Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.107589
Revised: April 12, 2025
Accepted: May 27, 2025
Published online: July 15, 2025
Processing time: 110 Days and 10.8 Hours
Colorectal cancer (CRC) is a common malignant tumor worldwide, and its tumor microenvironment (TME) plays a crucial role in tumor progression. Neutrophil extracellular traps (NETs), as an important component of the TME, have received widespread attention in recent years. This article explores the biological functions and molecular mechanisms of NETs in CRC and their impact on disease progression, while analyzing the application of single-cell sequencing technology (SCS) in this field. The development of SCS provides a new perspective for understanding the role of NETs in CRC. By combining SCS technology, targeting key regulatory nodes of NETs is expected to reverse the immunosuppressive microenvironment and provide a theoretical basis for developing novel diagnostic biomarkers and targeted therapeutic strategies, thereby promoting the development of precision medicine in CRC and helping enhance patient prognosis. Future research should further explore the integration of SCS technology with complementary methodologies to investigate NETs and develop specific detection methods and therapeutic strategies targeting NETs to enhance early diagnosis and treatment efficacy of tumors.
Core Tip: Neutrophil extracellular traps (NETs) play a critical role in the progression of colorectal cancer (CRC), impacting tumor metastasis, immune evasion, and angiogenesis. Recent advancements in single-cell sequencing (SCS) have provided deeper insights into the mechanisms behind NET formation and their interactions within the tumor microenvironment. SCS has revealed that NETs not only facilitate CRC cell invasion and metastasis but also promote an immunosuppressive environment by inhibiting T cell activity. This makes NETs a promising therapeutic target for CRC, potentially enhancing early diagnosis, treatment efficacy, and patient prognosis by targeting key regulatory pathways of NET formation.
- Citation: Xu ZX, Qu FY, Zhang Z, Luan WY, Lin SX, Miao YD. Exploring the role of neutrophil extracellular traps in colorectal cancer: Insights from single-cell sequencing. World J Gastrointest Oncol 2025; 17(7): 107589
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/107589.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.107589
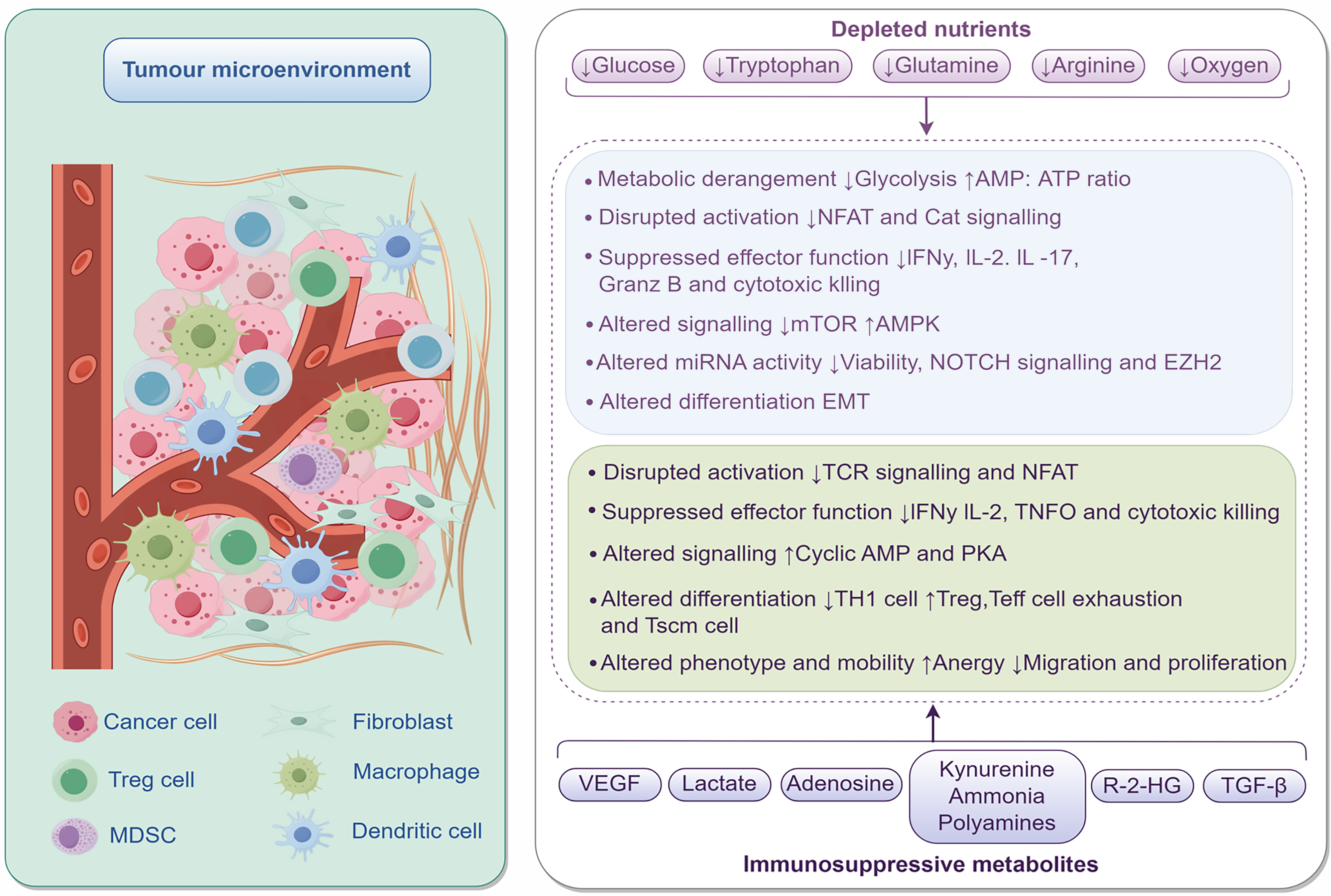
Colorectal cancer (CRC) is a common malignant tumor. According to data released by the International Agency for Research on Cancer of the World Health Organization, there were approximately 1.93 million new cases of CRC globally in 2022, with nearly 900000 patients succumbing to it. CRC ranks as the third most commonly diagnosed malignancy and the second leading cause of cancer-related deaths worldwide, posing a significant threat to global human health[1]. In China, CRC advanced from 4th to 2nd place in the ranking of malignant tumor incidence between 2013 and 2022, while its mortality ranking rose from 5th to 4th[2]. According to the National Cancer Center of China statistics, there were 517106 new cases of CRC in China in 2022, which accounted for 26.8% of all cases worldwide; there were 240010 deaths, which makes it the second deadliest cancer in China, just behind lung cancer[3]. Despite continuous innovations in screening methods and comprehensive treatment plans, the five-year survival rate for CRC still shows significant clinical stage dependence, plummeting from 91% in stage I to 12% in stage IV[4]. Behind this drastic decline in survival rates lies a complex multi-stage carcinogenesis mechanism: From the formation of adenomas triggered by mutations in driver genes such as APC and KRAS to malignant transformation caused by abnormal epigenetic modifications, ultimately completing the metastatic process through angiogenesis and Immune escape[5]. Among these processes, the tumor microenvironment (TME) is a crucial foundation for the growth, proliferation, and metastasis of tumor cells, closely related to the pathogenesis, progression, and clinical prognosis of CRC. Studies have shown that immunosuppression plays a dual regulatory role in the microenvironment. Studies demonstrate that immunosuppression exerts dual regulatory roles within the TME (Figure 1). Active immune evasion: Regulatory T cells (Tregs, Foxp3 +) suppress cluster of differentiation (CD) 8 + T cells via cytotoxic T-lymphocyte-associated protein 4 and interleukin (IL)-10, while myeloid-derived suppressor cells deplete local L-arginine through arginase-1 and inducible nitric oxide synthase, collectively driving a “cold tumor” phenotype (e.g., microsatellite instability-low/different mismatch repair-type CRC)[6,7]. Stromal-mediated tumor promotion: Tumor-associated macrophages (TAM) (M2 type) secrete vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β), which not only induce angiogenesis but also promote epithelial-mesenchymal transition (EMT), thereby accelerating adenoma-to-adenocarcinoma progression[8,9]. The TME, through the dynamic interplay of inflammation, immune response, and stroma, runs through the entire process of CRC from adenoma malignancy to metastasis. In these processes, neutrophil extracellular traps (NETs) play a crucial role.
NETs are primarily composed of a DNA backbone, histones, and various antimicrobial proteins. When neutrophils are stimulated by specific triggers, they undergo a unique cell death program known as NETosis. During this process neutrophils release their chromatin into the extracellular space, accompanied by the release of various antimicrobial substances that together form the structure of NETs. Activated neutrophils release NETs under various stimuli, and the NETosis process is driven by protein arginine deiminase 4 (PAD4). The components released have the role of capturing and eliminating microbes, but they also have adverse effects such as promoting inflammation[10].
In recent years, NETs are getting more attention in CRC research. Research indicates that NETs not only play an important role in immune defense but may also promote the proliferation and metastasis of tumor cells. The formation of NETs is induced by various factors released from tumor cells, which can activate neutrophils, prompting them to release DNA and antimicrobial proteins, thereby providing a favorable microenvironment for tumor cells. NETs promote tumor cell proliferation through various mechanisms, such as facilitating EMT, enabling tumor cells to acquire stronger migratory and invasive capabilities[11]. NETs can capture circulating tumor cells (CTCs), increase vascular permeability, and thus promote the invasion of tumor cells and the establishment of tumor micro metastases[12]. In CRC patients, high expression of NETs is also associated with tumor grade and poor prognosis, suggesting that NETs may become a new therapeutic target for CRC.
Single-cell sequencing technology (SCS) is a rapidly developing high-throughput genomics technology that can analyze gene expression, genomic, and epigenetic information at the single-cell level. The core of this technology lies in its ability to isolate, amplify, and sequence individual cells, revealing intercellular heterogeneity and complex biological processes. Compared to traditional bulk sequencing, SCS can capture the unique transcriptomic characteristics of each cell within a cell population, providing more precise analyses of cellular characteristics and intercellular interactions[13]. In 2009, the first single-cell message RNA whole-transcriptome sequencing was developed, significantly enhancing researchers’ ability to analyze the heterogeneity of single-cell transcriptomes during early mammalian embryonic development[14]. In 2011, the first single-cell DNA sequencing experiment of human cancer cells was conducted, followed by the first single-cell exome sequencing experiment in 2012[15]. Since then, SCS has been increasingly applied in cancer research, particularly in revealing tumor heterogeneity, TME, and its interactions with the immune system. Studies show that through SCS of CRC cells, researchers can identify different tumor cell subtypes, tumor stem cell populations, and their roles in tumor progression, as well as reveal the transcriptomic characteristics of CRC cells at different developmental stages, thus aiding in the identification of key genes associated with tumor metastasis[16]. In the field of CRC research, SCS can further reveal cellular clonal and differentiations, cellular heterogeneity in tissues, cell types involved in CRC, changes in immune cells, gene expression regulatory networks, dynamic changes between transcription and protein abundance, and the operational mechanisms of molecular networks or cellular networks in complex tissues[17]. By using SCS to explore intercellular heterogeneity and clinical pathological features of tumor cells, valuable insights can be gained for early diagnosis, treatment, and prognosis of CRC. This approach serves as a vital resource for identifying prognostic biomarkers, discovering novel therapeutic targets, and advancing personalized treatment interventions in CRC management.
In summary, the role of NETs in CRC is a complex and important research area. By integrating SCS, a deeper exploration of the specific mechanisms of NETs in CRC will help reveal their dual roles in TME and provide a theoretical basis for developing new therapeutic strategies. This review aims to systematically summarize the application progress of SCS in the study of NETs in CRC, integrating the latest findings of this technology in revealing the dynamic interactions between NETs and TME, addressing the unresolved molecular basis of its dual roles in tumor occurrence and development, and striving to clarify the precise mechanisms by which NETs influence tumor progression and immune responses, revealing their key roles in the pathological evolution of CRC. This will provide a theoretical foundation for developing novel diagnostic biomarkers and targeted therapeutic strategies, thereby advancing precision medicine in CRC and offering new scientific support for improving patient prognosis.
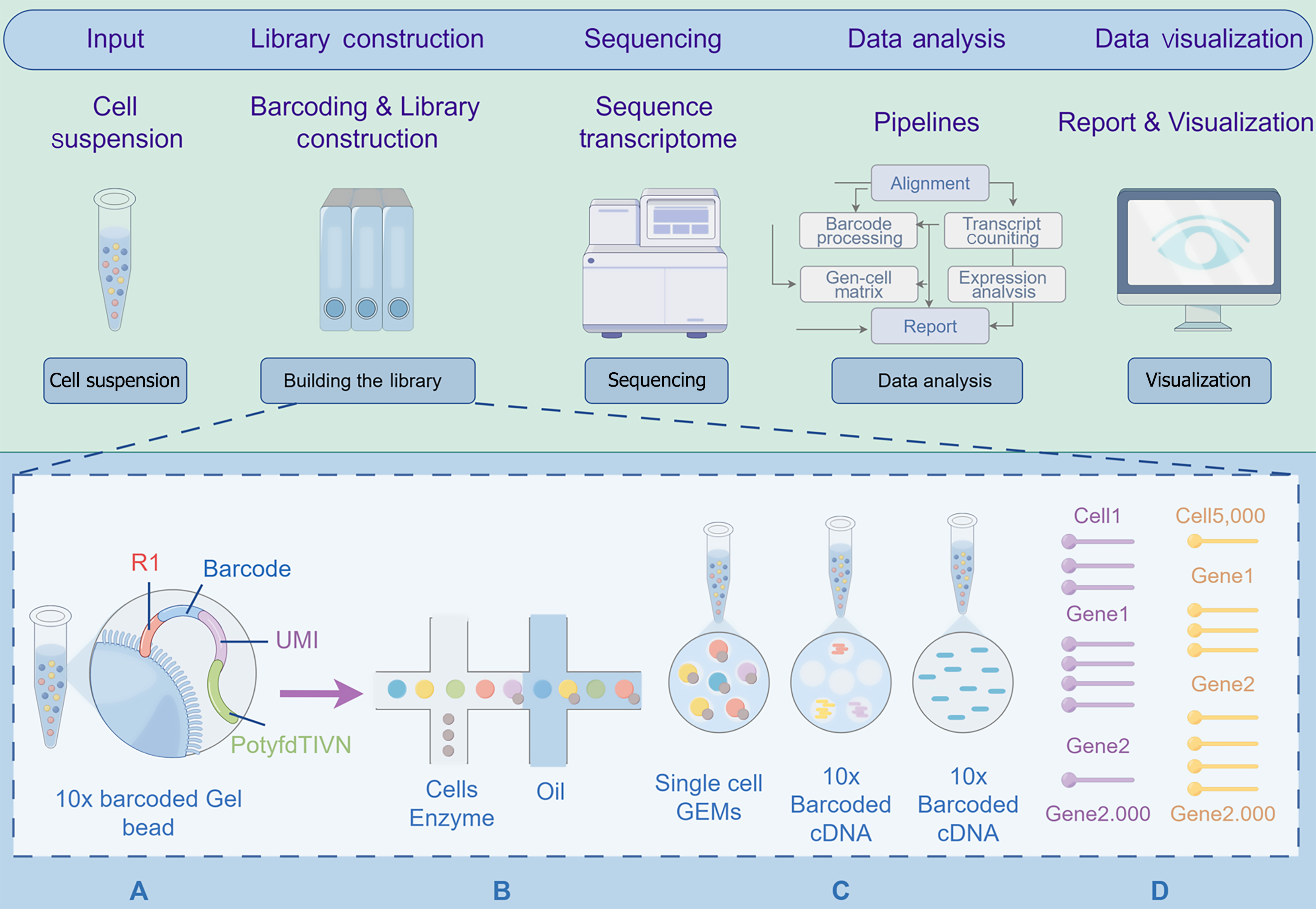
SCS is a second-generation sequencing method primarily used to analyze differences in genetic and protein information between cells, obtain genetic information from hard-to-culture microorganisms, and reveal the complex heterogeneous mechanisms of disease occurrence and development. The main methods of SCS include single-cell RNA sequencing (scRNA-seq), single-cell DNA sequencing, and single-cell epigenomic sequencing. The main steps include single-cell isolation, nucleic acid amplification, high-throughput sequencing, and data analysis. Single-cell isolation and nucleic acid amplification represent core technical components, with isolation methodologies encompassing serial dilution, micromanipulation, fluorescence-activated cell sorting (FACS), immunomagnetic separation, laser capture microdissection (LCM), and microfluidic platforms; amplification techniques primarily involve whole genome amplification and whole transcriptome amplification[18] (Figure 2). In recent years, with continuous technological advancements, the throughput and accuracy of SCS have significantly improved, demonstrating broad application potential in tumor research, immunology, and developmental biology[19]. Traditional sequencing technologies can only obtain average signals of cell populations during bulk sequencing, failing to specifically characterize the heterogeneity of tumor cells. SCS can accurately describe the genomic, transcriptomic, and other omics differences at the single-cell level, thereby revealing the evolutionary processes of tumor cells[20].
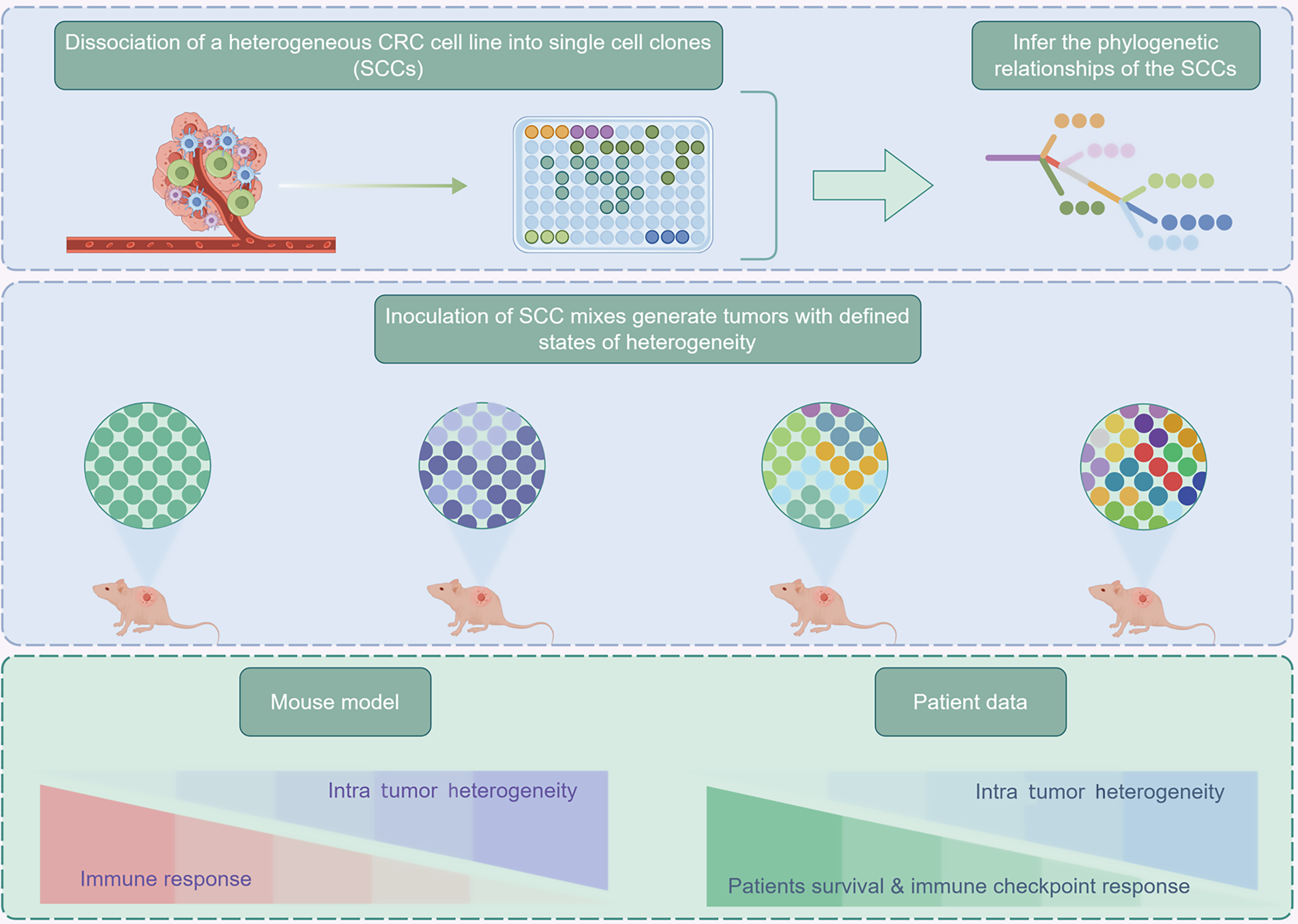
Organisms are composed of numerous different types of cells, which exhibit differences in gene expression, function, and phenotype. Tumor cells, in particular exhibit high heterogeneity, including clonal diversity and mutational evolution, making tumor heterogeneity a key feature of malignant tumors and a significant obstacle in cancer treatment and research[21]. Although previous bulk tissue sequencing had a wide coverage and high accuracy, it could only represent the dominant cellular signal information of each sample while masking the unique gene expression of rare cells. Therefore, it could not represent genes that are unstable in subgroups but remain constant in most cells. With advancements in genomic technology, the emergence of SCS has effectively tackled these issues. Genetic, transcriptomic, and epigenetic sequencing at the single-cell level provides an important foundation for correctly classifying heterogeneous tumor cell subpopulations and revealing the complex changes of tumor cells at the molecular level. The advent of SCS has made characterizing heterogeneity possible, and some studies have conducted SCS on CRC patients to study intratumorally heterogeneity at the single-cell level (Figure 3). Research shows that CRC tumor cells exhibit significant heterogeneity in gene expression, copy number variations, and metabolic characteristics; for example, single-cell transcriptome sequencing identified a high recurrence cell subpopulation that specifically expresses the EMP1 gene and possesses stem cell-like characteristics, capable of lying dormant in the liver post-surgery and triggering metastatic recurrence[22-25]. Additionally, single-cell multi-omics integrative analysis found that CRC epithelial cells could be further divided into intrinsic-consensus molecular subtypes (iCMS) 2 and iCMS3 subtypes, with the iCMS3 subtype associated with microsatellite instability status and had poorer clinical prognosis, while the iCMS2 subtype was enriched in pathways related to stroma remodeling. Spatial transcriptomics elucidates the spatial heterogeneity of TME, particularly revealing co-localization patterns between fibroblast activation protein + fibroblasts and SPP1 + macrophages at tumor invasive fronts, where they form an immune-rejecting connective tissue structure through the TGF-β and IL-1 signaling axis, inhibiting T cell infiltration and reducing responses to anti-programmed cell death ligand 1 (PD-L1) treatment. Notably, single-cell epigenomic analysis found that DNA methylation heterogeneity in tumor cells was associated with chemotherapy resistance; for instance, certain epithelial cell subpopulations in liver metastatic foci acquired pro-metastatic characteristics through epigenetic reprogramming[26]. These findings not only deepen the understanding of heterogeneity in CRC but also provide important theoretical support for developing personalized treatment strategies and precision medicine.
The diagnosis of CRC has traditionally relied on tissue origin and histological characteristics. Traditional diagnostic and prognostic assessment methods have certain limitations, while SCS opens up new opportunities for the development of precision medicine in the field of CRC. SCS can analyze individual cells, enhancing the understanding of the TME, detecting intercellular variations, and can also be applied to tumor or microenvironment transcriptomics, cell sorting, and phenotypic analysis, offering fresh ideas for early CRC diagnosis. Increasing evidence suggests that molecular characteristics can influence the TME, thereby altering clinical manifestations and responses to treatment. By identifying sensitive biomarkers, mutations, or gene expression profiles, a better understanding of molecular characteristics will provide new ideas for the early diagnosis of cancer[27].
SCS can generate comprehensive genetic information for all cell types within a tumor. By analyzing the gene expression patterns of different tumor subpopulations and comparing them with the gene expression patterns of normal tissues from the same patient or model system, the origin of the tumor can be accurately determined. Further analysis of differentially expressed genes and pathways in cancer cells also helps identify genes and pathways critical to cancer development, discovering potential therapeutic drug targets. SCS can reveal new drug targets and promote the development of combination therapies, such as combination treatments targeting different cancer cell subclones and the combination of immunotherapy with targeted therapy[28]. For example, a team led by Wang et al[29] at Sun Yat-sen University discovered through SCS analysis that the TME of metastatic microsatellite stable/proficient mismatch repair CRC patients contains immune suppressive cell infiltration (e.g., Tregs) and abnormal angiogenesis signals. Based on this, researchers proposed a combination of histone deacetylase inhibitors (to reshape epigenetic activation of immunity), programmed cell death protein 1 (PD-1) inhibitors (to relieve T cell exhaustion), and anti-VEGF drugs (to inhibit angiogenesis), significantly enhancing CD8 + T cell infiltration and tumor immune activity. Clinical data showed that this regimen increased the objective response rate by nearly three times and extended progression-free survival by five times, providing a new strategy for treating “cold tumors” with immunotherapy[29]. Furthermore, SCS methods provide a mechanism for studying the evolutionary structure of tumors and the genomic information of rare cell populations such as CTCs. For instance, studies have found that CTCs exhibit high genomic heterogeneity and possess certain gene mutations associated with tumor metastasis. These gene mutations may enable CTCs to have stronger invasive and metastatic capabilities, potentially affecting patient responses to treatment[30]. This finding provides new ideas for targeted therapeutic strategies. The latest advancements in SCS have empowered high-dimensional cellular mapping of tumorigenesis, metastatic dissemination, chemoresistance mechanisms, immunogenic antigen presentation, and immune editing evolutionary trajectories with unprecedented spatiotemporal resolution. Therefore, SCS technology enables a more detailed understanding of the genomic structure of cancer cell subpopulations, promoting the development of new tumor-targeted strategies against the backdrop of tumor heterogeneity[31].
Based on general characteristics, pathological types, and pathological stages, the prognosis of CRC patients can be roughly predicted. However, with the development of scRNA-seq, integrating gene expression characteristics and clinical information can more accurately predict the prognosis of CRC patients. Studies have found that specific subpopulations of cancer-associated fibroblasts (CAFs) and tumor-infiltrating lymphocytes (TILs) are significantly associated with patient survival rates[32]. Furthermore, scRNA-seq combined with machine learning algorithms, such as least absolute shrinkage and selection operator regression and random forests, can screen for high-predictive-value gene markers and construct multi-gene risk scoring models, achieving precise stratification of patient prognosis[33]. In experiments by Luo et al[34], SCS was used to construct and evaluate prognostic models for CRC patients. Survival analysis of 417 CRC patients with survival information from The Cancer Genome Atlas identified 27 autophagy-related genes (ARGs) significantly associated with overall survival (OS). Based on Boruta feature selection, 11 important ARGs were determined to calculate their regression coefficients, constructing a prognostic model that stratified patients into high-risk and low-risk groups based on risk scores. Survival analysis showed that the high-risk group had poor prognosis, with significant differences in OS between the two groups. Time-dependent receiver operating characteristic analysis indicated good predictive accuracy of the model, with risk scores unrelated to patient age and gender but related to pathological stage. The prognostic model constructed based on these genes demonstrated good predictive performance for CRC patient prognosis, aiding in revealing the molecular mechanisms of CRC occurrence and development[34]. The dynamic interplay between various immune cells and inflammatory chemokines within the TME reciprocally modulates tumor progression, impacts recurrence patterns and therapeutic responses, and ultimately contributes to unfavorable clinical outcomes in CRC patients. SCS enables comprehensive characterization of malignant cell features and TME influences in CRC, while concurrently identifying prognostic biomarkers and potential immunotherapeutic targets. This approach holds promise for optimizing personalized treatment strategies and enhancing both survival rates and therapeutic efficacy.
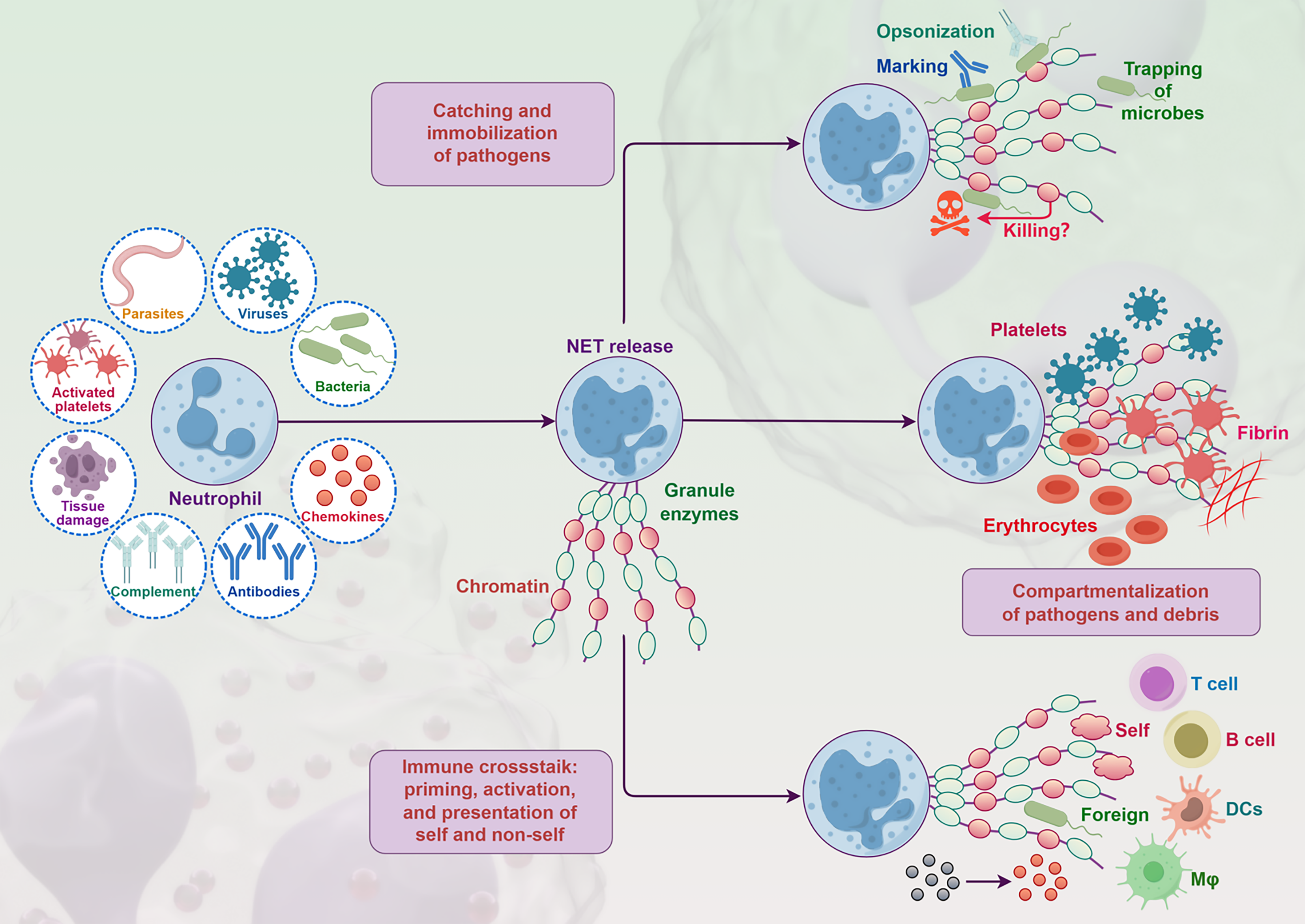
NETs are products of neutrophils dying in response to certain stimuli, initially thought to be a reaction to bacterial infections in infectious diseases (Figure 4). Subsequently, numerous studies have found that NETs are also associated with other non-infectious inflammatory diseases, including thromboembolism, autoimmune diseases, and cancer. CRC is one of the most common malignant tumors in the world. NETs are closely linked to the occurrence, development, and spread of CRC[35].
NETs are a mesh-like structure composed of chromatin DNA interspersed with cytoplasmic and granular proteins, extruded by activated neutrophils to capture and kill bacteria and fungi. The core scaffold of NETs is nuclear DNA extruded from neutrophils, forming a three-dimensional mesh structure with some interspersed specific cytoplasmic and granular proteins. However, increasing evidence suggests that NETs are related to the development and metastasis of cancer. In clinical studies, NETs infiltrate primary gastrointestinal cancer tissues and are found in higher quantities in metastatic lesions. Higher levels of NETs in the blood are linked to more advanced tumor stages, indicating that NETs are prognostic markers for gastrointestinal cancers[36].
Neutrophils are the first line of defense against pathogens, functioning through various mechanisms[10]. The release of NETs primarily occurs through a cell death process called NETosis, including steps like nuclear deformation and membrane breakdown; another form is non-lytic NETosis, which can rapidly release NETs within minutes, independent of cell death, by secreting chromatin and granular contents, accompanied by the release of granular proteins[37].
There are two pathways of NETosis: The classical pathway of NET formation requires reactive oxygen species (ROS): Phorbol 12-myristate 13-acetate stimulates neutrophils to produce nicotinamide adenine dinucleotide phosphate oxidase-2 dependent ROS, causing neutrophils to release neutrophil elastase (NE), partially degrading specific histones and myeloperoxidase (MPO), driving chromatin decondensation, leading to the extrusion of nuclear DNA to form a mesh structure[37-39]. Additionally, under special stimuli, mitochondrial ROS can also drive the formation of NETs, and in some cases, mitochondrial DNA can be extruded under ROS-dependent conditions to form NETs, with the discovery that optic atrophy protein 1 is crucial for the NET formation process[40].
Pathway of ROSindependent NET formation: NETs can be rapidly formed independently of ROS. In this process, NE translocates to the nucleus, chromatin decondenses, and protein-modified chromatin is packaged into vesicles that fuse with the cell membrane, releasing nuclear DNA through vesicular transport mechanisms. This process is rapid, lasting about 5-60 minutes, and does not affect neutrophil lifespan, triggered by Toll-like receptor (TLR) 2 or complement C3[41,42]. Additionally, bacterial toxins can induce the formation of pores in the host neutrophil membrane, with NE and caspase-11 processing gasdermin D to form pores on the nuclear membrane, granular membrane, and plasma membrane, facilitating NE migration to the nucleus, thereby inducing NET formation[43,44]. In addition to neutrophils, some myeloid cells can also release similar structures, but neutrophils have a higher secretion efficiency[45].
The changes in NETs at different stages of tumors provide important clues for understanding tumor progression. Studies have shown that as tumors develop from early to late stages, the expression levels and functions of NETs change significantly. NETs are highly expressed in both primary CRC tumor tissues and liver metastatic lesions. For example, the abundance of NETs in liver metastatic lesions may create a “soil” for metastatic cancer cells by inducing CAF activation and promoting T cell exhaustion[46]. Additionally, cytokines released by CRC cells (such as IL-8) can recruit neutrophils into the TME, inducing NET formation, which in turn promotes cancer cell migration and invasion[47,48].
In early tumors, NETs may primarily participate in anti-tumor immune responses, while in late tumors, NETs may promote tumor metastasis and immune evasion. For instance, in studies of CRC, high NETs expression is closely linked to tumor grade, metastatic risk, and patient prognosis[49]. The formation of NETs is also closely related to intercellular interactions in the TME, particularly in the activation and functional regulation of tumor-associated neutrophils (TANs)[50]. These findings emphasize the dynamic changes in NETs at different stages of tumors and their potential impact on tumor progression, and provide important evidence for the development of new therapeutic strategies.
NETs play a complex and critical role in the occurrence, development, and metastasis of CRC. On one hand, NETs may exert anti-tumor effects by directly capturing and killing tumor cells, activating anti-tumor immune responses, and inhibiting angiogenesis[51]. On the other hand, NETs, rich in DNA, histones, and granule proteins, can not only directly promote CRC cell proliferation, invasion, and metastasis but also indirectly affect CRC progression by remodeling the TME. For example, histone H3 and MPO in NETs can activate nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways within tumor cells, thereby enhancing the survival and invasive capabilities of CRC cells[52,53]. Furthermore, NETs can promote immune evasion and distant metastasis of CRC by inducing angiogenesis and inhibiting immune cell functions[54]. Notably, the formation of NETs is closely associated with poor prognosis in CRC patients, with high levels of NET markers (such as citrullinated histone H3) positively correlated with tumor staging and metastatic risk[55]. Therefore, targeting NETs may become a new strategy for CRC treatment, such as blocking CRC progression by inhibiting NETs formation or degrading their components[56]. However, the specific mechanisms of NETs in CRC still require further research to clarify their multifaceted roles in the TME and their potential therapeutic value.
The distribution and dynamic changes of NETs in CRC microenvironment exhibit significant spatiotemporal heterogeneity, with their formation and degradation regulated by various factors in the TME. Studies indicate that NETs are primarily enriched at the tumor invasive front, around blood vessels, and in metastatic lesion areas, which usually have higher levels of inflammatory factors (such as IL-8, tumor necrosis factor-α) and ROS that can induce neutrophils to release NETs[37,57]. During CRC progression, the dynamic changes in NETs are closely tied to tumor staging: In early tumors, NETs formation may be limited, while in late tumors and metastatic lesions, NET formation significantly increases, which is associated with enhanced tumor-related inflammatory responses and the formation of an immunosuppressive microenvironment[54,55]. Additionally, the distribution of NETs in the CRC microenvironment is also regulated by exosomes secreted by tumor cells and stromal cells (such as CAFs), which further promote the formation and stabilization of NETs by releasing pro-inflammatory factors and proteases[53,58]. Notably, NET degradation in the CRC microenvironment is relatively slow, and the residual DNA and histone components may further promote tumor cell proliferation and metastasis by activating TLR9 and receptor of advanced glycation end products signaling pathways[59]. Therefore, the distribution and dynamic changes of NETs in the CRC microenvironment not only reflect the biological characteristics of tumor progression but also provide potential theoretical basis for targeting NETs in therapeutic strategies.
NETs have a big impact on how CRC behaves through multidimensional mechanisms, playing a key role in angiogenesis, metastasis, and immune evasion. NETs promote tumor angiogenesis, a hallmark of malignant tumors, providing oxygen and nutrients for tumor proliferation and metastasis[60]. Neutrophils have high levels of VEGF and matrix metalloproteinases (MMP)-9, which are associated with angiogenesis. Angiopoietin family members ANGPT1 and ANGPT2 can induce neutrophil adhesion to endothelial cells, increasing NETs formation[61]. Experimental evidence shows that NE and MMPs released by NETs can degrade the extracellular matrix, stimulate endothelial cells to secrete pro-angiogenic factors (such as VEGF), and activate the coagulation cascade through tissue factor, promoting CRC angiogenesis[62]. NETs are also closely related to CRC metastasis, promoting tumor cell migration and invasion through various mechanisms. For example, Chen et al[63] found that high mobility group protein (HMGB1), as a NET-associated component protein, can promote CRC cell migration and metastasis by activating the TLR9 pathway and enhance tumor migration and invasion capabilities through EMT[63]. In terms of immune evasion, NETs obstruct CD8 + T cell contact with CRC cells through physical barriers and release histone modifications (such as citrullinated histone H3) that induce T cell exhaustion while upregulating PD-L1 expression, enhancing resistance to immune checkpoint inhibitors[64]. Some experiments have demonstrated that NETs induce M2 macrophage polarization through the TLR9/NF-κB signaling axis while inhibiting the cytotoxic functions of CD8 + T cells (such as downregulation of interferon-γ and granzyme B expression) and establishing an immunosuppressive microenvironment by upregulating immune checkpoint molecules like PD-L1, thereby achieving immune evasion[65,66]. Notably, citrullinated histone H3 released by NETs can directly damage the antigen-presenting function of dendritic cells, further weakening anti-tumor immune responses[66]. These findings not only elucidate the multifaceted mechanisms of NETs in tumor progression but also provide potential molecular targets for developing NET-targeted therapeutic strategies. In the future, combining single-cell multi-omics technologies will further reveal the dynamic regulatory networks of NETs in the TME and their clinical significance.
The prognostic significance of NETs in CRC remains contentious. While some studies associate NETs with favorable outcomes, Berry et al[67] demonstrated that elevated TANs correlate with improved OS in stage II CRC patients, and Galdiero et al[68] reported enhanced 5-fluorouracil chemotherapy response in TANs-high subgroups; however, others identify NETs as an adverse prognostic biomarker[69]. NETs worsen prognosis through mechanisms of promoting metastasis and immune suppression, as components released by NETs, such as elastase, can directly damage the basement membrane, facilitating CRC cell migration and distant metastasis (such as liver metastasis). Preclinical studies indicate that inhibiting NETs activity can significantly reduce the risk of metastasis[47]. NETs accumulate in metastatic lesions, providing a “supportive microenvironment” for metastatic cancer cells by inducing CAF activation and angiogenesis, leading to reduced patient survival[47,70]. Additionally, NETs recruit Tregs and inhibit the activity of cytotoxic T cells, forming an immunosuppressive microenvironment. Studies show that high expression of NETs is associated with increased markers of T cell exhaustion [such as PD-1, T cell immunoglobulin domain and mucin domain-3 (TIM-3)], weakening anti-tumor immune responses and accelerating disease progression[70]. Furthermore, NETs correlate with tumor node metastasis staging and survival rates, with NET levels positively correlated with CRC tumor node metastasis staging, where stage III/IV patients exhibit significantly higher NET expression than stage I/II patients, and the median survival in the high expression group (101 months) is significantly lower than that in the low expression group (116 months)[71,72]. These findings indicate that NETs are not only important drivers of disease progression but also potential markers of poor prognosis. Future targeted therapies against NETs may provide new strategies for improving patient prognosis.
Neutrophils, as a type of white blood cell, play an important role in immune defense, especially in the TME, where their role has garnered significant attention. Traditional research could only explore the role of neutrophils in CRC from a holistic perspective, while the emergence of SCS provides a powerful tool for in-depth analysis of their heterogeneity. SCS can comprehensively analyze genomes, transcriptomes, and epigenomes at the single-cell level. Among them, scRNA-seq plays a key role in studying the heterogeneity of neutrophils in CRC. Through scRNA-seq, researchers can accurately determine the gene expression profiles of different neutrophil subpopulations in CRC tissues, uncovering unique molecular characteristics of each subpopulation.
The application of SCS provides unprecedented resolution for in-depth analysis of the specific roles and molecular mechanisms of NETs in CRC. Through single-cell transcriptomic analysis, researchers found that TANs are the main source of NETs, with high expression of key genes such as PAD4, MPO, and NE, which drive NET formation by promoting chromatin decondensation and histone citrullination[73]. Single-cell data further reveal the multifaceted roles of NETs in the CRC immune microenvironment: NETs induce TAMs to polarize towards a pro-tumor phenotype (M2 type) and inhibit the activity of cytotoxic T cells, thereby promoting immune evasion[74,75]. Additionally, SCS has found that NETs enhance the invasiveness and metastatic potential of tumor cells by activating TLR9 and CXCR2 signaling pathways[76]. SCS reveals the interactions between NETs and specific immune cell subpopulations (such as regulatory T cells and exhausted T cells) in CRC, indicating that NETs play a core role in shaping the immunosuppressive microenvironment[77]. These findings not only elucidate the molecular mechanisms of NETs in CRC but also provide potential molecular targets for developing NET-targeted therapeutic strategies. In the future, integrating single-cell multi-omics technologies will further reveal the dynamic regulatory networks of NETs in CRC and their clinical significance.
Scientists have used scRNA-seq technology to conduct high-resolution analyses of neutrophils in the TME, discovering significant heterogeneity. For example, a pro-inflammatory subpopulation characterized by high expression of IL-1β, CXCL8, and other pro-inflammatory factors is associated with tumor invasion[78]; an immunosuppressive subpopulation expressing PD-L1, ARG1, and other molecules promotes immune evasion by inhibiting T cell activity[79]; and a pro-metastatic subpopulation enriched in metastatic lesions assists tumor cell colonization by releasing MMP9 and elastase to damage the basement membrane[80].
SCS can elucidate molecular changes during the NETs formation process at the gene expression level. After stimulation, polymorphonuclear neutrophils (PMNs) become activated to form NETs, a process accompanied by a series of changes in cell morphology and internal structure. Using SCS, researchers can analyze the dynamic changes in the expression of genes related to NETs formation in PMNs at different time points. Experiments have also shown that epigenetic and metabolic regulation are important mechanisms for NETs formation: Neutrophils upregulate glycolysis through hypoxia inducible factor-1α in a hypoxic TME, providing energy for NETosis[81]. Additionally, the synergistic effects of the immune microenvironment also significantly influence NETs production: HMGB1 released by NETs activates the TLR4 pathway in macrophages, secreting IL-1β to further induce NETs formation[82,83].
SCS reveals the functional heterogeneity of neutrophils in CRC and their dynamic transition mechanisms towards pro-cancer phenotypes. The formation of NETs is regulated by multiple factors, including tumor cells, microbes, and epigenetics, and promotes metastasis by remodeling the immune microenvironment.
In recent years, the rapid development of SCS has provided new perspectives for in-depth research on the mechanisms of NETs formation and its related genes and signaling pathways. Fang et al[84] discovered characteristics of NETs in single-cell transcriptomes through scRNA-seq analysis, finding significantly increased NETs activity in monocytes, dendritic cells, and macrophages, identifying 1276 differentially expressed genes. Other studies have shown that NETs are highly expressed in various tumors and are closely related to tumor progression, metastasis, and immune evasion. Through single-cell transcriptomic analysis, researchers found that the formation of NETs involves differential expression of several key genes, including PAD4, MPO, NE, and ELANE, which are significantly upregulated during NETs generation and participate in key steps such as chromatin decondensation and histone citrullination[85,86]. Additionally, single-cell data revealed the activation of various signaling pathways during NETs formation, such as ROS/NF-κB, phosphatidylinositol 3-kinase/protein kinase B, and MAPK pathways, which promote NETs release by regulating neutrophil activation, metabolic reprogramming, and modes of cell death[87,88]. SCS also identified specific subpopulations (such as low-density granulocytes) with a higher propensity for NETs generation, which may be closely related to pathological states like autoimmune diseases and cancer[74,89]. These findings not only provide a molecular basis for the role of NETs in tumors but also offer new ideas for future targeted therapeutic strategies. In the future, integrating single-cell multi-omics technologies will further reveal the dynamic regulatory networks of NETs in diseases and their clinical significance.
The TME refers to the complex ecosystem surrounding tumor cells, composed of various cell types, signaling molecules, extracellular matrix, and physical-chemical conditions (such as hypoxia and acidity). The TME not only supports tumor growth and metastasis but also participates in key processes such as immune evasion and treatment resistance[90,91]. In recent years, SCS has provided high-precision dynamic maps for elucidating the cellular interaction networks of NETs in the TME. Normal host cells in the TME, such as CAFs and TAMs, assist in the growth, invasion, and metastasis of cancer cells[92,93]. Many recent studies using SCS have shown that neutrophils are another type of leukocyte that coexist in various cancer tissues, capable of inducing chemotaxis, inflammation, and/or angiogenesis[94]. By utilizing various single-cell isolation techniques, such as LCM, FACS and Microfluidic Technology, individual cells, including tumor cells, immune cells, stromal cells, and neutrophils releasing NETs, are isolated from tumor tissues for nucleic acid amplification. Subsequently, high-throughput sequencing platforms are used to sequence the amplified nucleic acids, obtaining high-quality sequencing data[95]. By analyzing the expression of NETs-related genes and screening for key genes, after identifying the neutrophil population, further analysis of the expression of NETs-related genes, such as histone H3, MPO, and NE, can be conducted[50]. By screening the expression levels of these key genes, insights into the release and function of NETs in the TME can be gained. Constructing gene co-expression networks can analyze the interrelationships between NETs-related genes and other genes. Through gene co-expression network analysis, potential interactions between NETs and other cell types in the TME can be revealed[96]. By elucidating the intercellular interactions of NETs in the TME, a deeper understanding of the composition and function of the TME can be achieved, providing important clues for studying the mechanisms of tumor occurrence and development[97,98].
The application of SCS technology provides a high-precision perspective for analyzing the interactions between NETs and the immune microenvironment in CRC. Research indicates that NETs form an immunosuppressive microenvironment in CRC by releasing DNA-histone complexes, promoting tumor cells escape from immune surveillance. Ji et al[99] concluded through single-cell transcriptomic analysis that NETs can weaken anti-tumor immune responses by inhibiting the expression of CD8 + T cell activation-related genes (such as GZMB and IFNG) and upregulating immune checkpoint molecules (such as PD-L1). Other studies have found that in liver metastatic lesions, SCS revealed neutrophil subpopulations specifically expressing high levels of PAD4 and MPO, key genes for NET formation, with their spatial distribution significantly positively correlated with T cell exhaustion markers [TIM-3, lymphocyte activation gene-3 (LAG-3)] within the metastatic lesions[26,100]. Zhang et al[101] and Chu et al[102] demonstrated through single-cell clustering analysis of tumor-infiltrating B cells that NETs may induce B cells to transition to an immunosuppressive phenotype via the IL-8/CXCR2 axis, thereby promoting the expansion of Tregs. These findings suggest that NETs-related pathways (such as PAD4 or CXCR2) may reshape the CRC immune microenvironment, providing new strategies for combined immune checkpoint inhibitor therapy[99,100].
In recent years, NETs in CRC have received a lot of attention. This is especially true thanks to advances in single-cell technology, which has led to major progress in understanding their molecular mechanisms and clinical significance.
In the prevention and treatment of CRC, early diagnosis is crucial for improving patient prognosis and increasing survival rates. In recent years, SCS has emerged as a state-of-the-art method, demonstrating significant potential in the early diagnosis of CRC, particularly in revealing the role of NETs in the occurrence and development of CRC. Liquid biopsy is a non-invasive detection method which is significant for in-depth analysis of CTCs in the blood and cells associated with NETs using SCS[103]. Research has shown that the interaction between NETs and tumor cells in the TME is complex and diverse. Detecting gene expression characteristics related to the interaction between CTCs and NETs, as well as the cellular components and molecular markers associated with NETs in the blood, could help spot abnormal signals early in CRC, thereby improving the early diagnosis rate of the disease[104]. For example, SCS has revealed that CTC subpopulations carrying NETs formation-related genes (such as PAD4 and MPO) are more abundant in early CRC progression. Combining cell-free DNA methylation markers like SEPT9 with NETs profiles can boost the detection rate of stage I CRC to 79.3%, which is a 32% improvement over traditional carcinoembryonic antigen testing[105]. SCS can also help us spot cellular differences and key molecular events early in CRC. For instance, in precancerous lesions or early tumors, the abnormal activation of specific epithelial cell subpopulations or immune cells (such as Tregs) may promote microenvironment remodeling through the release of NETs, achieving early diagnosis of CRC by detecting Tregs[23]. Another study came up with a NETs-based risk scoring model to predict the prognosis and immune microenvironment characteristics of CRC patients. By identifying the gene expression characteristics of TANs through SCS and combining them with NETs markers (such as citrullinated histone H3) in peripheral blood or tissues, it may enhance the detection rate of early CRC[106,107]. SCS of CRC tissues can identify how NETs-related genes are expressed in different cell subpopulations, as well as the relationship between this gene expression and tumor occurrence and development, thus providing a more precise basis for early treatment.
SCS uniquely reveals how NETs contribute to immune evasion in CRC, providing potential for discovering new immunotherapy targets. NETs play a complex role in the tumor immune microenvironment, potentially exerting anti-tumor effects while also promoting tumor immune evasion[108]. Through SCS technology, the mechanisms of action of NETs-related immunosuppressive molecules or signaling pathways can be better understood, leading to the development of new immunotherapeutic drugs or the optimization of existing immunotherapy regimens[109]. For example, studies have found that certain components within NETs can inhibit T cell activity, leading to tumor immune evasion. Creating inhibitors that target these immunosuppressive molecules could boost the effectiveness and precision of immunotherapy. Combining the characteristics of NETs with SCS results to formulate personalized combination therapy strategies is an important approach to improving CRC treatment outcomes. The presence of NETs can affect the tumor’s sensitivity to different treatment methods[110]. Combining NETs-targeted therapies with chemotherapy, targeted therapy, or immunotherapy can overcome tumor heterogeneity and resistance, improving treatment efficacy[111]. For instance, SCS has revealed that CAFs promote CRC resistance through metabolic reprogramming and immunosuppressive molecules (such as CXCL12), and targeting CAFs-immune cell interactions can enhance the efficacy of chemotherapy or immunotherapy[110]. Understanding the formation mechanisms and cellular composition of NETs through SCS can facilitate the targeted development of NETs-targeted therapies, used in conjunction with other treatment methods to enhance therapeutic efficacy.
During CRC treatment, dynamically monitoring changes in NETs-related cells and molecules through SCS is of great significance for assessing treatment efficacy. Different treatment methods, such as chemotherapy, targeted therapy, or immunotherapy, have varying impacts on the TME, and since NETs are an important component of the TME, their changes can reflect the tumor’s response to treatment[108]. For example, using scRNA-seq, it has been found that NETs can inhibit T cell function, allowing for the assessment of changes in T cell status (especially TILs) after treatment. NETs-targeted therapies or their combination with immune checkpoint inhibitors can lead to a decrease in the proportion of exhausted T cells (expressing PD-1, TIM-3, LAG-3, etc.) and an increase in the proportion or function of effector T cells (expressing IFNG, GZMB, etc.), indicating good therapeutic efficacy[101,112]. SCS provides unprecedented opportunities for dynamically and high-resolution assessing the effects of CRC treatment. By monitoring changes in NETs-related cell subpopulations (mainly neutrophils) and evaluating how treatment reshapes the immune microenvironment, identifying response and resistance markers through scRNA-seq is expected to guide the development of more effective, personalized CRC treatment plans, ultimately improving patient prognosis[110].
Using SCS data to build NETs-related prognostic models can give a more accurate basis for assessing CRC patient prognosis. By comprehensively considering NETs-related cell subpopulations, gene expression characteristics, and clinical pathological factors, we can predict the recurrence risk and survival outcomes of CRC patients[113]. Different NETs-related cell subpopulations play distinct roles in tumor occurrence, development, and metastasis, and SCS can accurately identify these cell subpopulations and analyze their gene expression characteristics[54]. SCS has revealed that the high expression of key genes involved in NETs formation (such as PAD4 and NE) is significantly associated with poor prognosis in CRC. For example, the S100A8/A9 + neutrophil subpopulation has been identified through single-cell transcriptomic data as a major source of NETs, with its abundance closely related to the risk of liver metastasis and decreased survival rates[78]. In multi-gene prognostic models based on single-cell data, markers containing NETs-related genes can effectively distinguish patient risk stratification, guiding personalized treatment[114]. Another study showed that TAMs and CAFs in NETs rich areas worsen immunosuppression by secreting factors like CXCL12, which further harms prognosis[115]. Therefore, studying NETs through SCS and combining clinical pathological factors, such as tumor staging, grading, and lymph node metastasis, to construct prognostic models can provide more scientific references for clinical treatment decisions, helping doctors formulate more reasonable treatment plans and improving patient survival rates and quality of life.
As an emerging biomarker, NETs show potential applications in various diseases, especially in tumor diagnosis and prognosis assessment. NETs are highly expressed in various malignant tumors, and their formation is closely related to tumor progression, metastasis, and prognosis[116,117]. In CRC, the levels of NETs are associated with tumor aggressiveness and metastatic potential, suggesting their potential as a biomarker for assessing patient prognosis[49]. Additionally, NETs-related biomarkers, such as cell-free DNA and intracellular histone modifications, have been confirmed to have good diagnostic value in various tumor types[118]. With a deeper understanding of the biological characteristics of NETs, future research can focus on developing specific detection methods for NETs to improve early diagnosis rates and the accuracy of prognosis assessments in tumors.
Therapeutic strategies based on NETs are becoming an important direction in cancer research. Existing studies have shown that NETs promote tumor cell proliferation and metastasis in the TME, thus targeting NETs may help inhibit tumor progression[119,120]. For example, de Buhr and von Köckritz-Blickwede[121] proposed the application of DNase as a potential therapeutic strategy to alleviate inflammatory responses in the TME by degrading NETs, thereby inhibiting tumor growth and metastasis. Future research can combine multi-omics technologies to further explore the mechanisms of NETs in different tumor types and develop corresponding targeted therapeutic strategies to improve the efficacy and safety of cancer treatment. By integrating existing therapeutic approaches, NETs-based treatment strategies are expected to provide new treatment options for CRC patients.
The rapid development of SCS has provided new insights into the role of NETs in CRC, gradually highlighting their significance in the TME. NETs are closely linked to CRC progression and metastasis, influencing tumor growth through interactions with tumor cells, immune cells, and other microenvironment components. These findings not only deepen our understanding of CRC’s complexity but also offer potential diagnostic and therapeutic strategies. However, NETs exhibit variable roles at different stages of CRC, sometimes promoting tumor growth while also inhibiting progression. This complexity is influenced by factors such as tumor molecular characteristics, microenvironment conditions, and patient differences. Future research should focus on using SCS to better understand the dynamic changes in NETs within CRC and explore their interactions with other immune cell types. This will help identify new biomarkers and therapeutic targets for early diagnosis and treatment. In summary, while progress has been made in understanding NETs in CRC, further research integrating methods like SCS is essential to reveal their mechanisms and guide clinical practice, ultimately advancing early diagnosis and personalized treatment.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12684] [Article Influence: 6342.0] [Reference Citation Analysis (6)] |
| 2. | Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 1221] [Article Influence: 610.5] [Reference Citation Analysis (0)] |
| 3. | Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. [Cancer incidence and mortality in China, 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 245] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 10910] [Article Influence: 3636.7] [Reference Citation Analysis (2)] |
| 5. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8097] [Article Influence: 224.9] [Reference Citation Analysis (1)] |
| 6. | Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, Li J, Wang G, Lan Z, Li J, Tang M, Jiang S, Ma X, Chen P, Katkhuda R, Korphaisarn K, Chakravarti D, Chang A, Spring DJ, Chang Q, Zhang J, Maru DM, Maeda DY, Zebala JA, Kopetz S, Wang YA, DePinho RA. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell. 2019;35:559-572.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 486] [Article Influence: 69.4] [Reference Citation Analysis (1)] |
| 7. | Shiri AM, Zhang T, Bedke T, Zazara DE, Zhao L, Lücke J, Sabihi M, Fazio A, Zhang S, Tauriello DVF, Batlle E, Steglich B, Kempski J, Agalioti T, Nawrocki M, Xu Y, Riecken K, Liebold I, Brockmann L, Konczalla L, Bosurgi L, Mercanoglu B, Seeger P, Küsters N, Lykoudis PM, Heumann A, Arck PC, Fehse B, Busch P, Grotelüschen R, Mann O, Izbicki JR, Hackert T, Flavell RA, Gagliani N, Giannou AD, Huber S. IL-10 dampens antitumor immunity and promotes liver metastasis via PD-L1 induction. J Hepatol. 2024;80:634-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 90] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 8. | Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2921] [Cited by in RCA: 3459] [Article Influence: 247.1] [Reference Citation Analysis (0)] |
| 9. | Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 2743] [Article Influence: 391.9] [Reference Citation Analysis (0)] |
| 10. | Mutua V, Gershwin LJ. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin Rev Allergy Immunol. 2021;61:194-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 441] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 11. | Li J, Chen J, Sun J, Li K. The Formation of NETs and Their Mechanism of Promoting Tumor Metastasis. J Oncol. 2023;2023:7022337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 12. | De Meo ML, Spicer JD. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin Immunol. 2021;57:101595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Wang D, Peng M, Tang L, Ouyang J, Xiong F, Guo C, Tang Y, Zhou Y, Liao Q, Wu X, Wang H, Yu J, Li Y, Li X, Li G, Zeng Z, Tan Y, Xiong W. Single-cell RNA sequencing in cancer research. J Exp Clin Cancer Res. 2021;40:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 268] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 14. | Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA. mRNA-Seq whole-transcriptome analysis of a single cell. Nat Methods. 2009;6:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3043] [Cited by in RCA: 2625] [Article Influence: 154.4] [Reference Citation Analysis (0)] |
| 15. | Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, Cook K, Stepansky A, Levy D, Esposito D, Muthuswamy L, Krasnitz A, McCombie WR, Hicks J, Wigler M. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1880] [Cited by in RCA: 1979] [Article Influence: 131.9] [Reference Citation Analysis (0)] |
| 16. | Bazyari MJ, Saadat Z, Firouzjaei AA, Aghaee-Bakhtiari SH. Deciphering colorectal cancer progression features and prognostic signature by single-cell RNA sequencing pseudotime trajectory analysis. Biochem Biophys Rep. 2023;35:101491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (3)] |
| 17. | Zhao L, Wang Q, Yang C, Ye Y, Shen Z. Application of Single-Cell Sequencing Technology in Research on Colorectal Cancer. J Pers Med. 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Yasen A, Aini A, Wang H, Li W, Zhang C, Ran B, Tuxun T, Maimaitinijiati Y, Shao Y, Aji T, Wen H. Progress and applications of single-cell sequencing techniques. Infect Genet Evol. 2020;80:104198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Slovin S, Carissimo A, Panariello F, Grimaldi A, Bouché V, Gambardella G, Cacchiarelli D. Single-Cell RNA Sequencing Analysis: A Step-by-Step Overview. Methods Mol Biol. 2021;2284:343-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 20. | Bai X, Li Y, Zeng X, Zhao Q, Zhang Z. Single-cell sequencing technology in tumor research. Clin Chim Acta. 2021;518:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Valdeolivas A, Amberg B, Giroud N, Richardson M, Gálvez EJC, Badillo S, Julien-Laferrière A, Túrós D, Voith von Voithenberg L, Wells I, Pesti B, Lo AA, Yángüez E, Das Thakur M, Bscheider M, Sultan M, Kumpesa N, Jacobsen B, Bergauer T, Saez-Rodriguez J, Rottenberg S, Schwalie PC, Hahn K. Profiling the heterogeneity of colorectal cancer consensus molecular subtypes using spatial transcriptomics. NPJ Precis Oncol. 2024;8:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 68] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 22. | Cañellas-Socias A, Cortina C, Hernando-Momblona X, Palomo-Ponce S, Mulholland EJ, Turon G, Mateo L, Conti S, Roman O, Sevillano M, Slebe F, Stork D, Caballé-Mestres A, Berenguer-Llergo A, Álvarez-Varela A, Fenderico N, Novellasdemunt L, Jiménez-Gracia L, Sipka T, Bardia L, Lorden P, Colombelli J, Heyn H, Trepat X, Tejpar S, Sancho E, Tauriello DVF, Leedham S, Attolini CS, Batlle E. Metastatic recurrence in colorectal cancer arises from residual EMP1(+) cells. Nature. 2022;611:603-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 23. | Joanito I, Wirapati P, Zhao N, Nawaz Z, Yeo G, Lee F, Eng CLP, Macalinao DC, Kahraman M, Srinivasan H, Lakshmanan V, Verbandt S, Tsantoulis P, Gunn N, Venkatesh PN, Poh ZW, Nahar R, Oh HLJ, Loo JM, Chia S, Cheow LF, Cheruba E, Wong MT, Kua L, Chua C, Nguyen A, Golovan J, Gan A, Lim WJ, Guo YA, Yap CK, Tay B, Hong Y, Chong DQ, Chok AY, Park WY, Han S, Chang MH, Seow-En I, Fu C, Mathew R, Toh EL, Hong LZ, Skanderup AJ, DasGupta R, Ong CJ, Lim KH, Tan EKW, Koo SL, Leow WQ, Tejpar S, Prabhakar S, Tan IB. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat Genet. 2022;54:963-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 273] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 24. | Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z, Ding X, Bao R, Hong L, Jia W, Fang F, Liu H, Chen L, Zhong J, Zou D, Liu L, Han L, Ginhoux F, Liu Y, Ye Y, Su B. Single-cell and spatial analysis reveal interaction of FAP(+) fibroblasts and SPP1(+) macrophages in colorectal cancer. Nat Commun. 2022;13:1742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 606] [Article Influence: 151.5] [Reference Citation Analysis (0)] |
| 25. | Li R, Liu X, Huang X, Zhang D, Chen Z, Zhang J, Bai R, Zhang S, Zhao H, Xu Z, Zeng L, Zhuang L, Wen S, Wu S, Li M, Zuo Z, Lin J, Lin D, Zheng J. Single-cell transcriptomic analysis deciphers heterogenous cancer stem-like cells in colorectal cancer and their organ-specific metastasis. Gut. 2024;73:470-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (1)] |
| 26. | Wang F, Long J, Li L, Wu ZX, Da TT, Wang XQ, Huang C, Jiang YH, Yao XQ, Ma HQ, Lian ZX, Zhao ZB, Cao J. Single-cell and spatial transcriptome analysis reveals the cellular heterogeneity of liver metastatic colorectal cancer. Sci Adv. 2023;9:eadf5464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 27. | Lei Y, Tang R, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Applications of single-cell sequencing in cancer research: progress and perspectives. J Hematol Oncol. 2021;14:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 28. | Wu F, Fan J, Fang J, Dalvi PS, Odenthal M, Fang N. Single Cell Sequencing: A New Dimension in Cancer Diagnosis and Treatment. Adv Exp Med Biol. 2020;1255:109-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Wang F, Jin Y, Wang M, Luo HY, Fang WJ, Wang YN, Chen YX, Huang RJ, Guan WL, Li JB, Li YH, Wang FH, Hu XH, Zhang YQ, Qiu MZ, Liu LL, Wang ZX, Ren C, Wang DS, Zhang DS, Wang ZQ, Liao WT, Tian L, Zhao Q, Xu RH. Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial. Nat Med. 2024;30:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 30. | Wang Y, Guo L, Feng L, Zhang W, Xiao T, Di X, Chen G, Zhang K. Single nucleotide variant profiles of viable single circulating tumour cells reveal CTC behaviours in breast cancer. Oncol Rep. 2018;39:2147-2159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Ye B, Gao Q, Zeng Z, Stary CM, Jian Z, Xiong X, Gu L. Single-Cell Sequencing Technology in Oncology: Applications for Clinical Therapies and Research. Anal Cell Pathol (Amst). 2016;2016:9369240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Li GM, Xiao GZ, Qin PF, Wan XY, Fu YJ, Zheng YH, Luo MY, Ren DL, Liu SP, Chen HX, Lin HC. Single-Cell RNA Sequencing Reveals Heterogeneity in the Tumor Microenvironment between Young-Onset and Old-Onset Colorectal Cancer. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 33. | Hu S, Qin J, Ding M, Gao R, Xiao Q, Lou J, Chen Y, Wang S, Pan Y. Bulk integrated single-cell-spatial transcriptomics reveals the impact of preoperative chemotherapy on cancer-associated fibroblasts and tumor cells in colorectal cancer, and construction of related predictive models using machine learning. Biochim Biophys Acta Mol Basis Dis. 2025;1871:167535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Luo Y, Deng X, Liao W, Huang Y, Lu C. Prognostic value of autophagy-related genes based on single-cell RNA-sequencing in colorectal cancer. Front Genet. 2023;14:1109683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 35. | Li D, Shao J, Cao B, Zhao R, Li H, Gao W, Chen P, Jin L, Cao L, Ji S, Dong G. The Significance of Neutrophil Extracellular Traps in Colorectal Cancer and Beyond: From Bench to Bedside. Front Oncol. 2022;12:848594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Chu ZQ, Zhang KC, Chen L. Neutrophil extracellular traps in gastrointestinal cancer. World J Gastroenterol. 2021;27:5474-5487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (3)] |
| 37. | Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 2434] [Article Influence: 270.4] [Reference Citation Analysis (0)] |
| 38. | Sorvillo N, Cherpokova D, Martinod K, Wagner DD. Extracellular DNA NET-Works With Dire Consequences for Health. Circ Res. 2019;125:470-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 39. | Thiam HR, Wong SL, Wagner DD, Waterman CM. Cellular Mechanisms of NETosis. Annu Rev Cell Dev Biol. 2020;36:191-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 433] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 40. | Amini P, Stojkov D, Felser A, Jackson CB, Courage C, Schaller A, Gelman L, Soriano ME, Nuoffer JM, Scorrano L, Benarafa C, Yousefi S, Simon HU. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat Commun. 2018;9:2958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 41. | Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18:1386-1393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 676] [Cited by in RCA: 918] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 42. | Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A. 2015;112:2817-2822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 544] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 43. | Hirschfeld J, Roberts HM, Chapple IL, Parčina M, Jepsen S, Johansson A, Claesson R. Effects of Aggregatibacter actinomycetemcomitans leukotoxin on neutrophil migration and extracellular trap formation. J Oral Microbiol. 2016;8:33070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Björnsdottir H, Dahlstrand Rudin A, Klose FP, Elmwall J, Welin A, Stylianou M, Christenson K, Urban CF, Forsman H, Dahlgren C, Karlsson A, Bylund J. Phenol-Soluble Modulin α Peptide Toxins from Aggressive Staphylococcus aureus Induce Rapid Formation of Neutrophil Extracellular Traps through a Reactive Oxygen Species-Independent Pathway. Front Immunol. 2017;8:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Chen F, Liu Y, Shi Y, Zhang J, Liu X, Liu Z, Lv J, Leng Y. The emerging role of neutrophilic extracellular traps in intestinal disease. Gut Pathog. 2022;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 46. | Li C, Chen T, Liu J, Wang Y, Zhang C, Guo L, Shi D, Zhang T, Wang X, Li J. FGF19-Induced Inflammatory CAF Promoted Neutrophil Extracellular Trap Formation in the Liver Metastasis of Colorectal Cancer. Adv Sci (Weinh). 2023;10:e2302613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 47. | Okamoto M, Mizuno R, Kawada K, Itatani Y, Kiyasu Y, Hanada K, Hirata W, Nishikawa Y, Masui H, Sugimoto N, Tamura T, Inamoto S, Sakai Y, Obama K. Neutrophil Extracellular Traps Promote Metastases of Colorectal Cancers through Activation of ERK Signaling by Releasing Neutrophil Elastase. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 48. | Ronchetti L, Boubaker NS, Barba M, Vici P, Gurtner A, Piaggio G. Neutrophil extracellular traps in cancer: not only catching microbes. J Exp Clin Cancer Res. 2021;40:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 49. | Huang X, Sun T, Wang J, Hong X, Chen H, Yan T, Zhou C, Sun D, Yang C, Yu T, Su W, Du W, Xiong H. Metformin Reprograms Tryptophan Metabolism to Stimulate CD8+ T-cell Function in Colorectal Cancer. Cancer Res. 2023;83:2358-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 50. | Lv X, Ma W, Miao X, Hu S, Xie H. Navigating colorectal cancer prognosis: A Treg-related signature discovered through single-cell and bulk transcriptomic approaches. Environ Toxicol. 2024;39:3512-3522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 51. | Xia L, Yan X, Zhang H. Mitochondrial DNA-activated cGAS-STING pathway in cancer: Mechanisms and therapeutic implications. Biochim Biophys Acta Rev Cancer. 2025;1880:189249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 52. | Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1085] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 53. | Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, Bružas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JT, Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1134] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 54. | Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F, Liu J, Xing Y, Chen X, Su S, Song E. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 723] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 55. | Thålin C, Lundström S, Seignez C, Daleskog M, Lundström A, Henriksson P, Helleday T, Phillipson M, Wallén H, Demers M. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One. 2018;13:e0191231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 56. | Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109:13076-13081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 725] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 57. | Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5773] [Cited by in RCA: 7627] [Article Influence: 346.7] [Reference Citation Analysis (0)] |
| 58. | Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, Wagner DD. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016;5:e1134073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 59. | Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016;76:1367-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 555] [Article Influence: 55.5] [Reference Citation Analysis (1)] |
| 60. | Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 61. | Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 401] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 62. | Erpenbeck L, Schön MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene. 2017;36:2483-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 63. | Chen Y, Hu H, Tan S, Dong Q, Fan X, Wang Y, Zhang H, He J. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp Hematol Oncol. 2022;11:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 64. | Tan H, Jiang Y, Shen L, Nuerhashi G, Wen C, Gu L, Wang Y, Qi H, Cao F, Huang T, Liu Y, Xie W, Deng W, Fan W. Cryoablation-induced neutrophil Ca(2+) elevation and NET formation exacerbate immune escape in colorectal cancer liver metastasis. J Exp Clin Cancer Res. 2024;43:319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 65. | Wu R, Sun C, Chen X, Yang R, Luan Y, Zhao X, Yu P, Luo R, Hou Y, Tian R, Bian S, Li Y, Dong Y, Liu Q, Dai W, Fan Z, Yan R, Pan B, Feng S, Wu J, Chen F, Yang C, Wang H, Dai H, Shu M. NSUN5/TET2-directed chromatin-associated RNA modification of 5-methylcytosine to 5-hydroxymethylcytosine governs glioma immune evasion. Proc Natl Acad Sci U S A. 2024;121:e2321611121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 66. | Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front Immunol. 2020;11:1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 67. | Berry RS, Xiong MJ, Greenbaum A, Mortaji P, Nofchissey RA, Schultz F, Martinez C, Luo L, Morris KT, Hanson JA. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS One. 2017;12:e0188799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S, Polentarutti N, Malesci A, Marone G, Roncalli M, Laghi L, Garlanda C, Mantovani A, Jaillon S. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139:446-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 69. | Zheng W, Wu J, Peng Y, Sun J, Cheng P, Huang Q. Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 70. | Zheng K, Duan J, Wang R, Chen H, He H, Zheng X, Zhao Z, Jing B, Zhang Y, Liu S, Xie D, Lin Y, Sun Y, Zhang N, Cai M. Deep learning model with pathological knowledge for detection of colorectal neuroendocrine tumor. Cell Rep Med. 2024;5:101785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 71. | Shi H, Pan Y, Xiang G, Wang M, Huang Y, He L, Wang J, Fang Q, Li L, Liu Z. A novel NET-related gene signature for predicting DLBCL prognosis. J Transl Med. 2023;21:630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 72. | Peng JF, Peng JS, Wang R, Liu C, Wang ZB. [Study on MOS gene expression in colorectal cancer patients and its relationship with patients' clinicopathologic features and prognosis]. Zhongguo Quanke Yixue. 2021;24:3077-3081+3086. [DOI] [Full Text] |
| 73. | Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria I, Andueza MP, Nieto CP, Resano L, Azpilikueta A, Allegretti M, de Pizzol M, Ponz-Sarvisé M, Rouzaut A, Sanmamed MF, Schalper K, Carleton M, Mellado M, Rodriguez-Ruiz ME, Berraondo P, Perez-Gracia JL, Melero I. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020;52:856-871.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 585] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 74. | Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, Merad M, Frenette PS. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 670] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 75. | Cedervall J, Zhang Y, Olsson AK. Tumor-Induced NETosis as a Risk Factor for Metastasis and Organ Failure. Cancer Res. 2016;76:4311-4315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 76. | Li Q, Chen Y, Zhang D, Grossman J, Li L, Khurana N, Jiang H, Grierson PM, Herndon J, DeNardo DG, Challen GA, Liu J, Ruzinova MB, Fields RC, Lim KH. IRAK4 mediates colitis-induced tumorigenesis and chemoresistance in colorectal cancer. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 77. | Zhang Y, Wang Z, Lu Y, Sanchez DJ, Li J, Wang L, Meng X, Chen J, Kien TT, Zhong M, Gao WQ, Ding X. Region-Specific CD16(+) Neutrophils Promote Colorectal Cancer Progression by Inhibiting Natural Killer Cells. Adv Sci (Weinh). 2024;11:e2403414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 78. | Tang N, Huang HH, Liu XJ. [A 9-gene prognostic model of colorectal cancer immune cells using single-cell sequencing and transcriptome sequencing]. Zhongguo Aizheng Zazhi. 2024;34:548-560. [DOI] [Full Text] |
| 79. | Li J, Wu C, Hu H, Qin G, Wu X, Bai F, Zhang J, Cai Y, Huang Y, Wang C, Yang J, Luan Y, Jiang Z, Ling J, Wu Z, Chen Y, Xie Z, Deng Y. Remodeling of the immune and stromal cell compartment by PD-1 blockade in mismatch repair-deficient colorectal cancer. Cancer Cell. 2023;41:1152-1169.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 217] [Reference Citation Analysis (0)] |
| 80. | Zhao Y, Zhang B, Ma Y, Guo M, Zhao F, Chen J, Wang B, Jin H, Zhou F, Guan J, Zhao Q, Liu Q, Wang H, Zhao F, Wang X. Distinct molecular profiles drive multifaceted characteristics of colorectal cancer metastatic seeds. J Exp Med. 2024;221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 81. | Wang XQ, Zhang B, Zhang Q, Dai Z, Zhang JX, Liang XL, Li L, Wu L, Liu SS. [Single-cell sequencing-based analysis of platelet number and functional changes in early sepsis]. Shiyong Yixue Zazhi. 2024;40:1218-1224. [DOI] [Full Text] |
| 82. | Fang Q, Stehr AM, Naschberger E, Knopf J, Herrmann M, Stürzl M. No NETs no TIME: Crosstalk between neutrophil extracellular traps and the tumor immune microenvironment. Front Immunol. 2022;13:1075260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 83. | Kaltenmeier C, Yazdani HO, Morder K, Geller DA, Simmons RL, Tohme S. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front Immunol. 2021;12:785222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 84. | Fang Z, Liu C, Yu X, Yang K, Yu T, Ji Y, Liu C. Identification of neutrophil extracellular trap-related biomarkers in non-alcoholic fatty liver disease through machine learning and single-cell analysis. Sci Rep. 2024;14:21085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 85. | Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1163] [Cited by in RCA: 1660] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 86. | Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207:1853-1862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 856] [Cited by in RCA: 1183] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 87. | Silvestre-Roig C, Braster Q, Ortega-Gomez A, Soehnlein O. Neutrophils as regulators of cardiovascular inflammation. Nat Rev Cardiol. 2020;17:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 423] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 88. | Ambler WG, Kaplan MJ. Vascular damage in systemic lupus erythematosus. Nat Rev Nephrol. 2024;20:251-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 89. | Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2015;74:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 371] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 90. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48760] [Article Influence: 3250.7] [Reference Citation Analysis (12)] |
| 91. | Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2019] [Cited by in RCA: 4258] [Article Influence: 532.3] [Reference Citation Analysis (1)] |
| 92. | Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2625] [Cited by in RCA: 2921] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 93. | Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2555] [Cited by in RCA: 2469] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 94. | Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 95. | Zhang Q, Liu Y, Wang X, Zhang C, Hou M, Liu Y. Integration of single-cell RNA sequencing and bulk RNA transcriptome sequencing reveals a heterogeneous immune landscape and pivotal cell subpopulations associated with colorectal cancer prognosis. Front Immunol. 2023;14:1184167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 96. | Chen H, Zhao T, Fan J, Yu Z, Ge Y, Zhu H, Dong P, Zhang F, Zhang L, Xue X, Lin X. Construction of a prognostic model for colorectal adenocarcinoma based on Zn transport-related genes identified by single-cell sequencing and weighted co-expression network analysis. Front Oncol. 2023;13:1207499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 97. | Cui P, Wang H, Bai Z. Integrated single-cell and bulk RNA-seq analysis identifies a prognostic T-cell signature in colorectal cancer. Sci Rep. 2024;14:20177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 98. | Zhang JP, Yan BZ, Liu J, Wang W. Action of circulating and infiltrating B cells in the immune microenvironment of colorectal cancer by single-cell sequencing analysis. World J Gastrointest Oncol. 2024;16:2683-2696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 99. | Ji L, Fu G, Huang M, Kao X, Zhu J, Dai Z, Chen Y, Li H, Zhou J, Chu X, Lei Z. scRNA-seq of colorectal cancer shows regional immune atlas with the function of CD20(+) B cells. Cancer Lett. 2024;584:216664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 100. | Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu K, Hu X, Bu Z, Peng J, Ren X, Zhang Z. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell. 2022;40:424-437.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 290] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 101. | Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O'Brien SA, He Y, Wang L, Zhang Q, Kim A, Gao R, Orf J, Wang T, Sawant D, Kang J, Bhatt D, Lu D, Li CM, Rapaport AS, Perez K, Ye Y, Wang S, Hu X, Ren X, Ouyang W, Shen Z, Egen JG, Zhang Z, Yu X. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell. 2020;181:442-459.e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 1043] [Article Influence: 173.8] [Reference Citation Analysis (5)] |
| 102. | Chu X, Li X, Zhang Y, Dang G, Miao Y, Xu W, Wang J, Zhang Z, Cheng S. Integrative single-cell analysis of human colorectal cancer reveals patient stratification with distinct immune evasion mechanisms. Nat Cancer. 2024;5:1409-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 103. | Tieng FYF, Abu N, Nasir SN, Lee LH, Ab Mutalib NS. Liquid Biopsy-Based Colorectal Cancer Screening via Surface Markers of Circulating Tumor Cells. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Yin H, Xie J, Xing S, Lu X, Yu Y, Ren Y, Tao J, He G, Zhang L, Yuan X, Yang Z, Huang Z. Machine learning-based analysis identifies and validates serum exosomal proteomic signatures for the diagnosis of colorectal cancer. Cell Rep Med. 2024;5:101689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 105. | Gao Y, Cao D, Li M, Zhao F, Wang P, Mei S, Song Q, Wang P, Nie Y, Zhao W, Wang S, Yan H, Wang X, Jiao Y, Liu Q. Integration of multiomics features for blood-based early detection of colorectal cancer. Mol Cancer. 2024;23:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 106. | Dong J, Zhao J, Wu Z, Liu J, Wang B, Qi X. The Predictive Value of Neutrophil Extracellular Trap-Related Risk Score in Prognosis and Immune Microenvironment of Colorectal Cancer Patients. Mol Biotechnol. 2025;67:1509-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 107. | Chen C, Cai Y, Hu W, Tan K, Lu Z, Zhu X, Liu Z, He C, Xu G, Zhang R, Ning C, Ruan S, Gao J, Yang X, Wei Y, Zhu X, Li X, Wang F, Wang F, Li J, Jin M, Li B, Zhu Y, Tian J, Miao X. Single-cell eQTL mapping reveals cell subtype-specific genetic control and mechanism in malignant transformation of colorectal cancer. Cancer Discov. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 108. | Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 882] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 109. | Bao X, Wang D, Dai X, Liu C, Zhang H, Jin Y, Tong Z, Li B, Tong C, Xin S, Li X, Wang Y, Liu L, Zhu X, Fu Q, Zheng Y, Deng J, Tian W, Guo T, Zhao P, Chen W, Fang W. An immunometabolism subtyping system identifies S100A9(+) macrophage as an immune therapeutic target in colorectal cancer based on multiomics analysis. Cell Rep Med. 2023;4:100987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 110. | Chen Y, Wang D, Li Y, Qi L, Si W, Bo Y, Chen X, Ye Z, Fan H, Liu B, Liu C, Zhang L, Zhang X, Li Z, Zhu L, Wu A, Zhang Z. Spatiotemporal single-cell analysis decodes cellular dynamics underlying different responses to immunotherapy in colorectal cancer. Cancer Cell. 2024;42:1268-1285.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 94] [Reference Citation Analysis (0)] |
| 111. | Ito Y, Pan D, Zhang W, Zhang X, Juan TY, Pyrdol JW, Kyrysyuk O, Doench JG, Liu XS, Wucherpfennig KW. Addressing Tumor Heterogeneity by Sensitizing Resistant Cancer Cells to T cell-Secreted Cytokines. Cancer Discov. 2023;13:1186-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 112. | Pelka K, Hofree M, Chen JH, Sarkizova S, Pirl JD, Jorgji V, Bejnood A, Dionne D, Ge WH, Xu KH, Chao SX, Zollinger DR, Lieb DJ, Reeves JW, Fuhrman CA, Hoang ML, Delorey T, Nguyen LT, Waldman J, Klapholz M, Wakiro I, Cohen O, Albers J, Smillie CS, Cuoco MS, Wu J, Su MJ, Yeung J, Vijaykumar B, Magnuson AM, Asinovski N, Moll T, Goder-Reiser MN, Applebaum AS, Brais LK, DelloStritto LK, Denning SL, Phillips ST, Hill EK, Meehan JK, Frederick DT, Sharova T, Kanodia A, Todres EZ, Jané-Valbuena J, Biton M, Izar B, Lambden CD, Clancy TE, Bleday R, Melnitchouk N, Irani J, Kunitake H, Berger DL, Srivastava A, Hornick JL, Ogino S, Rotem A, Vigneau S, Johnson BE, Corcoran RB, Sharpe AH, Kuchroo VK, Ng K, Giannakis M, Nieman LT, Boland GM, Aguirre AJ, Anderson AC, Rozenblatt-Rosen O, Regev A, Hacohen N. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021;184:4734-4752.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 113. | Lee HO, Hong Y, Etlioglu HE, Cho YB, Pomella V, Van den Bosch B, Vanhecke J, Verbandt S, Hong H, Min JW, Kim N, Eum HH, Qian J, Boeckx B, Lambrechts D, Tsantoulis P, De Hertogh G, Chung W, Lee T, An M, Shin HT, Joung JG, Jung MH, Ko G, Wirapati P, Kim SH, Kim HC, Yun SH, Tan IBH, Ranjan B, Lee WY, Kim TY, Choi JK, Kim YJ, Prabhakar S, Tejpar S, Park WY. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat Genet. 2020;52:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 542] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 114. | Xu WD, Shapar T, Liu KJ, Han T, Zhao H. [Construction and validation of a prognostic immune-related gene signature model for colorectal cancer]. Zhongguo Putong Waike Zazhi. 2021;30:449-463. [DOI] [Full Text] |
| 115. | Mo SB, Feng B, Peng JJ, Zou H. [The current research status of circulating tumor DNA in colorectal cancer]. Zhongguo Linchuang Yaolixue Zazhi. 2024;40:3186-3190. [DOI] [Full Text] |
| 116. | Chen Y, Han L, Qiu X, Wang G, Zheng J. Neutrophil Extracellular Traps in Digestive Cancers: Warrior or Accomplice. Front Oncol. 2021;11:766636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Xu Y, Ying K. [Research progress on neutrophil extracellular traps in tumor]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 118. | Kalligeros M, Diamantopoulos L, Toumpanakis C. Biomarkers in Small Intestine NETs and Carcinoid Heart Disease: A Comprehensive Review. Biology (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | Bonilha CS, Veras FP, de Queiroz Cunha F. NET-targeted therapy: effects, limitations, and potential strategies to enhance treatment efficacy. Trends Pharmacol Sci. 2023;44:622-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 120. | Hijioka S, Yamashige D, Esaki M, Honda G, Higuchi R, Masui T, Shimizu Y, Ohtsuka M, Kumamoto Y, Katanuma A, Gotohda N, Akita H, Unno M, Endo I, Yokoyama Y, Yamada S, Matsumoto I, Ohtsuka T, Hirano S, Yasuda H, Kawai M, Aoki T, Nakamura M, Hashimoto D, Rikiyama T, Horiguchi A, Fujii T, Mizuno S, Hanada K, Tani M, Hatori T, Ito T, Okuno M, Kagawa S, Tajima H, Ishii T, Sugimoto M, Onoe S, Takami H, Takada R, Miura T, Kurita Y, Kamei K, Mataki Y, Okazaki K, Takeyama Y, Yamaue H, Satoi S; Japan Pancreas Society Clinical Research Promotion Committee Group (Corporate Authorship). Factors Affecting Nonfunctioning Small Pancreatic Neuroendocrine Neoplasms and Proposed New Treatment Strategies. Clin Gastroenterol Hepatol. 2024;22:1416-1426.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 121. | de Buhr N, von Köckritz-Blickwede M. The Balance of Neutrophil Extracellular Trap Formation and Nuclease Degradation: an Unknown Role of Bacterial Coinfections in COVID-19 Patients? mBio. 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/