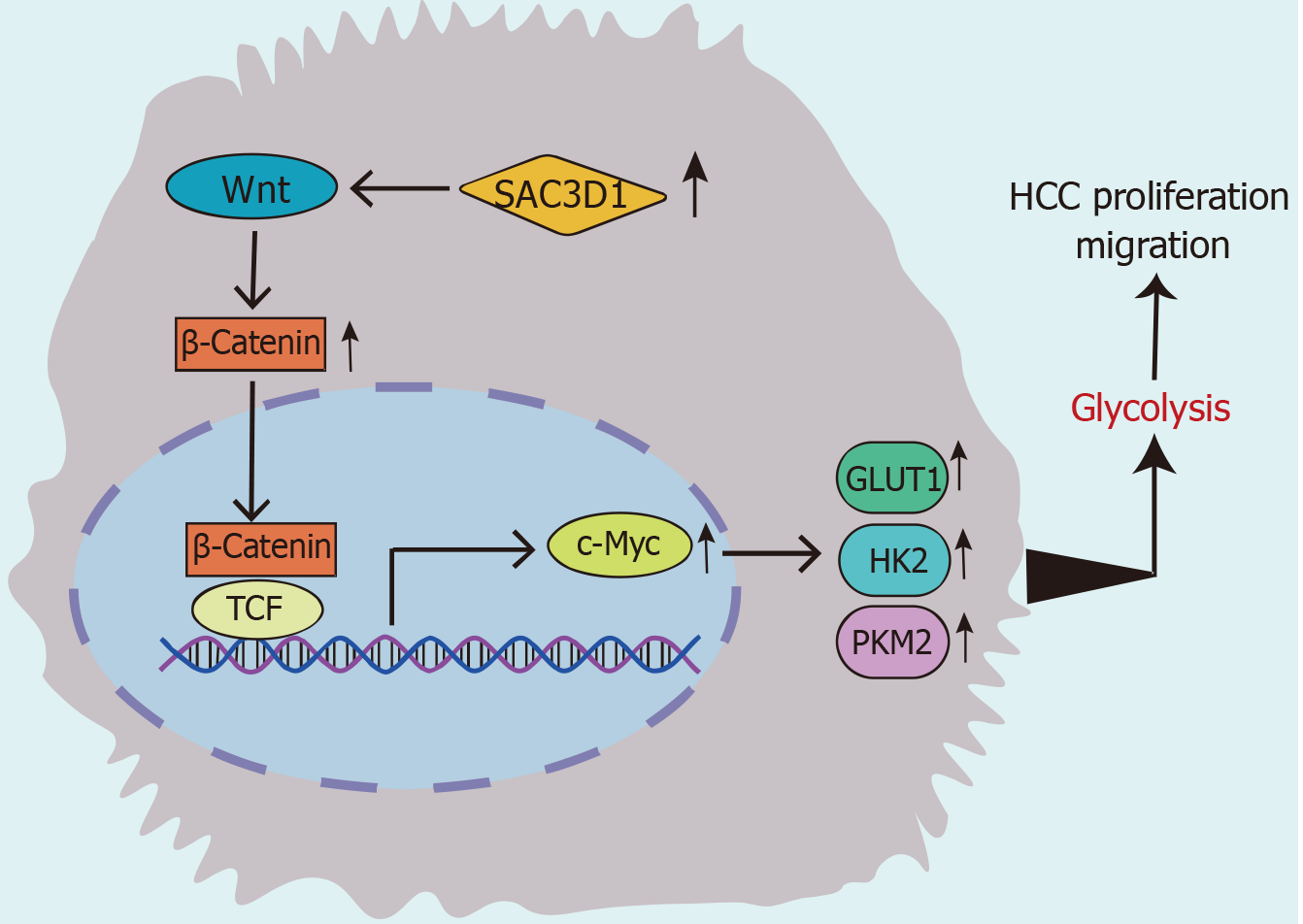
To investigate the effects of SAC3D1 in animals, MHCC-97H Ctrl and MHCC-97H SAC3D1 OE cell sublines were injected subcutaneously into nude mice. The investigational data demonstrated that the tumor volume and weight in mice injected with the MHCC-97H SAC3D1 OE cell subline were markedly greater relative to those in the control group (Figure 7A and B). Immunohistochemistry analysis revealed the expression levels of SAC3D1, β-Catenin, c-Myc, GLUT1, HK2, PKM2, and LDHA in the tissues, which were consistent with the in vitro cell experiments. In vivo, the upregulation of SAC3D1 expression also led to a significant increase in the expression of β-Catenin, c-Myc, GLUT1, HK2, and PKM2 (Figure 7C and D). Therefore, in vivo, SAC3D1 could also significantly enhance the development of HCC by regulating the β-Catenin/c-Myc signaling axis, thereby affecting cellular energy metabolism (Figure 8).
Metabolic reprogramming drives the occurrence and development of tumors. Metabolic dysregulation is considered a hallmark of cancer, particularly in the progression of HCC[28]. SAC3D1 has been reported to be highly expressed in various cancers and is associated with poor prognosis in cancer patients[13,14]. However, the precise mechanism by which SAC3D1 mediates the occurrence of HCC and whether it regulates metabolic reprogramming remains unclear. To our knowledge, we elucidated the expression of SAC3D1 in HCC and its regulatory effects upon the biological behaviors of HCC cells. Additionally, we propose the first evidence that SAC3D1 exerts a regulatory function in the metabolic reprogramming of HCC.
Through our research, we found that upregulating SAC3D1 expression promoted the proliferation and colony formation ability of HCC cells, while also affecting their migration. Utilizing transcriptome sequencing, differential gene expression screening, and GSEA analysis, we discovered that SAC3D1 facilitated the triggering of the insulin molecular cascade, which is crucial for the regulation of glucose and lipid metabolism in the liver[29]. It is known that, unlike normal cells, malignant tumor cells primarily metabolize glucose through aerobic glycolysis rather than mitochondrial oxidation. Consequently, the increased uptake of glucose by malignant tumor cells also leads to elevated levels of other intermediate metabolites, resulting in the production of more ATP for tumor cell use[30]. Therefore, we validated the promoting effect of SAC3D1 on glycolysis in HCC cells using the Seahorse XFe96 metabolic analyzer. The glucose transporter GLUT1 and critical glycolytic catalysts HK2, PKM2, and LDHA are important proteins in the glycolytic process[31]. Our study also found that SAC3D1 enhanced the transcription and translation of these glycolysis-related genes, further confirming its role in promoting glycolysis in HCC cells.
KEGG analysis suggested that SAC3D1 promoted triggering of Wnt/β-Catenin molecular cascade, which is known to play pivotal functions in embryonic development and tissue homeostasis. Dysregulation of this signaling pathway is often associated with various severe diseases, including both cancerous and non-cancerous conditions. Numerous studies have indicated that abnormal regulation of Wnt/β-Catenin signaling was involved in the onset and progression of a range of diseases, such as cardiovascular diseases, pulmonary disorders, hepatic disorders, neurodegenerative diseases, and tumors[32]. A potential common molecular mechanism underlying these diseases involves processes including cell proliferation and specialization, inflammation, and fibrosis[32]. Furthermore, the Wnt/β-Catenin molecular cascade has also been considered closely related to drug resistance, metastasis, stemness, and metabolic reprogramming among various malignant tumors[33,34]. β-Catenin, serving as a central protein in the Wnt signaling pathway, plays a critical role by translocating to the nucleus and binding to TCF/LEF, thereby promoting the transcription of downstream target genes. Our investigation revealed that overexpression of SAC3D1 increases the expression of β-Catenin simultaneously in vitro and in vivo. c-Myc, an important target gene of TCF/LEF, can directly upregulate GLUT1, HK2, LDHA, ASCT2, and GLS1, thereby participating in glycolysis and glutamine metabolism[27]. This study demonstrated that upregulation of SAC3D1 expression promoted c-Myc expression in both in vitro and in vivo settings. Treatment of HCC cells with the β-Catenin antagonist iCRT3 showed that iCRT3 reduced the increase in c-Myc induced by SAC3D1, subsequently inhibiting the expression of GLUT1, HK2, and PKM2 proteins. This further validated that SAC3D1 participated in the regulation of energy metabolism in HCC cells by influencing the β-Catenin/c-Myc signaling axis.
Nevertheless, we have not yet validated the specific mechanism by which SAC3D1 regulates the expression of β-Catenin. Previous studies indicate that SAC3D1 interacts with Axin to inhibit β-Catenin ubiquitination, thereby stabilizing β-Catenin protein levels[35]. SAC3D1 is predicted to localize to the microtubule cytoskeleton, participate in centrosome duplication and spindle assembly, and function as part of a protein complex. Based on its biological roles, we hypothesize that SAC3D1 regulates β-Catenin post-translationally rather than transcriptionally. Our qPCR experiments confirmed this hypothesis, as SAC3D1 OE did not significantly alter β-Catenin mRNA levels (Supplementary Figure 2E). This implies that SAC3D1 overexpression may inhibit β-Catenin degradation. While the exact regulatory mechanism remains to be fully elucidated, we have incorporated this discussion to contextualize our findings and guide future research.
In summary, this research elucidated the expression and biological function of SAC3D1 in HCC. For the first time, it proposed the regulatory role of SAC3D1 in the metabolic reprogramming of HCC. SAC3D1 promoted glycolysis in cancer cells through triggering the β-Catenin/c-Myc signaling axis, thereby facilitating the proliferation and metastasis of HCC. These findings offer new insights for the treatment, diagnosis, and prognostic assessment of HCC.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12233] [Article Influence: 6116.5] [Reference Citation Analysis (6)] |
| 2. | Sun LY, Zhang KJ, Xie YM, Liu JW, Xiao ZQ. Immunotherapies for advanced hepatocellular carcinoma. Front Pharmacol. 2023;14:1138493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 3. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1484] [Article Influence: 296.8] [Reference Citation Analysis (1)] |
| 4. | Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 239] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 5. | Frenette C. Advances in Hepatocellular Carcinoma. Clin Liver Dis. 2020;24:xiii-xxiv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4078] [Article Influence: 509.8] [Reference Citation Analysis (5)] |
| 7. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 5242] [Article Influence: 873.7] [Reference Citation Analysis (27)] |
| 8. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 1143] [Article Influence: 285.8] [Reference Citation Analysis (0)] |
| 9. | Yang X, Yang C, Zhang S, Geng H, Zhu AX, Bernards R, Qin W, Fan J, Wang C, Gao Q. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell. 2024;42:180-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 246] [Reference Citation Analysis (0)] |
| 10. | Qin Y, Han S, Yu Y, Qi D, Ran M, Yang M, Liu Y, Li Y, Lu L, Liu Y, Li Y. Lenvatinib in hepatocellular carcinoma: Resistance mechanisms and strategies for improved efficacy. Liver Int. 2024;44:1808-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 11. | Chen R, Hu X, Huang Y, Jiang Y, Chen G, Shan Q, Xu X, Zheng S. Regulated Cell Death in Lenvatinib Resistance of Hepatocellular Carcinoma: from Molecular Mechanisms to Therapeutic Strategies. Int J Biol Sci. 2025;21:2012-2026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Bauer A, Kölling R. The SAC3 gene encodes a nuclear protein required for normal progression of mitosis. J Cell Sci. 1996;109 ( Pt 6):1575-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Khuda SE, Yoshida M, Xing Y, Shimasaki T, Takeya M, Kuwahara K, Sakaguchi N. The Sac3 homologue shd1 is involved in mitotic progression in mammalian cells. J Biol Chem. 2004;279:46182-46190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Fan J, Yan D, Teng M, Tang H, Zhou C, Wang X, Li D, Qiu G, Peng Z. Digital transcript profile analysis with aRNA-LongSAGE validates FERMT1 as a potential novel prognostic marker for colon cancer. Clin Cancer Res. 2011;17:2908-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Yang H, Li G, Qiu G. Bioinformatics Analysis Using ATAC-seq and RNA-seq for the Identification of 15 Gene Signatures Associated With the Prediction of Prognosis in Hepatocellular Carcinoma. Front Oncol. 2021;11:726551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Fan Q, Wang Y, Cheng J, Pan B, Zang X, Liu R, Deng Y. Single-cell RNA-seq reveals T cell exhaustion and immune response landscape in osteosarcoma. Front Immunol. 2024;15:1362970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 17. | Liu AG, Zhong JC, Chen G, He RQ, He YQ, Ma J, Yang LH, Wu XJ, Huang JT, Li JJ, Mo WJ, Qin XG. Upregulated expression of SAC3D1 is associated with progression in gastric cancer. Int J Oncol. 2020;57:122-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Icard P, Simula L. Metabolic oscillations during cell-cycle progression. Trends Endocrinol Metab. 2022;33:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Han ME, Kim JY, Kim GH, Park SY, Kim YH, Oh SO. SAC3D1: a novel prognostic marker in hepatocellular carcinoma. Sci Rep. 2018;8:15608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Liu Y, He M, Ke X, Chen Y, Zhu J, Tan Z, Chen J. Centrosome amplification-related signature correlated with immune microenvironment and treatment response predicts prognosis and improves diagnosis of hepatocellular carcinoma by integrating machine learning and single-cell analyses. Hepatol Int. 2024;18:108-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 21. | Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK, Varambally S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2365] [Cited by in RCA: 4430] [Article Influence: 492.2] [Reference Citation Analysis (0)] |
| 22. | Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556-W560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1991] [Cited by in RCA: 3469] [Article Influence: 495.6] [Reference Citation Analysis (0)] |
| 23. | Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1438] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 24. | Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022;13:877-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 500] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 25. | Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, Yi P, Tang L, Pan Q, Rao S, Liang J, Tang Y, Su M, Luo X, Yang Y, Shi Y, Wang H, Zhou Y, Liao Q. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 866] [Article Influence: 173.2] [Reference Citation Analysis (9)] |
| 26. | Liu HH, Xu Y, Li CJ, Hsu SJ, Lin XH, Zhang R, Chen J, Chen J, Gao DM, Cui JF, Yang XR, Ren ZG, Chen RX. An SCD1-dependent mechanoresponsive pathway promotes HCC invasion and metastasis through lipid metabolic reprogramming. Mol Ther. 2022;30:2554-2567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 27. | Dong LW, Yang GZ, Pan YF, Chen Y, Tan YX, Dai RY, Ren YB, Fu J, Wang HY. The oncoprotein p28GANK establishes a positive feedback loop in β-catenin signaling. Cell Res. 2011;21:1248-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Du D, Liu C, Qin M, Zhang X, Xi T, Yuan S, Hao H, Xiong J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm Sin B. 2022;12:558-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 451] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 29. | Norton L, Shannon C, Gastaldelli A, DeFronzo RA. Insulin: The master regulator of glucose metabolism. Metabolism. 2022;129:155142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 30. | Fang G, Zhang P, Liu J, Zhang X, Zhu X, Li R, Wang H. Inhibition of GSK-3β activity suppresses HCC malignant phenotype by inhibiting glycolysis via activating AMPK/mTOR signaling. Cancer Lett. 2019;463:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Fang Y, Shen ZY, Zhan YZ, Feng XC, Chen KL, Li YS, Deng HJ, Pan SM, Wu DH, Ding Y. CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat Commun. 2019;10:3981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 32. | Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 1580] [Article Influence: 395.0] [Reference Citation Analysis (0)] |
| 33. | Liu R, Li Y, Tian L, Shi H, Wang J, Liang Y, Sun B, Wang S, Zhou M, Wu L, Nie J, Lin B, Tang S, Zhang Y, Wang G, Zhang C, Han J, Xu B, Liu L, Gong K, Zheng T. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019;443:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Han Q, Chen CA, Yang W, Liang D, Lv HW, Lv GS, Zong QN, Wang HY. ATP-citrate lyase regulates stemness and metastasis in hepatocellular carcinoma via the Wnt/β-catenin signaling pathway. Hepatobiliary Pancreat Dis Int. 2021;20:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Wang H, Shi X. SAC3D1 activates Wnt/βcatenin signalling in hepatocellular carcinoma. Mol Med Rep. 2022;26:317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |