Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.107670
Revised: April 23, 2025
Accepted: June 5, 2025
Published online: July 15, 2025
Processing time: 108 Days and 3.2 Hours
Pancreatic cancer is recognized as one of the leading causes of cancer mortality, representing the second most common source of cancer-related deaths within the gastrointestinal domain. Surgical resection is currently the only definitive treatment; however, the subtle emergence of symptoms often leads to a diagnosis at an advanced stage, with merely 10%-15% of patients being eligible for surgical intervention. The primary obstacle to achieving a potential radical resection is the presence of distant metastatic disease or invasion of adjacent major vascular structures. This review aims to highlight the critical role of endoscopic ultrasound in the diagnosis and staging of pancreatic tumors. We systematically searched PubMed, MEDLINE and Web of Science by using ‘pancreatic cancer’ and ‘endoscopic ultrasonography’ as keywords. Relevant studies were reviewed and analyzed. Endoscopic ultrasonography (EUS) is efficient in the diagnosis and staging of pancreatic cancer, past studies reported the accuracy of EUS is 63% to 94% for T-staging and 44% to 82% for N-staging but there are still limitations that need to be comprehensively applied with other diagnostic methods to evaluation of distant metastasis for surgical resectability. Our review aims to reveal the value for the staging of pancreatic cancer.
Core Tip: Pancreatic cancer is a lethal health problem. Most pancreatic cancers asymptomatic in the early stages, without noticeable clinical presentation in the early stage. Surgical resection is the only approach for pancreatic cancer. Endoscopic ultrasonography (EUS) provides precise evaluations of tumor, invasion, and lymph node involvement, but a singular imaging modality appears inadequate for the preoperative staging of pancreatic cancer. Our review aims to reveal the diagnosis and staging of EUS in pancreatic cancer.
- Citation: Yang X, Ge N. Diagnostic value of endoscopic ultrasound in staging of pancreatic cancer. World J Gastrointest Oncol 2025; 17(7): 107670
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/107670.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.107670
Pancreatic cancer is a lethal health problem, and its global burden has increased steeply in the past years[1,2]. Pancreatic cancer ranks seventh among the causes of cancer-related deaths. It is among the leading contributors to cancer mortality and holds the second position in the of fatalities associated with digestive system malignancies according to statistics[3]. Most pancreatic cancers are pathologically pancreatic ductal adenocarcinomas, which is asymptomatic in the early stages, without noticeable clinical presentation in the early stage, however pancreatic malignant tumors were diagnosed in the late stage of the disease when symptoms appear. The early diagnosis is less than 20%. Most patients are in a progressive stage at the time of diagnosis and have a poor prognosis[4]. Timely surgical resection is the only approach for pancreatic cancer. However, most patients are asymptomatic in the early stages, and only 15% of patients are eligible for surgery[5,6]. The global 5-year survival rate for pancreatic cancer is approximately 9%-12%[7].
According to recent tumor node metastases (TNM) criteria (Tables 1 and 2) and international guideline[8], the TNM staging has a direct impact on surgical resectability. The TNM staging system provides a key basis for therapeutic decision-making and prognostic assessment. Early-stage patients with radical surgery have better prognosis, whereas advanced patients usually require neoadjuvant or palliative treatment due to vascular invasion or metastasis[9], which means that the cancer has grown into nearby large blood vessels or distant organ. Accurate evaluation of resectable pancreatic cancer is essential for determining surgical indications and predicting prognosis, further emphasizing the importance of TNM staging[10]. Generally, endoscopic ultrasonography (EUS) provides precise evaluations of tumor, invasion, and lymph node involvement, but a singular imaging modality appears inadequate for the preoperative staging of pancreatic cancer. Our review aims to reveal the diagnosis and staging of EUS in pancreatic cancer.
| Stage | |
| T1 | Tumor ≤ 2 cm in greatest dimension |
| T1a | Tumor ≤ 0.5 cm in greatest dimension |
| T1b | Tumor > 0.5 and < 1 cm in greatest dimension |
| T1c | Tumor 1 to 2 cm in greatest dimension |
| T2 | Tumor > 2 and ≤ 4 cm in greatest dimension |
| T3 | Tumor > 4 cm in greatest dimension |
| T4 | Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery |
| N1 | Metastasis in one to three regional lymph nodes |
| N2 | Metastasis in four or more regional lymph nodes |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| T | N | M | Stage |
| T1 | N0 | M0 | IA |
| T2 | N0 | M0 | IB |
| T3 | N0 | M0 | IIA |
| T1-3 | N1 | M0 | IIB |
| T1-3 | N2 | M0 | III |
| T4 | Any N | M0 | III |
| Any T | Any N | M1 | IV |
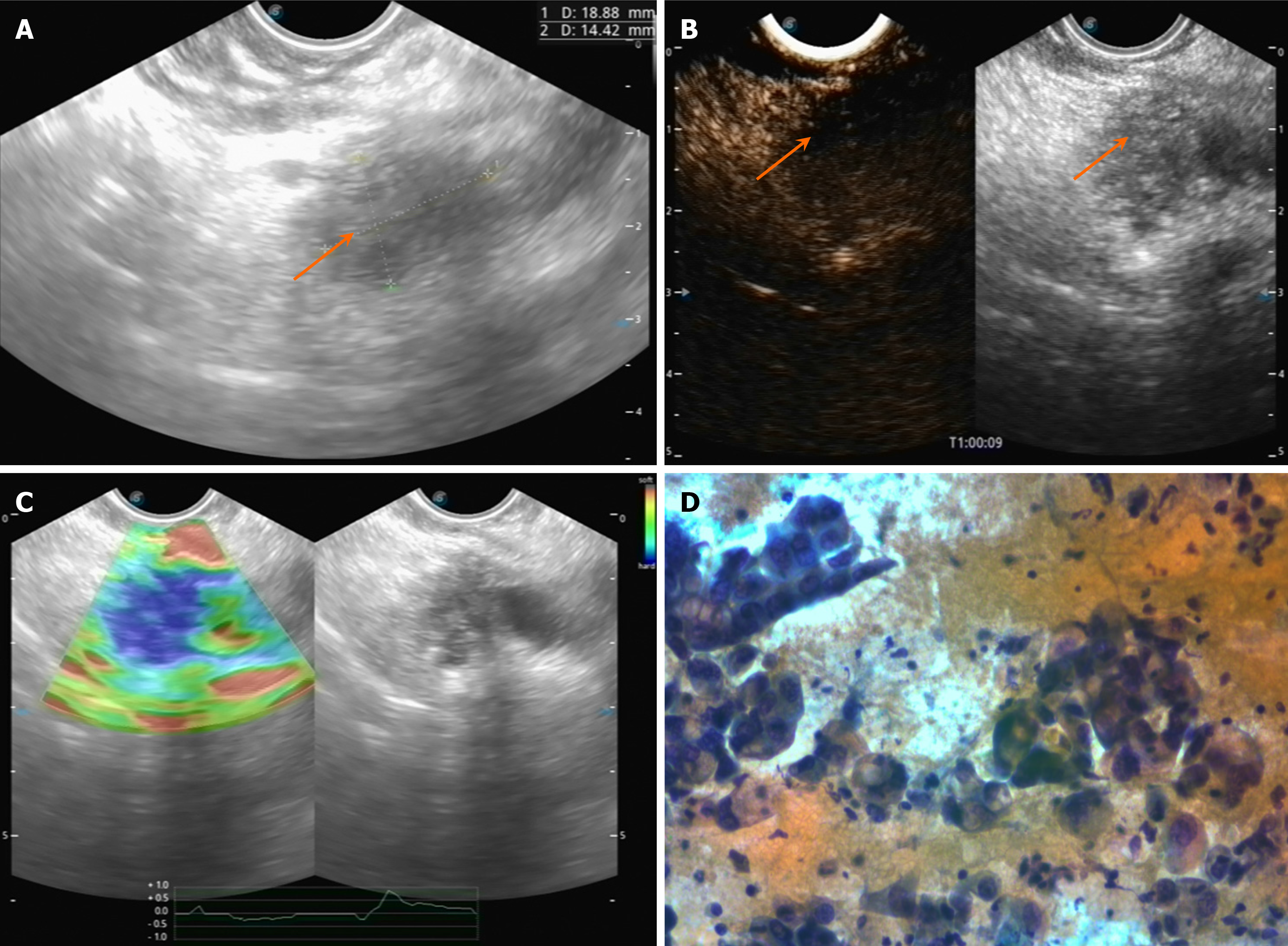
EUS plays an important role in the diagnosis of pancreatic mass, particularly for small masses. Pancreatic cancer usually presents as a hypoechoic mass relative to the surrounding pancreatic parenchyma with irregular margins or poorly defined contours[11] (Figure 1A). It often shows a hypovascular pattern on contrast-enhanced EUS (Figure 1B)[12,13]. EUS-elastography (EUS-EG) can assesses tissue stiffness, which is a critical parameter in identifying malignant. Pancreatic cancer typically presents as areas of intense blue coloration on EUS-EG, indicating high stiffness. In contrast, the benign lesions were shown in a mixed coloration pattern with green to red on elastography[14] (Figure 1C). Eventually the pancreatic cancer was histopathologically confirmed with EUS-fine-needle aspiration (FNA; Figure 1D).
The staging accuracy of EUS reported in different studies varies widely, ranging from 63%-94% for T-staging and 44%-82% for N-staging. A meta-analysis from Li et al[15] reported the pooled sensitivity and specificity of EUS for T1-2 staging was 72% and 90%, and for T3-4 staging was 90% and 72%. This indicated that EUS is particularly more effective in vascular invasion or identifying more advanced tumors(T-staging > T3). But a study showed EUS provides effective staging of pancreatic lesions, especially high-resolution imaging and evaluation of lesions < 1 cm[16]. In patients with small pancreatic masses, the accuracy of EUS in vascular invasion may be lower. Nevertheless, the difference in sensitivity of EUS for identifying vascular invasion is significant[17]. One study also supports that, with a sensitivity of 85% and specificity of 91% for vascular invasion[18]. A meta-analysis including 1308 patients showed the pooled sensitivity was 73%, but the pooled specificity was 90.2% for vascular invasion[17]. Diameter of the pancreas lesions may affect the accuracy of EUS[19]. And the accuracy of EUS was affected by the vascular localization, studies showed that the portal or splenic vein is more accurate than the superior mesenteric artery and vein[20].
The metastasis of lymph node is one of the most important predictors for prognosis of pancreatic cancer[21], and EUS allows direct evaluation and tissue sampling of lymph nodes in the region adjacent to the pancreas, avoiding surgical intervention[16,22]. And a study from Soriano et al[23] demonstrated that EUS had the highest accuracy in N-staging than computed tomography (CT; 65%). And the study from Puli et al[24] showed the sensitivity and accuracy of EUS-FNA for retroperitoneal lymph node samples were 83% and 63%. The sensitivity and specificity of EUS-FNA in diagnosing positive para-aortic lymph nodes were 96.7% and 100%, while the sensitivity of positron emission tomography (PET)-CT was only 53.3%[16]. During the development of pancreatic cancer, tumor-associated lymphatic vessels connect the tumor to the vast lymphatic vascular system. It has been found that lymphatic vessels density (LVD) levels significantly correlate with lymph node metastasis[25]. EUS can access to tumor sample to quantify LVD by microscopic examination[26], and pancreatic cancer patients with higher levels of LVD in their tumors have a worse prognosis after radical resection compared to patients with lower levels[27].
The liver is the most common distant metastatic organ of pancreatic cancer, and the left liver can be explored by EUS through the stomach. If the lesion is appropriate, which would be confirmed by EUS-tissue acquisition (EUS-TA)[28]. CT or PET-CT is an ideal imaging modality for the detection of distant metastases, with a sensitivity of up to 97% for metastatic tumors > 1 cm but decreasing for smaller lesions. In addition, EUS-FNA has a sensitivity of 82%-94% for the diagnosis of malignant ascites or liver metastases[29], but the EUS is limited in distant place and therefore it is not completely reliable in assessing distant metastasis of tumors.
EUS is a highly sensitive and radiation-free modality for detecting pancreatic mass lesions, especially it can acquire tissue for histologic test[30]. EUS-FNA improved the accuracy of diagnosis and staging of pancreatic cancer. EUS-FNA is essential tool for obtaining a definitive cytological or histological diagnosis. EUS-FNA has a sensitivity and specificity of up to 95% and 100%[31], which is considered as a preferred method for diagnosing pancreatic masses pathologically. The EUS and its related techniques improved diagnosis and staging of pancreatic lesions. A meta-analysis including 15 studies revealed the sensitivity of EUS-FNA for pancreatic cancer was 92% and the specificity was 96%[31]. Another meta-analysis including 4766 patients, EUS-FNA diagnosed solid pancreatic mass with a sensitivity of 89% and specificity of 96%[24].
And a study including 109 patients with pancreatic lesions ≤ 10 mm showed diagnostic accuracy of EUS with rapid on-site evaluation was 87.5%, the study identified a Tis-stage patient whose lesion was no longer visible on CT scan before surgery[32]. Additionally, EUS can be complemented by advanced techniques such as contrast-enhanced EUS (CE-EUS) and EUS elastography, which improve diagnostic accuracy and aid in differentiating malignant from benign lesions.
CE-EUS improved the diagnostic rate and accuracy of EUS[33]. CE-EUS is particularly useful in cases where conventional EUS and other imaging modalities are inconclusive, and a study show that the diagnostic rate of pancreatic lesions by CE-EUS has significantly increased from 64% to 91%[34]. A meta-analysis reported pooled sensitivity and specificity of 98% and 63% for qualitative EUS-EG[35]. Moreover, other study reported that EUS-EG had a sensitivity of 91.7% and specificity of 90.0% for diagnosing vascular invasion, which was superior to CT[36].
EUS is highly effective for diagnosing and staging of pancreatic cancer, particularly when compared to other diagnostic tools such as CT and magnetic resonance imaging (MRI), but imaging modality should be selected regarding local centers’ diagnosis level[37,38]. EUS is superior for tumor detection and staging, while exhibiting comparable performance in the diagnosis of lymph node and resectability for patients with pancreatic cancer preoperatively[39-41]. A systematic review showed that EUS was more accurate than CT for staging and vascular invasion in earlier research, but similar in later research[42,43].
A retrospective study including 63 patients with surgery showed that the sensitivity of EUS and MRI for tumor resectability was 61% and 73%[44]. EUS is more sensitive to diagnose small pancreatic lesions, nevertheless MRI is more efficient to detect tumor metastasis or vascular invasion[11,45]. Combining EUS and MRI would improve the accuracy of staging. Studies have shown that when the two modalities were identical on resectability, the accuracy is reliably[23,44]. EUS with other additional technology has a sensitivity of 91%-100% and a specificity of up to 100% for detecting pancreatic cancer, which is significantly higher than CT, which has a sensitivity of 53%-91% for pancreatic cancer[18]. EUS provides superior high-resolution images of the pancreas, which is particularly useful for detecting small lesions (< 2 cm) that may be missed by CT or MRI[46].
EUS has become an essential modality for preoperative diagnosis and resectability of pancreatic cancer. EUS plays a crucial role in differentiating pancreatic lesions, preoperative staging of cancer, and identifying lymph node metastasis[47]. EUS is particularly important in the diagnosis and management of borderline resectable pancreatic cancer[48]. Since patients with borderline resectable pancreatic cancer are typically treated with neoadjuvant therapy, radical resection may become feasible if the tumor regresses following evaluation by imaging modalities such as EUS. EUS is particularly advantageous in assessing localized tumor extent and treatment response[49,50]. Those patients might still benefit from therapy[51]. EUS, in combination with other diagnostic modality and new technologies, will further enhance its role in diagnosis of pancreatic cancer. EUS-TA is the gold standard for confirming the diagnosis of pancreatic cancer because it can safely, reproducibly, and non-invasively obtain pathological specimens that can only be obtained by laparoscopic or open surgery in the past[52]. But EUS-FNA was recommended to applied to pancreatic carcinoma in situ, for that ERCP-based cytology may be helpful[53,54]. However, the criteria for TNM staging are constantly changing, and those studies we discussed were heterogeneous which were difficult to compare different T staging studies. The data from different studies is difficult due to review in the criteria for exclusion and inclusion. And the diagnostic accuracy of EUS is highly dependent on the experience of the endoscopist. EUS facilitates early diagnosis and treatment planning and improves survival rate in pancreatic cancer, but EUS is difficult to diagnose tumors over 4 cm in diameter, and ultrasound attenuation can cause the surrounding structures to be poorly visualized, affecting judgments of staging and resectability. Artificial intelligence (AI)-assisted EUS has the potential to improve the diagnostic accuracy for pancreatic cancer[55,56]. AI can assist EUS-FNA/fine-needle biopsy (FNB) by providing real-time feedback to endoscopists in selecting the appropriate needle size and type, guiding the depth and location of the puncture, and helping assess sample quality[57]. Additionally, AI is expected to reduce the number of punctures required to obtain an adequate sample, improve puncture accuracy, and reduce the risk of complications[58]. Some studies have assessed EUS-FNB specimens with the help of AI, achieving the same diagnostic accuracy as macroscopic on-site evaluation[59,60]. However, challenges remain in integrating AI into clinical practice, including the need for extensive validation and generalization of AI models[61,62]. Nowadays, quantitative parameters in CE-EUS such as relative peak enhancement and in-slope derived from time-intensity curve analysis can improve the characterization of pancreatic lesions, and many studies are exploring the diagnostic value of Harmonic CE-EUS for pancreatic masses[63]. Moreover, EUS can guide radiofrequency ablation or inject antitumor agents to treat pancreatic cancer, though which was designed for unresectable cancer[64,65]. In summary, EUS has the indispensable role in staging and therapeutic decision-making in pancreatic cancer[66,67].
| 1. | Schwartz SM. Epidemiology of Cancer. Clin Chem. 2024;70:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 112] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 2. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12644] [Article Influence: 6322.0] [Reference Citation Analysis (6)] |
| 3. | Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75:10-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 1510] [Article Influence: 1510.0] [Reference Citation Analysis (3)] |
| 4. | Mosalem OM, Abdelhakeem A, Abdel-Razeq NH, Babiker H. Pancreatic ductal adenocarcinoma (PDAC): clinical progress in the last five years. Expert Opin Investig Drugs. 2025;34:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Stoop TF, Theijse RT, Seelen LWF, Groot Koerkamp B, van Eijck CHJ, Wolfgang CL, van Tienhoven G, van Santvoort HC, Molenaar IQ, Wilmink JW, Del Chiaro M, Katz MHG, Hackert T, Besselink MG; International Collaborative Group on Locally Advanced Pancreatic Cancer. Preoperative chemotherapy, radiotherapy and surgical decision-making in patients with borderline resectable and locally advanced pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2024;21:101-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 112] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 6. | Blackford AL, Canto MI, Dbouk M, Hruban RH, Katona BW, Chak A, Brand RE, Syngal S, Farrell J, Kastrinos F, Stoffel EM, Rustgi A, Klein AP, Kamel I, Fishman EK, He J, Burkhart R, Shin EJ, Lennon AM, Goggins M. Pancreatic Cancer Surveillance and Survival of High-Risk Individuals. JAMA Oncol. 2024;10:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 7. | Wei H, Ren H. Precision treatment of pancreatic ductal adenocarcinoma. Cancer Lett. 2024;585:216636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | van Roessel S, Kasumova GG, Verheij J, Najarian RM, Maggino L, de Pastena M, Malleo G, Marchegiani G, Salvia R, Ng SC, de Geus SW, Lof S, Giovinazzo F, van Dam JL, Kent TS, Busch OR, van Eijck CH, Koerkamp BG, Abu Hilal M, Bassi C, Tseng JF, Besselink MG. International Validation of the Eighth Edition of the American Joint Committee on Cancer (AJCC) TNM Staging System in Patients With Resected Pancreatic Cancer. JAMA Surg. 2018;153:e183617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (1)] |
| 9. | Court CM, Hines OJ. The New American Joint Committee on Cancer TNM Staging System for Pancreatic Cancer-Balancing Usefulness With Prognostication. JAMA Surg. 2018;153:e183629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hu ZI, O'Reilly EM. Therapeutic developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2024;21:7-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 237] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 11. | Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20:7864-7877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 264] [Article Influence: 22.0] [Reference Citation Analysis (4)] |
| 12. | Kitano M, Kudo M, Sakamoto H, Komaki T. Endoscopic ultrasonography and contrast-enhanced endoscopic ultrasonography. Pancreatology. 2011;11 Suppl 2:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M, Murakami T, Takeyama Y. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 14. | Shin CM, Villa E. The efficiency of contrast-enhanced endoscopic ultrasound (EUS) combined with EUS elastography for pancreatic cancer diagnosis: a systematic review and meta-analysis. Ultrasonography. 2023;42:20-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 15. | Li JH, He R, Li YM, Cao G, Ma QY, Yang WB. Endoscopic ultrasonography for tumor node staging and vascular invasion in pancreatic cancer: a meta-analysis. Dig Surg. 2014;31:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 16. | Fujita A, Ryozawa S, Mizuide M, Tanisaka Y, Ogawa T, Suzuki M, Katsuda H, Saito Y, Tashima T, Miyaguchi K, Arai E, Kawasaki T, Mashimo Y. Diagnosis of Pancreatic Solid Lesions, Subepithelial Lesions, and Lymph Nodes Using Endoscopic Ultrasound. J Clin Med. 2021;10:1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Puli SR, Singh S, Hagedorn CH, Reddy J, Olyaee M. Diagnostic accuracy of EUS for vascular invasion in pancreatic and periampullary cancers: a meta-analysis and systematic review. Gastrointest Endosc. 2007;65:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Expert Panel on Gastrointestinal Imaging:, Qayyum A, Tamm EP, Kamel IR, Allen PJ, Arif-Tiwari H, Chernyak V, Gonda TA, Grajo JR, Hindman NM, Horowitz JM, Kaur H, McNamara MM, Noto RB, Srivastava PK, Lalani T. ACR Appropriateness Criteria(®) Staging of Pancreatic Ductal Adenocarcinoma. J Am Coll Radiol. 2017;14:S560-S569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Tellez-Avila FI, Chavez-Tapia NC, López-Arce G, Franco-Guzmán AM, Sosa-Lozano LA, Alfaro-Lara R, Chan-Nuñez C, Giovannini M, Elizondo-Rivera J, Ramírez-Luna MA. Vascular invasion in pancreatic cancer: predictive values for endoscopic ultrasound and computed tomography imaging. Pancreas. 2012;41:636-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Rösch T, Dittler HJ, Strobel K, Meining A, Schusdziarra V, Lorenz R, Allescher HD, Kassem AM, Gerhardt P, Siewert JR, Höfler H, Classen M. Endoscopic ultrasound criteria for vascular invasion in the staging of cancer of the head of the pancreas: a blind reevaluation of videotapes. Gastrointest Endosc. 2000;52:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Ji H, Hu C, Yang X, Liu Y, Ji G, Ge S, Wang X, Wang M. Lymph node metastasis in cancer progression: molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduct Target Ther. 2023;8:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 22. | Levine I, Trindade AJ. Endoscopic ultrasound fine needle aspiration vs fine needle biopsy for pancreatic masses, subepithelial lesions, and lymph nodes. World J Gastroenterol. 2021;27:4194-4207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 23. | Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, Real MI, Gilabert R, Quintó L, Trilla A, Feu F, Montanyà X, Fernández-Cruz L, Navarro S. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 268] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 24. | Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 25. | Li J, Wang QB, Liang YB, Chen XM, Luo WL, Li YK, Chen X, Lu QY, Ke Y. Tumor-associated lymphatic vessel density is a reliable biomarker for prognosis of esophageal cancer after radical resection: a systemic review and meta-analysis. Front Immunol. 2024;15:1453482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 26. | Zhang S, Zhang D, Yi S, Gong M, Lu C, Cai Y, Tang X, Zou L. The relationship of lymphatic vessel density, lymphovascular invasion, and lymph node metastasis in breast cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:2863-2873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Li J, Liang YB, Wang QB, Li YK, Chen XM, Luo WL, Lakang Y, Yang ZS, Wang Y, Li ZW, Ke Y. Tumor-associated lymphatic vessel density is a postoperative prognostic biomarker of hepatobiliary cancers: a systematic review and meta-analysis. Front Immunol. 2024;15:1519999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 28. | Ichim VA, Chira RI, Nagy GA, Chira A, Mircea PA. Endoscopic Ultrasound-guided Biopsy of Liver Tumors. In Vivo. 2022;36:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | ASGE Standards of Practice Committee; Eloubeidi MA, Decker GA, Chandrasekhara V, Chathadi KV, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JR, Pasha SF, Saltzman JR, Sharaf R, Shergill AK, Cash BD, DeWitt JM. The role of endoscopy in the evaluation and management of patients with solid pancreatic neoplasia. Gastrointest Endosc. 2016;83:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Teshima CW, Sandha GS. Endoscopic ultrasound in the diagnosis and treatment of pancreatic disease. World J Gastroenterol. 2014;20:9976-9989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012;138:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 32. | Kawasaki Y, Hijioka S, Nagashio Y, Ohba A, Maruki Y, Takeshita K, Takasaki T, Agarie D, Hagiwara Y, Hara H, Okamoto K, Yamashige D, Kondo S, Morizane C, Ueno H, Mizui T, Takamoto T, Nara S, Ban D, Esaki M, Saito Y, Hiraoka N, Okusaka T. Diagnostic performance of EUS-guided tissue acquisition for solid pancreatic lesions ≤10 mm. Endosc Ultrasound. 2024;13:115-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 33. | Yamashita Y, Shimokawa T, Napoléon B, Fusaroli P, Gincul R, Kudo M, Kitano M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig Endosc. 2019;31:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Buxbaum J, Ko C, Varghese N, Lee A, Sahakian A, King K, Serna J, Lee H, Tchelepi H, Van Dam J, Duddalwar V. Qualitative and Quantitative Contrast-enhanced Endoscopic Ultrasound Improves Evaluation of Focal Pancreatic Lesions. Clin Gastroenterol Hepatol. 2020;18:917-925.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Zhang B, Zhu F, Li P, Yu S, Zhao Y, Li M. Endoscopic ultrasound elastography in the diagnosis of pancreatic masses: A meta-analysis. Pancreatology. 2018;18:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Yamada K, Kawashima H, Ohno E, Ishikawa T, Tanaka H, Nakamura M, Miyahara R, Ishigami M, Hirooka Y, Fujishiro M. Diagnosis of vascular invasion in pancreatic ductal adenocarcinoma using endoscopic ultrasound elastography. BMC Gastroenterol. 2020;20:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology. 2022;163:386-402.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 603] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 38. | Guo T, Xu T, Zhang S, Lai Y, Wu X, Wu D, Feng Y, Jiang Q, Wang Q, Qian J, Yang A. The role of EUS in diagnosing focal autoimmune pancreatitis and differentiating it from pancreatic cancer. Endosc Ultrasound. 2021;10:280-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Iglesias-Garcia J, de la Iglesia-Garcia D, Olmos-Martinez JM, Lariño-Noia J, Dominguez-Muñoz JE. Differential diagnosis of solid pancreatic masses. Minerva Gastroenterol Dietol. 2020;66:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Yang R, Lu M, Qian X, Chen J, Li L, Wang J, Zhang Y. Diagnostic accuracy of EUS and CT of vascular invasion in pancreatic cancer: a systematic review. J Cancer Res Clin Oncol. 2014;140:2077-2086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Gorris M, Janssen QP, Besselink MG, van den Broek BLJ, van Eijck CHJ, van Gils MJ, Koerkamp BG, Struik F, van Driel LMJW, van Hooft JE. Sensitivity of CT, MRI, and EUS-FNA/B in the preoperative workup of histologically proven left-sided pancreatic lesions. Pancreatology. 2022;22:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Fukushima H, Itoh S, Takada A, Mori Y, Suzuki K, Sawaki A, Iwano S, Satake H, Ota T, Ikeda M, Ishigaki T. Diagnostic value of curved multiplanar reformatted images in multislice CT for the detection of resectable pancreatic ductal adenocarcinoma. Eur Radiol. 2006;16:1709-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4:717-25; quiz 664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Ahmad NA, Lewis JD, Siegelman ES, Rosato EF, Ginsberg GG, Kochman ML. Role of endoscopic ultrasound and magnetic resonance imaging in the preoperative staging of pancreatic adenocarcinoma. Am J Gastroenterol. 2000;95:1926-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Harrington KA, Shukla-Dave A, Paudyal R, Do RKG. MRI of the Pancreas. J Magn Reson Imaging. 2021;53:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Kitano M, Yoshida T, Itonaga M, Tamura T, Hatamaru K, Yamashita Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J Gastroenterol. 2019;54:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (1)] |
| 47. | Grützmeier SE, Sodal HMM, Kovacevic B, Vilmann P, Karstensen JG, Klausen P. EUS-guided biopsies versus surgical specimens for establishing patient-derived pancreatic cancer organoids: a systematic review and meta-analysis. Gastrointest Endosc. 2024;100:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 48. | Maruo M, Ikeura T, Takaori A, Ikeda M, Nakamaru K, Ito T, Masuda M, Mitsuyama T, Nakayama S, Shimatani M, Takaoka M, Shibata N, Boku S, Yasuda T, Miyazaki H, Matsumura K, Yamaki S, Hashimoto D, Satoi S, Naganuma M. Impact of endoscopic ultrasound-guided tissue acquisition on prognosis and peritoneal lavage cytology in resectable or borderline resectable pancreatic ductal adenocarcinoma. Pancreatology. 2024;24:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 49. | Sugimoto M, Takahashi N, Farnell MB, Smyrk TC, Truty MJ, Nagorney DM, Smoot RL, Chari ST, Carter RE, Kendrick ML. Survival benefit of neoadjuvant therapy in patients with non-metastatic pancreatic ductal adenocarcinoma: A propensity matching and intention-to-treat analysis. J Surg Oncol. 2019;120:976-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Oshima M, Okano K, Kamada H, Suto H, Ando Y, Ibuki E, Ishikawa R, Masaki T, Haba R, Suzuki Y. P53 immunolabeling in EUS-FNA biopsy can predict low resection rate and early recurrence in resectable or borderline resectable pancreatic cancer treated with neoadjuvant therapy. J Hepatobiliary Pancreat Sci. 2023;30:802-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 51. | Hu H, Xu Y, Zhang Q, Gao Y, Wu Z. The survival effect of neoadjuvant therapy and neoadjuvant plus adjuvant therapy on pancreatic ductal adenocarcinoma patients with different TNM stages: a propensity score matching analysis based on the SEER database. Expert Rev Anticancer Ther. 2024;24:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 52. | Salom F, Prat F. Current role of endoscopic ultrasound in the diagnosis and management of pancreatic cancer. World J Gastrointest Endosc. 2022;14:35-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 53. | Kawamura R, Ishii Y, Serikawa M, Tsuboi T, Tsushima K, Nakamura S, Hirano T, Ikemoto J, Kiyoshita Y, Saeki S, Tamura Y, Miyamoto S, Nakamura K, Furukawa M, Ishida K, Arihiro K, Uemura K, Aikata H. Optimal indication of endoscopic retrograde pancreatography-based cytology in the preoperative pathological diagnosis of pancreatic ductal adenocarcinoma. Pancreatology. 2022;22:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Sagami R, Nakahodo J, Minami R, Yamao K, Yoshida A, Nishikiori H, Takenaka M, Mizukami K, Murakami K. True diagnostic ability of EUS-guided fine-needle aspiration/biopsy sampling for small pancreatic lesions ≤10 mm and salvage diagnosis by pancreatic juice cytology: a multicenter study. Gastrointest Endosc. 2024;99:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 55. | Zhang D, Wu C, Yang Z, Yin H, Liu Y, Li W, Huang H, Jin Z. The application of artificial intelligence in EUS. Endosc Ultrasound. 2024;13:65-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 56. | Dahiya DS, Al-Haddad M, Chandan S, Gangwani MK, Aziz M, Mohan BP, Ramai D, Canakis A, Bapaye J, Sharma N. Artificial Intelligence in Endoscopic Ultrasound for Pancreatic Cancer: Where Are We Now and What Does the Future Entail? J Clin Med. 2022;11:7476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Zhang S, Zhou Y, Tang D, Ni M, Zheng J, Xu G, Peng C, Shen S, Zhan Q, Wang X, Hu D, Li WJ, Wang L, Lv Y, Zou X. A deep learning-based segmentation system for rapid onsite cytologic pathology evaluation of pancreatic masses: A retrospective, multicenter, diagnostic study. EBioMedicine. 2022;80:104022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 58. | Mahajan S, Siyu S, Bhutani MS. What can artificial intelligence do for EUS? Endosc Ultrasound. 2025;14:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 59. | Huang J, Fan X, Liu W. Applications and Prospects of Artificial Intelligence-Assisted Endoscopic Ultrasound in Digestive System Diseases. Diagnostics (Basel). 2023;13:2815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 60. | Ishikawa T, Hayakawa M, Suzuki H, Ohno E, Mizutani Y, Iida T, Fujishiro M, Kawashima H, Hotta K. Development of a Novel Evaluation Method for Endoscopic Ultrasound-Guided Fine-Needle Biopsy in Pancreatic Diseases Using Artificial Intelligence. Diagnostics (Basel). 2022;12:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Dhali A, Kipkorir V, Srichawla BS, Kumar H, Rathna RB, Ongidi I, Chaudhry T, Morara G, Nurani K, Cheruto D, Biswas J, Chieng LR, Dhali GK. Artificial intelligence assisted endoscopic ultrasound for detection of pancreatic space-occupying lesion: a systematic review and meta-analysis. Int J Surg. 2023;109:4298-4308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Yin H, Yang X, Sun L, Pan P, Peng L, Li K, Zhang D, Cui F, Xia C, Huang H, Li Z. The value of artificial intelligence techniques in predicting pancreatic ductal adenocarcinoma with EUS images: A meta-analysis and systematic review. Endosc Ultrasound. 2023;12:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 63. | Binda C, Coluccio C, Marocchi G, Sbrancia M, Fabbri C. The Role of Contrast-Enhanced Harmonic Endoscopic Ultrasound in Interventional Endoscopic Ultrasound. Medicina (Kaunas). 2021;57:1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 64. | Yang D, Ning J, Liao X, Jiang H, Qin S. Local Sustained Chemotherapy of Pancreatic Cancer Using Endoscopic Ultrasound-Guided Injection of Biodegradable Thermo-Sensitive Hydrogel. Int J Nanomedicine. 2023;18:3989-4005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 65. | Levy MJ, Alberts SR, Bamlet WR, Burch PA, Farnell MB, Gleeson FC, Haddock MG, Kendrick ML, Oberg AL, Petersen GM, Takahashi N, Chari ST. EUS-guided fine-needle injection of gemcitabine for locally advanced and metastatic pancreatic cancer. Gastrointest Endosc. 2017;86:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Gornals JB. EUS-guided radiofrequency ablation in pancreatic cancer: Promising but still questionable! Gastrointest Endosc. 2024;100:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 67. | Kongkam P, Tiankanon K, Seo DW, Luangsukrerk T, Sriuranpong V, Nantavithya C, Jantarattana T, Cañones A, Kerr SJ, Tantitanawat K, Angsuwatcharakon P, Ridtitid W, Kullavanijaya P, Rerknimitr R. EUS-guided radiofrequency ablation plus chemotherapy versus chemotherapy alone for pancreatic cancer (ERAP): An observational open-label pilot study. Endosc Ultrasound. 2023;12:402-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/