Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.106684
Revised: April 21, 2025
Accepted: June 10, 2025
Published online: July 15, 2025
Processing time: 108 Days and 5.2 Hours
Advanced hepatocellular carcinoma (HCC) with ascites (AS) lacks reliable biomarkers for predicting treatment outcomes. The combined prognostic value of the systemic immune-inflammation index (SII) and prognostic nutritional index (PNI) remains underexplored for novel therapies.
To evaluate the clinical efficacy of combining intraperitoneal compound Kushen injection (CKI) with immunotherapy in patients with advanced HCC using a scoring system that combines SII and PNI.
SII and PNI were calculated prior to treatment from peripheral blood samples, and critical values were determined by receiver operating characteristic analysis. SII-PNI scores were categorized as follows: 2, high SII (≥ 558.5) and low PNI (≤ 33.58); 1, high SII or low PNI; and 0, neither high SII nor low PNI. After immunotherapy combined with CKI, patients with advanced HCC were evaluated using the SII-PNI scoring criteria.
The SII-PNI score was significantly lower in patients without concomitant AS than in those with AS (P = 0.017). Progression-free survival was significantly longer in patients with a low SII-PNI score than in those with a high SII-PNI score (P = 0.0125). Multivariate analysis identified the SII-PNI score as an independent prognostic factor for 2-year overall survival in patients with advanced HCC and AS (P < 0.001).
The pretreatment SII-PNI score is an important indicator of treatment sensitivity for patients with advanced HCC receiving intraperitoneal CKI. It also represents a crucial basis for evaluating treatment efficacy and prognosis, aiding in the identification of high-risk groups and prognosis prediction.
Core Tip: A scoring system that combines the systemic immune-inflammation index and prognostic nutritional index is effective in evaluating treatment response and prognosis in advanced hepatocellular carcinoma patients receiving immunotherapy and compound Kushen injection. The systemic immune-inflammation index-prognostic nutritional index score identifies patients at high risk, predicts progression-free and overall survival, and supports personalized treatment planning. By improving the inflammatory and nutritional status, compound Kushen injection enhances the therapeutic outcomes of immunotherapy, particularly in hepatocellular carcinoma patients with ascites. This scoring system contributes to a more refined assessment of patient prognosis and a deeper understanding of the interplay between nutrition, immunity, and inflammation in hepatocellular carcinoma management.
- Citation: Zhu YF, Zhang DW, Zhang M, Yu MH, Zhang SH, Wu YY. Prognostic value of immune-inflammation and nutritional indices in advanced hepatocellular carcinoma patients receiving immunotherapy with compound Kushen injection. World J Gastrointest Oncol 2025; 17(7): 106684
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/106684.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.106684
Primary liver cancer is among the deadliest cancers globally, posing a serious threat to human life and health[1]. Hepatocellular carcinoma (HCC) is the most common pathological type of primary liver cancer, and together, HCC, intrahepatic cholangiocarcinoma and mixed HCC/cholangiocarcinoma account for approximately 75% of primary liver cancers[2].
Surgical resection is currently the primary and most effective treatment for liver cancer. However, postoperative metastasis and recurrence rates remain high, ranging from 40% to 70%[3]. Studies have found that the liver’s inflammatory state is an important factor in promoting liver tumor progression. Liver inflammation induces cytokine and chemokine secretion, leading to the degradation of damaged cells and further liver injury[4].
The predictive role of inflammatory indicators in tumor progression and prognosis has become increasingly studied. Ascites (AS, also termed intraperitoneal effusion) is a serious and common complication of advanced HCC. Patients with advanced HCC complicated by AS often have a poor nutritional condition, low tolerance, poor prognosis, short survival time, and limited treatment options, making recurrence more likely[5]. The selection of late-stage treatments for advanced HCC with malignant AS is a crucial factor influencing HCC prognosis.
Recent research has demonstrated that HCC pathogenesis is closely related to inflammation and the immune microenvironment[6]. The levels of some common inflammatory indicators, such as lymphocytes, are significantly increased in patients with cancerous AS, thereby representing important indicators of the presence of AS[7]. The systemic immune-inflammation index (SII) is an inflammatory index derived from a comprehensive analysis of neutrophil, platelet, and lymphocyte counts in peripheral blood, and it reflects different inflammation types and immune infiltration[8]. Numerous studies have reported that SII can predict malignant tumor prognosis, including non-small cell lung cancer[9], colorectal cancer[10], and HCC[11]. Similar to SII, the prognostic nutritional index (PNI) has significant value in predicting the prognosis of patients with various tumors[12]. Multiple analyses have confirmed the correlation between SII and PNI. Ding et al[13] used combined SII-PNI scores to assess the prognosis of patients with gastric cancer undergoing neoadjuvant chemotherapy, suggesting that the combined index could be important for formulating treatment strategies and clinical risk stratification. Wang et al[14] applied SII and PNI to assess the prognosis of patients with HCC after hepatic resection, demonstrating that both indices can assess liver function and independently predict the prognosis of these patients.
Inflammation and nutrition play crucial roles in HCC progression[15]. Recent studies revealed that SII and PNI are closely related to the prognosis of HCC[16]. A meta-analysis published in 2021 demonstrated that SII has significant prognostic value in HCC, with higher values being associated with poorer prognosis[14]. Another study published in 2022 found that the inflammation–immunity–nutrition score could predict overall survival (OS) and progression-free survival (PFS) in patients with HCC undergoing radical surgery[17]. These findings highlight the importance of inflammation and nutrition in HCC progression and suggest that SII and PNI could serve as potential biomarkers for predicting treatment outcomes[18].
Currently, traditional Chinese medicine (TCM) is extensively applied in anti-tumor therapy. Compound Kushen injection (CKI) has been used for more than 15 years in treating various malignant tumors. Its clinical efficacy and pharmacological mechanisms of action against gastrointestinal tumors, including HCC, are being actively investigated[19,20]. Studies on CKI as an anti-cancer compound have demonstrated its ability to significantly reduce liver inflammation and improve immune function. In addition, CKI can potentially reduce immunosuppressive cell counts in patients with triple-negative breast cancer, thereby enhancing their sensitivity to immune checkpoint inhibitors[21].
CKI holds promise in modulating the immune response during cancer treatment[14]. It can induce immune cell proliferation and activation, including T cells and natural killer cells, and enhance their cytotoxic activity against tumor cells. Moreover, CKI can promote cytokine secretion, including interferon-γ and tumor necrosis factor-α, further strengthening the immune system’s ability to recognize and eliminate tumor cells[22]. These mechanisms provide a theoretical basis for combining CKI with other therapies to enhance their overall anti-tumor effects[17].
HCC treatment primarily involves surgical and non-surgical approaches (e.g., vascular embolism, radiofrequency ablation, radiotherapy, chemoradiotherapy). Although these treatments can effectively remove local lesions, the postoperative recurrence rate remains high and patient prognosis is poor. The multiple-kinase inhibitor sorafenib can prolong the survival of patients with HCC, but its efficacy is not ideal. However, the combined use of sorafenib and the TCM Banxia Xiexin decoction significantly prolongs OS and PFS[23]. Based on this research, we hypothesized that adding CKI to immunotherapy could have a synergistic effect against HCC. Therefore, this study explored adjuvant therapy for immunotherapy in liver cancer, particularly for treating serious complications such as advanced liver cancer with AS, while improving the efficacy and sensitivity of immunotherapy.
This study was approved by the Ethics Committee of The First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, approval No. 2024MCZQ09. The inclusion criteria were as follows: Diagnosis of HCC confirmed by pathological tissue examination; Child-Pugh grade A and B and a projected survival of ≥ 3 months; Eastern Cooperative Oncology Group performance status ≤ 2; and tumor, node, metastasis stage III-IV. The exclusion criteria were as follows: Use of proprietary Chinese medicine adjuvant therapies other than CKI or other drugs that affect immune status; psychiatric disorders; other tumor diseases; and renal insufficiency or cardiovascular and cerebrovascular disease. Patients were screened for inclusion in the study based on these criteria. Those who met the requirements were randomly assigned to either the control or treatment group using a random number table method.
Both groups underwent the same basic work-up, including magnetic resonance imaging, computed tomography, biochemical examination, routine blood examination, coagulation function tests, and alpha-fetoprotein estimation. Both groups received immunotherapy with sintilimab (Sinopharm S20180016; Cinda Biopharmaceutical (Suzhou) Co., Ltd., specification: 100 mg/stick) administered intravenously for 1 hour at a dose of 2 mg/kg every 21 days for four cycles. During treatment, symptomatic supportive therapy was provided, including liver protection, antiemetics, pain relief, acid suppression, and gastric protection.
The treatment group also received CKI (Shanxi Zhendong Pharmaceutical Co. Ltd., Sinopharm Z14021231, specification: 5 mL per stick) as part of the immunotherapy regimen. CKI was administered as 20 mL mixed with 500 mL of 5% glucose infusion. CKI treatment began on the same day as immunotherapy and continued for 14 days.
PNI combines albumin levels and lymphocyte counts to assess nutritional and immune status. Lower PNI indicates a poorer nutritional status and weakened immune function, which are associated with worse outcomes in patients with advanced HCC. PNI was calculated as follows: PNI= albumin (g/L) + 5 × totallymphocyte count (× 109/L).
SII integrates platelet, neutrophil, and lymphocyte counts to reflect systemic inflammation and immune responses. Higher SII indicates increased inflammation and immune dysregulation, which are associated with poorer prognosis in patients with cancer, including those with HCC. SII was calculated as follows:
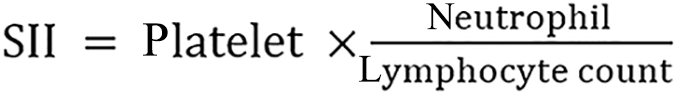
All cell counts were measured in units of 1 × 109 g/L.
SPSS version 21.0 (https://www.ibm.com/cn-zh/analytics/spss-statistics-software) was used for statistical analysis. Descriptive and comparative statistical tests were performed, and the relationship between PNI and SII was evaluated by Spearman’s correlation analysis in GraphPad Prism (GraphPad, Boston, MA, Unites States). The area under the receiver operating characteristic curve (AUC) was used to evaluate the ability to distinguish between patients with or without AS, and the optimal cutoffs for SII and PNI were determined using the highest Youden index. Multivariate analysis was employed to identify independent risk factors affecting prognosis. Survival analysis was performed using the Kaplan–Meier method, and the log-rank test was used to assess differences in survival between the groups. The hazard ratio (HR) and 95% confidence interval (CI) were used to describe relative risk factors. P < 0.05 was considered statistically significant.
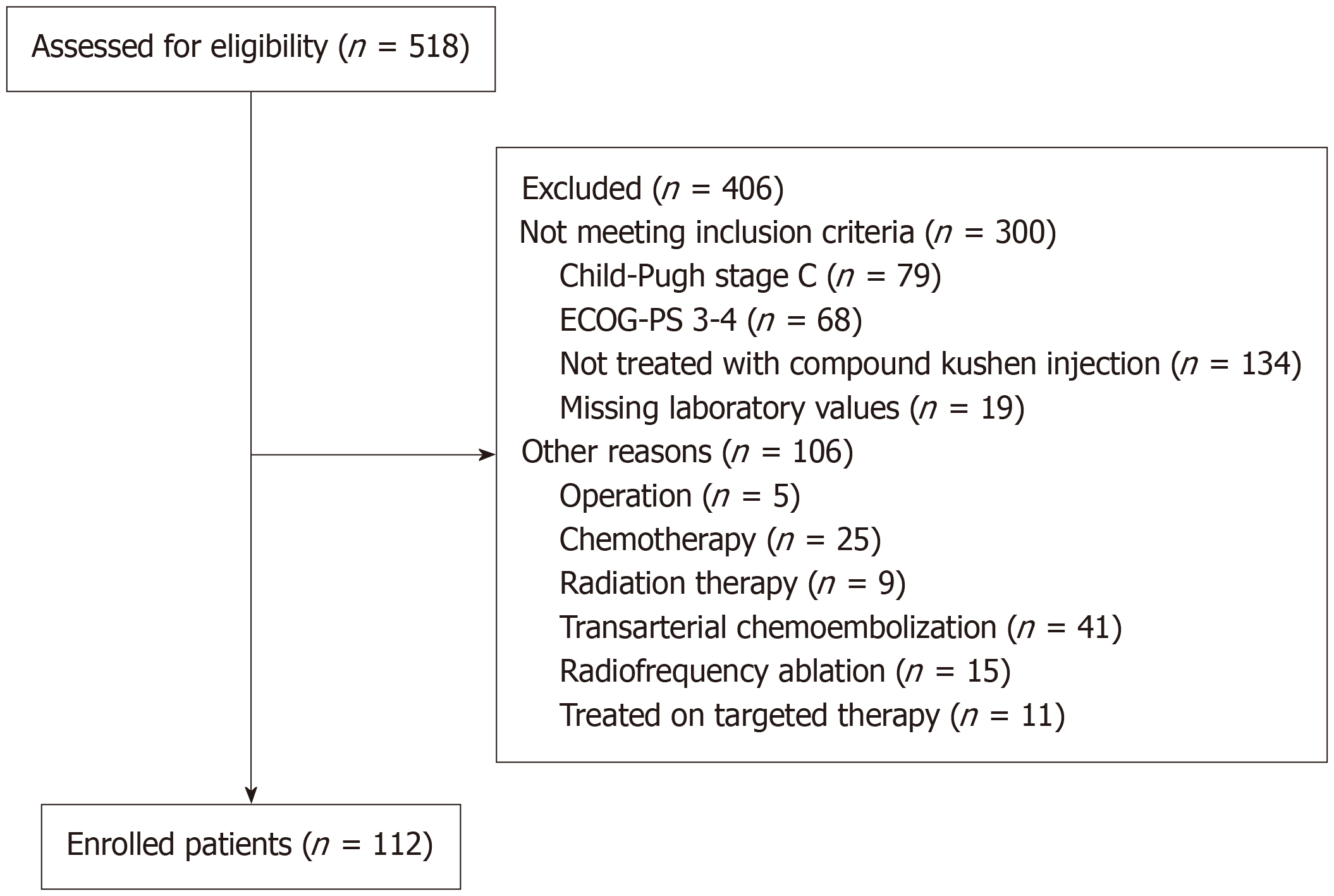
A total of 518 patients with liver cancer were selected from the electronic database of the First Affiliated Hospital of Anhui University of TCM between January 2020 and January 2024. Of these, 300 patients did not meet the inclusion criteria, and 106 patients had received other treatments. Ultimately, 112 patients with advanced HCC were included in the study (Figure 1). The clinical characteristics of these patients are summarized in Table 1.
| Characteristics | Treatment group (n = 58) | Control group (n = 54) | P value |
| WBC (109/L), mean ± SD | 6.87 ± 6.60 | 7.15 ± 4.44 | 0.796 |
| Neutrophil (109/L), mean ± SD | 66.26 ± 15.58 | 69.9 ± 14.86 | 0.209 |
| Lymphocyte (109/L), mean ± SD | 1.15 ± 0.41 | 0.98 ± 0.51 | 0.060 |
| Monocyte (109/L), mean ± SD | 0.64 ± 0.44 | 0.89 ± 1.74 | 0.309 |
| Age (year), mean ± SD | 63.98 ± 10.17 | 67.4 ± 11.91 | 0.097 |
| Sex | 0.056 | ||
| Male | 42 (72.4) | 7(13.0) | |
| Female | 16 (27.6) | 47(87.0) | |
| HBV | 0.054 | ||
| Yes | 18 (31) | 27 (50) | |
| No | 40 (69) | 27 (50) | |
| Child-Pugh grade | 0.076 | ||
| A | 45 (77.6) | 26 (48.1) | |
| B | 13 (22.4) | 28 (51.9) | |
| ECOG-PS | 0.249 | ||
| 0 | 12 (20.7) | 9 (16.7) | |
| 1 | 39 (67.2) | 32 (59.3) | |
| 2 | 7 (12.1) | 12 (24.1) | |
| Tumor size (cm) | 0.938 | ||
| ≥ 5 | 20 (34.5) | 19 (35.2) | |
| < 5 | 38 (65.5) | 35 (64.8) | |
| Lenvatinib/sorafenib treatment | 0.982 | ||
| Yes | 46 (79) | 43 (80) | |
| No | 12 (21) | 11(20) | |
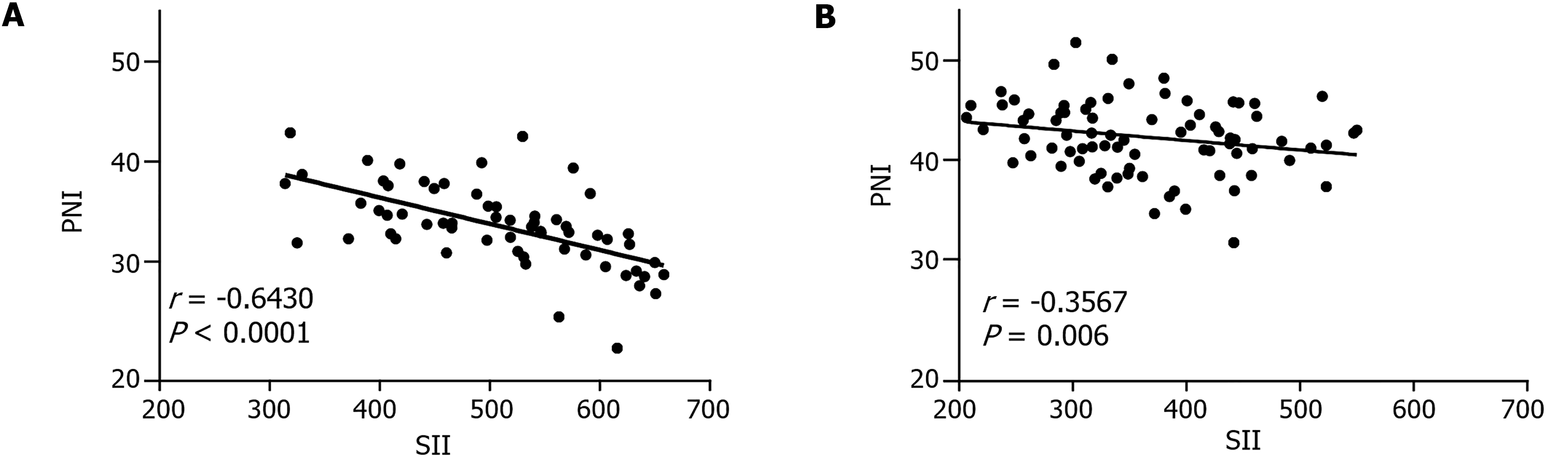
The correlation between SII and PNI was analyzed using Pearson’s correlation coefficient. There was a strong negative correlation in the control group (r = -0.6430, P < 0.0001; Figure 2A). In contrast, a weaker negative correlation was recorded in the treatment group (r = -0.3567, P = 0.006; Figure 2B).
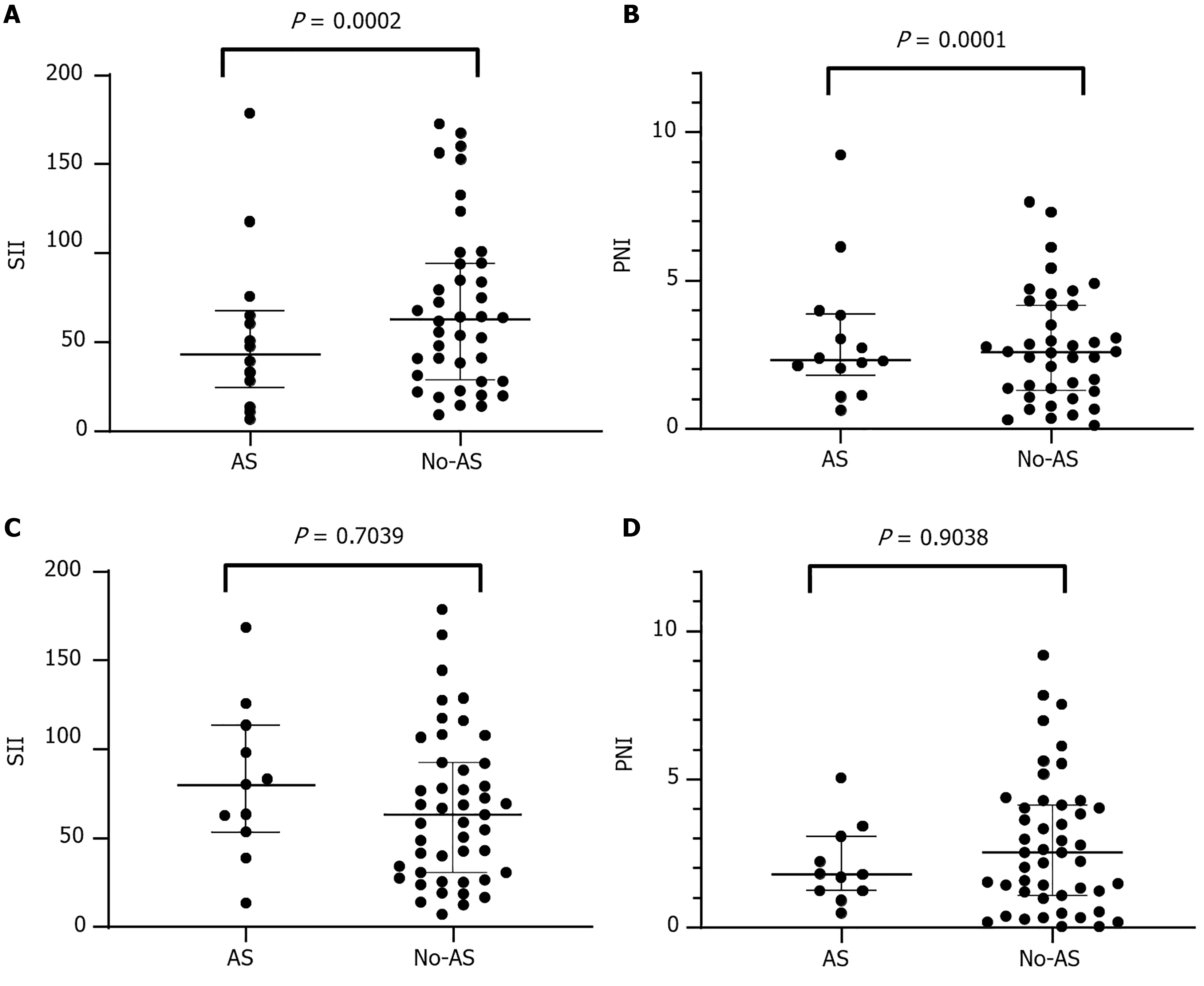
Prior to treatment, SII was significantly higher among patients with AS than those without AS in the control group (P = 0.0002; Figure 3A), whereas PNI was significantly higher among patients with AS (P < 0.0001; Figure 3B). After two cycles of CKI combined with immunotherapy, there was no significant difference in SII or PNI between patients with or without AS (both P > 0.05; Figure 3C and D). Post-treatment analysis revealed no significant differences in SII (369.6 vs 380.6, P = 0.704) or PNI (42.67 vs 42.53, P = 0.904) between the 87 patients without AS and the 25 patients with AS. We did not observe any significant improvement in SII or PNI in the control group after treatment. In contrast, there were pronounced improvements in both inflammatory markers and nutritional parameters in the treatment group. These findings suggest that compared to immunotherapy alone, CKI can improve the therapeutic effect on AS management in patients with HCC, particularly in those with concurrent hyperinflammatory and hypoproteinemic conditions.
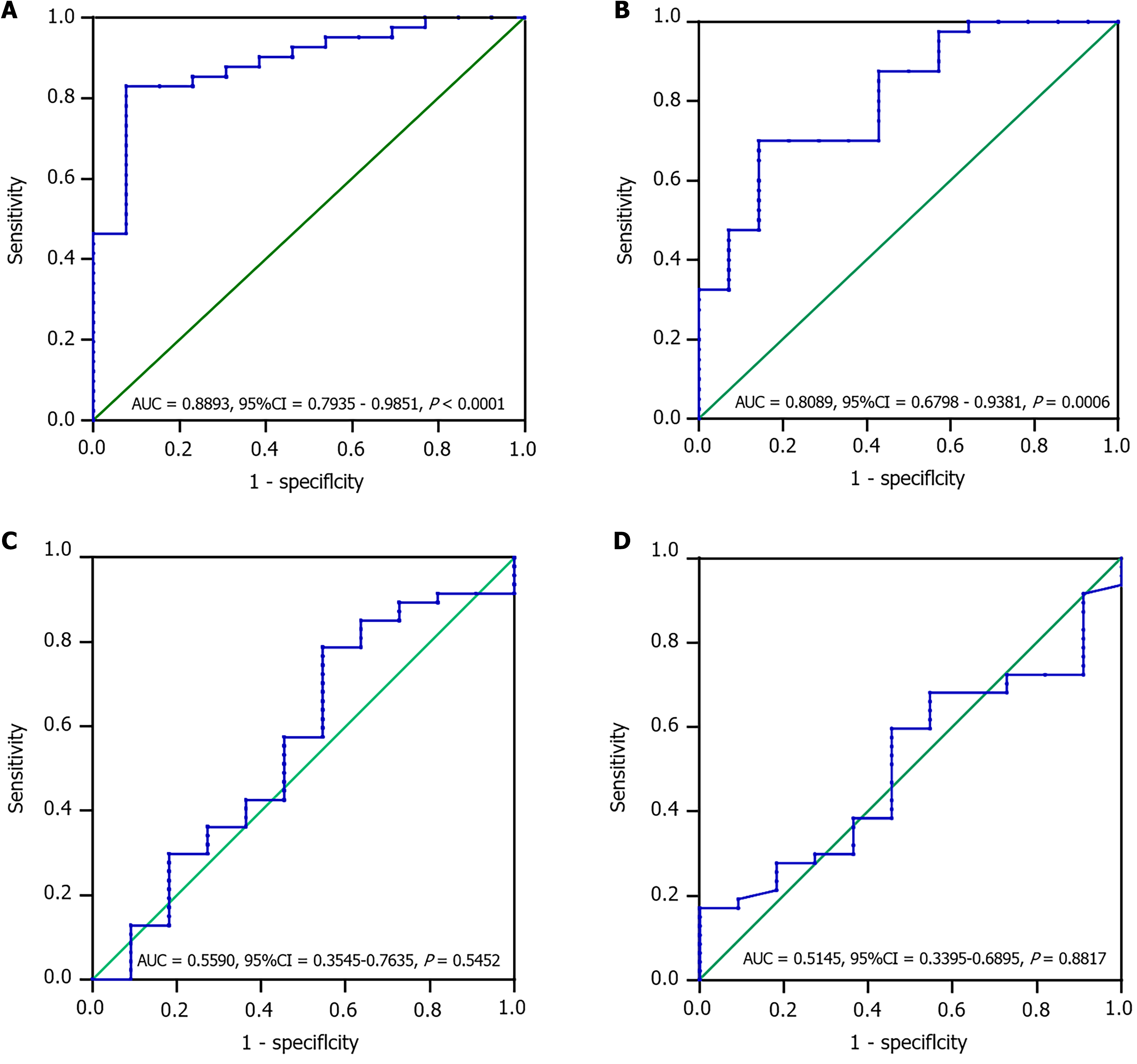
The optimal cutoff for SII before CKI treatment was 558.5 (AUC = 0.8893, 95%CI: 0.7935-0.9851, P < 0.0001), and this cutoff had a sensitivity of 0.8293 and specificity of 0.9231 (Figure 4A). The optimal cutoff for PNI was 33.58 (AUC = 0.8089, 95%CI: 0.6798-0.9381, P = 0.0006), which had a sensitivity of 0.7 and specificity of 0.8571 (Figure 4B). However, the best critical value for PNI was 46.05 (AUC = 0.5145, 95%CI: 0.3395-0.6895, P = 0.8817), which failed to accurately distinguish between patients with and without AS. Based on the optimal cutoffs for SII and PNI before CKI treatment, patients were divided into three groups: 2 points (n = 15), high SII (≥ 558.5) and low PNI (≤ 33.58); 1 point (n = 14), high SII or low PNI; and 0 points (n = 83), neither high SII nor low PNI. The results demonstrated that the combined SII-PNI score before CKI treatment could accurately predict the AS status of patients with HCC. However, after CKI treatment, the sensitivity and specificity of SII-PNI were reduced (Figure 4C and D). This suggests that the addition of CKI may improve the inflammatory and nutritional status of patients with AS, thereby reducing the sensitivity and specificity of SII and PNI in predicting AS status. Taken together, these data suggest that CKI may improve the inflammatory response and nutritional status of patients with AS.
In the treatment group, the treatment response was classified as partial response (PR) in 25 patients (44.6%), stable disease (SD) in 20 patients (35.7%), and progressive disease (PD) in 11 patients (17.2%). In the control group, the response rates were 7 (12.5%), 31 (55.4%), and 18 patients (32.1%), respectively (Figure 5). The SII-PNI score in the control group was significantly lower among patients with PR or SD than among those with PD (P < 0.001), and the SII-PNI score was significantly lower among patients without AS than among those with AS (P < 0.001; Tables 2 and 3).
| Treatment response | SII-PNI score | P value | ||
| 0 (n = 83) | 1 (n = 14) | 2 (n = 15) | ||
| Non-PD (n = 75) | 63 (75.9) | 9 (64.3) | 3 (20.0) | < 0.0001 |
| PD (n = 37) | 20 (24.0) | 5 (35.7) | 12 (80.0) | |
| AS status | SII-PNI score | P value | ||
| 0 (n = 83) | 1 (n = 14) | 2 (n = 15) | ||
| Non-AS (n = 87) | 71 (85.5) | 12 (85.7) | 4 (26.7) | < 0.0001 |
| AS (n = 25) | 12 (14.5) | 2 (14.3) | 11 (73.3) | |
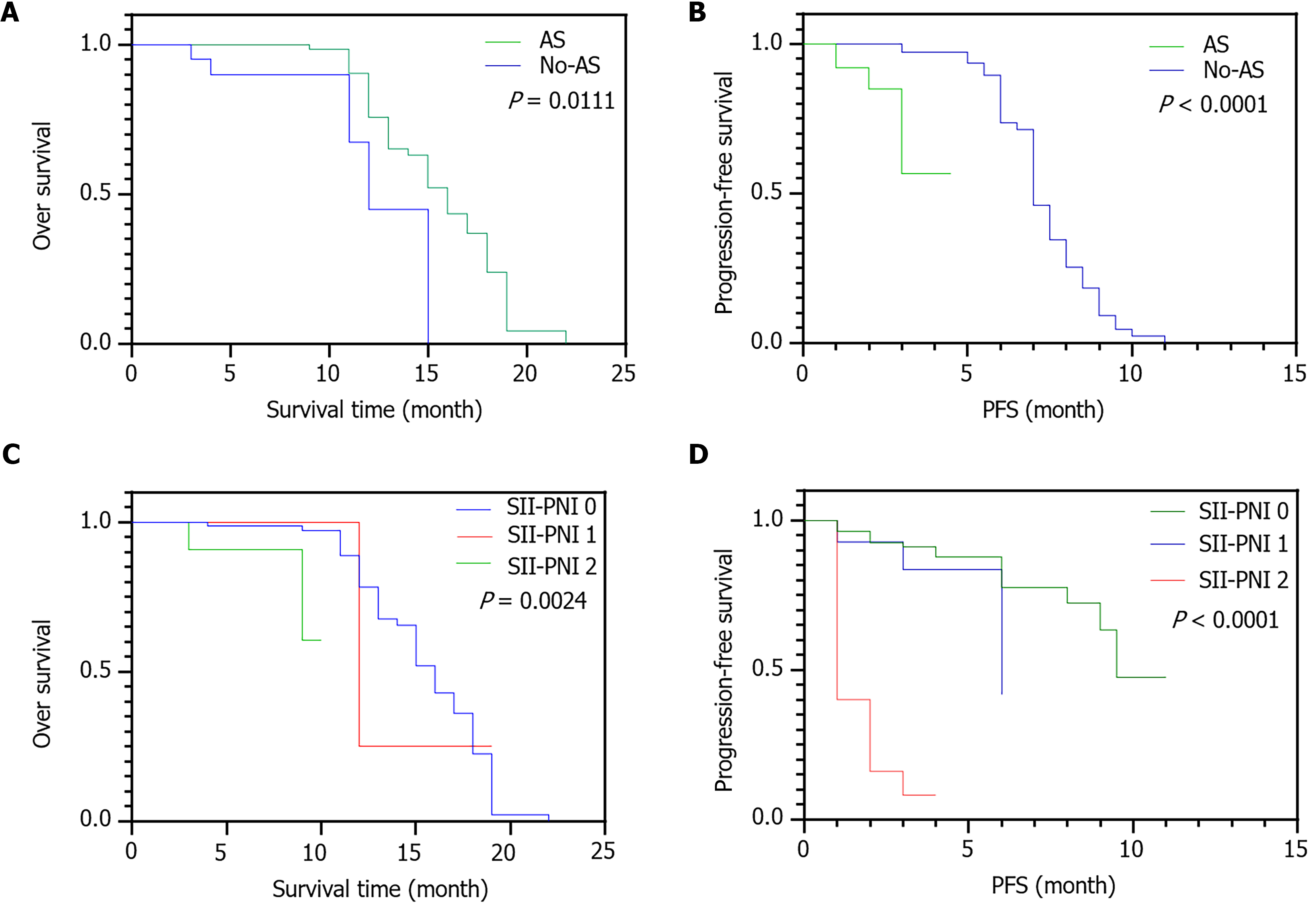
Patients who received CKI and immunotherapy were followed up for a median of 25.5 months (interquartile range: 15.6-38.4). The OS rate at 2 years was 54.5%, and median OS was 15 months (95%CI: 13.6-16.3). The 2-year PFS rate was 54.8%, and median PFS was 7 months (95%CI: 6.6-7.3 months). The 2-year OS (80.00% vs 47.1%, P = 0.0111) and PFS rates (71.43% vs 12.50%, P < 0.0001) were significantly higher in patients without AS than in those with AS (Figure 6A and B). The OS rate was significantly higher for patients with a higher SII–PNI score than among those with a lower SII-PNI score (P < 0.05; Figure 6C). The 2-year PFS rates in patients with SII-PNI scores of 2, 1, and 0 were 79.5%, 71.4%, and 13.3%, respectively (P < 0.05; Figure 6D).
Univariate Cox regression analysis (Table 4) illustrated that serum albumin (HR = 0.91387, 95%CI: 0.849-0.982, P = 0.015), PNI (HR = 0.907, 95%CI: 0.871-0.987, P = 0.016), SII (HR = 1.005, 95%CI: 1.002-1.008, P = 0.002), and concomitant AS (HR = 20.308, 95%CI: 3.783-109.043, P < 0.0001) were important prognostic factors associated with recurrence-free survival. Meanwhile, serum albumin (HR = 0.87, 95%CI: 0.832-0.988, P = 0.028), neutrophils (HR = 1.194, 95%CI: 1.062-1.343, P = 0.008), concomitant AS (HR = 3.175, 95%CI: 1.182-8.530, P = 0.042), and SII (HR = 1.004, 95%CI: 1.000-1.007, P = 0.031) were important prognostic factors associated with OS.
| Independent factor | OS | PFS | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Univariate analysis | ||||
| Age | 1.108 (0.635-1.934) | 0.718 | 1.296 (0.732-2.296) | 0.374 |
| Sex | 1.03 (0.572-1.857) | 0.921 | 1.263 (0.691-2.309) | 0.443 |
| Albumin | 0.907 (0.832-0.988) | 0.028 | 0.913 (0.849-0.982) | 0.015 |
| WBC | 0.999 (0.956-1.044) | 0.959 | 1.008 (0.973-1.044) | 0.685 |
| Neutrophil | 1.194 (1.062-1.343) | 0.008 | 1.107 (0.991-1.236) | 0.102 |
| Lymphocyte | 1.064 (0.559-2.023) | 0.851 | 0.765 (0.404-1.447) | 0.407 |
| Monocyte | 0.759 (0.349-1.649) | 0.460 | 0.653 (0.313-1.365) | 0.205 |
| AFP | 0.97 (0.517-1.850) | 0.948 | 0.907 (0.474-1.738) | 0.768 |
| HBV | 1.83 (0.898-3.730) | 0.116 | 0.865 (0.425-1.761) | 0.686 |
| Child-Pugh grade | 1.629 (0.836-3.176) | 0.169 | 1.688 (0.866-3.289) | 0.141 |
| Tumor size | 1.096 (0.623-1.928) | 0.750 | 1.096 (0.623-1.928) | 0.750 |
| ECOG performance status | 1.505 (0.628-3.607) | 0.382 | 0.535 (0.207-1.336) | 0.142 |
| SII | 1.004 (1.000-1.007) | 0.031 | 1.005 (1.002-1.008) | 0.002 |
| PNI | 0.944 (0.882-1.012) | 0.105 | 0.927 (0.871-0.987) | 0.016 |
| AS | 3.175 (1.182-8.530) | 0.042 | 20.308 (3.783-109.043) | 0.000 |
| Multivariate analysis | ||||
| Albumin | 0.895 (0.804-0.995) | 0.041 | 0.962 (0.833-1.111) | 0.597 |
| AS | 1.21 (11.4-0.986) | 0.053 | 2.764 (1.967-2.602) | 0.003 |
| PNI | 1.002 (0.998-1.006) | 0.403 | 1.003 (0.999-1.007) | 0.131 |
| SII | 1.043 (0.920-1.181) | 0.512 | 1.000 (0.881-1.134) | 0.999 |
| SII-PNI | 2.357 (1.458-2.433) | 0.000 | 0.626 (6.572-0.532) | 0.197 |
Multivariate analysis (Table 4) revealed that the presence of AS (HR = 2.764, 95%CI: 1.967-2.602, P = 0.003) was an important prognostic factor for PFS, and albumin (HR = 0.895, 95%CI: 0.804-0.995, P = 0.041) and the SII-PNI score (HR = 2.357, 95%CI: 1.458-2.433, P < 0.0001) were independent risk factors that influenced the 2-year OS of patients with advanced HCC.
This study revealed that OS at 2 years was worse among patients with advanced HCC and AS than in their counterparts without AS. The addition of CKI improved patients’ inflammatory status and prolonged survival in patients with AS. Additionally, multivariate analysis demonstrated that the SII-PNI score can be used as an independent risk factor in predicting the prognosis of patients with liver cancer. We aim to establish immunotherapy evaluation criteria based on the SII–PNI score to achieve more significant treatment benefits in patients with a low score (0-1) such that they have a better prognosis.
The anti-inflammatory and nutritional benefits of CKI have been highlighted in HCC, but its relationships with immunotherapy [e.g., programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) modulation] and the immune microenvironment require further exploration[24]. Recent studies indicated that tumor-associated macrophages (TAMs) play a significant role in the immune microenvironment of HCC. The PD-1/PD-L1 axis is highly active in TAMs, and it can induce their polarization toward the M2 phenotype. M2-type TAMs promote resistance to PD-1/PD-L1 blockade therapy by inhibiting T cell function and inducing T cell exclusion[25]. Moreover, PD-L1 can promote macrophage polarization toward the M2 phenotype via extracellular signal-regulated kinase/protein kinase B/mammalian target of rapamycin signaling pathway, enhancing their mitochondrial function and metabolic reprogramming[26]. Therefore, inducing TAM polarization from the M2 to the M1 phenotype may be a potential strategy to overcome resistance to PD-1/PD-L1 blockade therapy. As a TCM with immunomodulatory effects, CKI might enhance the efficacy of immunotherapy by affecting the TAM polarization state and activity of the PD-1/PD-L1 axis[20]. However, the specific mechanisms by which CKI acts in the HCC immune microenvironment, especially its relationships with PD-1/PD-L1 modulation and macrophage polarization, require further research to elucidate.
Furthermore, this study found that combination treatment significantly reduced SII and increased PNI, reflecting improvements in patients’ inflammatory status and nutritional condition. Published studies indicate that CKI can promote HepG2 HCC cell apoptosis and inhibit proliferation[27]. In addition, CKI is primarily composed of bitter ginseng and soil poria, which can enhance the cytotoxic effects of other treatments and activate immune-related pathways. CKI can increase the efficacy of immunotherapy and improve the sensitivity of patients with HCC to treatment[28]. The overall response rate of patients treated with CKI was 33.03%, significantly exceeding the rate of 16.1% in the control group.
Chronic inflammation, such as that associated with hepatitis B virus and hepatitis C virus infection, toxic chemical ingestion, and non-alcoholic fatty liver disease, is the main cause of HCC development[15]. Compared to that in the control group (0.02 ± 0.131), the SII-PNI score was significantly higher in the treatment group (0.8 ± 0.855). This suggests that the addition of CKI can significantly reduce inflammation and improve the nutritional status of patients with HCC. A previous study revealed that CKI inhibited HCC invasion and metastasis and confirmed that CKI inhibited HCC progression by suppressing key glycolysis metabolites[29]. Blocking glycolysis can inhibit inflammatory transitions, reduce pro-inflammatory cytokine secretion, and decrease the expression of co-stimulating molecules associated with relieving infectious inflammation in vitro and in vivo[30]. Based on the aforementioned research and clinical observations, it is possible that the positive therapeutic effect of CKI in patients with AS could involve extracellular matrix remodeling through its ability to target the HBP pathway, thereby regulating the number of immune cells infiltrating the tumor microenvironment. Further research is required to determine the specific mechanism and explore its consequences.
The role of the inflammatory and nutritional condition in post-tumor prognosis has attracted substantial research[31], and its prognostic value has been widely recognized. Wang et al[32] found that CKI can inhibit tumor growth in the livers of mice by inhibiting angiogenesis, resulting in clear anti-inflammatory and immune effects. CKI altered the inflammatory state of the liver and helped animals resist tumor invasion. This was consistent with our findings in this study. In patients with AS, PNI was significantly higher in the combined treatment group (42.5 ± 2.54) than in the control group (30.8 ± 3.89). Meanwhile, SII was significantly lower in the treatment group (380.6 ± 96.05) than in the control group (594.6 ± 72.82). This suggests that the addition of CKI to immunotherapy can alleviate inflammation index and improve the nutritional status of patients with AS compared to the effects of immunotherapy alone.
PNI and SII are commonly used biomarkers in the routine assessment of patients with cancer. Currently, the roles of inflammation and nutritional status in post-tumor prognosis have attracted increasing attention from researchers. In this study, we found that CKI combined with immunotherapy can improve patients’ inflammatory and nutritional status, thereby enhancing the effect of immunotherapy. The SII-PNI score can reflect patients’ physical condition and prognosis, potentially offering clinical value for evaluating the efficacy of immunotherapy.
We evaluated the efficacy of immunotherapy combined with CKI in the treatment of patients with HCC and AS. The study had multiple inherent limitations associated with its retrospective design. First, patients received different anti-tumor therapies prior to study enrollment, which might have resulted in the inclusion of a non-homogeneous group. Second, the reporting of side effects and symptom evaluation lacked precision. This bias might have led to a subjective evaluation of efficacy. Third, the sample size of this study was small, and the conclusions cannot be judged without a power calculation. Although the results require further validation and are not sufficient to prompt changes in clinical practice, future research should assess the complex interactions and mechanisms between TCM and immunotherapy and aim to develop more refined evaluation indicators and standards for the efficacy of immunotherapy.
In this study, our results demonstrated that the pretreatment SII-PNI score had predictive value for patients with HCC and AS. Higher SII-PNI scores might be predictive of AS and sensitivity to CKI. The addition of CKI significantly improved patients’ nutritional status and reduced inflammatory markers, thereby lowering the SII-PNI score. The SII-PNI score effectively reflected the inflammatory and nutritional status of patients with HCC, and it can also be used to predict the prognosis of patients with AS, helping to identify patients with advanced HCC and AS. In patients with advanced HCC, CKI-assisted immunotherapy improved treatment responses and survival outcomes. These findings might facilitate the development of therapeutic strategies and clinical risk stratification to identify the patients most likely to benefit from this therapeutic strategy, improve treatment outcomes, and avoid unnecessary resource use and side effects.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68596] [Article Influence: 13719.2] [Reference Citation Analysis (201)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13325] [Article Influence: 1332.5] [Reference Citation Analysis (4)] |
| 3. | Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2016;379:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 4. | Akkiz H, Carr BI, Bag HG, Karaoğullarından Ü, Yalçın K, Ekin N, Özakyol A, Altıntaş E, Balaban HY, Şimşek H, Uyanıkoğlu A, Balkan A, Kuran S, Üsküdar O, Ülger Y, Güney B, Delik A. Serum levels of inflammatory markers CRP, ESR and albumin in relation to survival for patients with hepatocellular carcinoma. Int J Clin Pract. 2021;75:e13593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Wen Q, Zhang Y, Luo J, Xiong K, Lu Y, Wu Z, Wang BQ, Wu J, Chen Y, Fu S. Therapeutic efficacy of thermosensitive Pluronic hydrogel for codelivery of resveratrol microspheres and cisplatin in the treatment of liver cancer ascites. Int J Pharm. 2020;582:119334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Fujimori M, Tsuchihashi H, Fujimori S, Kobayashi S, Nomi Y, Hirato J, Oyama T, Fukuda T, Saio M. High neutrophil incorporation rate of ascitic fluid cytology as an indicator of cancerous ascites. Int J Oncol. 2020;57:1214-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Da BB, Luo S, Huang M, Song F, Ding R, Xiao Y, Fu Y, Yang YS, Wang HL. Prediction of Hepatocellular Carcinoma Prognosis and Immune Cell Infiltration Using Gene Signature Associated with Inflammatory Response. Comput Math Methods Med. 2022;2022:2415129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Wang Q, Zhu D. The prognostic value of systemic immune-inflammation index (SII) in patients after radical operation for carcinoma of stomach in gastric cancer. J Gastrointest Oncol. 2019;10:965-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Gao Y, Guo W, Cai S, Zhang F, Shao F, Zhang G, Liu T, Tan F, Li N, Xue Q, Gao S, He J. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J Cancer. 2019;10:3188-3196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 10. | Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 250] [Cited by in RCA: 531] [Article Influence: 59.0] [Reference Citation Analysis (12)] |
| 11. | Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2020;99:e18571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Mirili C, Yılmaz A, Demirkan S, Bilici M, Basol Tekin S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. 2019;24:1301-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 13. | Ding P, Yang P, Sun C, Tian Y, Guo H, Liu Y, Li Y, Zhao Q. Predictive Effect of Systemic Immune-Inflammation Index Combined With Prognostic Nutrition Index Score on Efficacy and Prognosis of Neoadjuvant Intraperitoneal and Systemic Paclitaxel Combined With Apatinib Conversion Therapy in Gastric Cancer Patients With Positive Peritoneal Lavage Cytology: A Prospective Study. Front Oncol. 2021;11:791912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Wang D, Hu X, Xiao L, Long G, Yao L, Wang Z, Zhou L. Prognostic Nutritional Index and Systemic Immune-Inflammation Index Predict the Prognosis of Patients with HCC. J Gastrointest Surg. 2021;25:421-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 15. | Yang Y, Sun M, Yao W, Wang F, Li X, Wang W, Li J, Gao Z, Qiu L, You R, Yang C, Ba Q, Wang H. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer. 2020;8:e000317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 16. | Luo H, Zhou X. [Researche advances on CIK cells and their clinical use in lung cancer]. Zhongguo Fei Ai Za Zhi. 2011;14:954-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Liang Y, Zhang Z, Zhong D, Lai C, Dai Z, Zou H, Feng T, Shang J, Shi Y, Huang X. The prognostic significance of inflammation-immunity-nutrition score on postoperative survival and recurrence in hepatocellular carcinoma patients. Front Oncol. 2022;12:913731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Song L, Su X, Lu Y, Hua D, Gao Z. An Inflammation-Associated Prognosis Model for Hepatocellular Carcinoma Based on Adenylate Uridylate- (AU-) Rich Element Genes. Mediators Inflamm. 2023;2023:2613492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Yang Y, Sun M, Li W, Liu C, Jiang Z, Gu P, Li J, Wang W, You R, Ba Q, Li X, Wang H. Rebalancing TGF-β/Smad7 signaling via Compound kushen injection in hepatic stellate cells protects against liver fibrosis and hepatocarcinogenesis. Clin Transl Med. 2021;11:e410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 20. | Lu S, Meng Z, Tan Y, Wu C, Huang Z, Huang J, Fu C, Stalin A, Guo S, Liu X, You L, Li X, Zhang J, Zhou W, Zhang X, Wang M, Wu J. An advanced network pharmacology study to explore the novel molecular mechanism of Compound Kushen Injection for treating hepatocellular carcinoma by bioinformatics and experimental verification. BMC Complement Med Ther. 2022;22:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Liu X, Wu Y, Zhang Y, Bu D, Wu C, Lu S, Huang Z, Song Y, Zhao Y, Guo F, Ye P, Fu C, Shen L, Zhang J, Wang H, Duan X, Wu J. High Throughput Transcriptome Data Analysis and Computational Verification Reveal Immunotherapy Biomarkers of Compound Kushen Injection for Treating Triple-Negative Breast Cancer. Front Oncol. 2021;11:747300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | He T, Xu B, Wang LN, Wang ZY, Shi HC, Zhong CJ, Zhu XD, Shen YH, Zhou J, Fan J, Sun HC, Hu B, Huang C. The prognostic value of systemic immune-inflammation index in patients with unresectable hepatocellular carcinoma treated with immune-based therapy. Biomark Res. 2025;13:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 23. | Wang L, Ke J, Wang C, Li Y, Wu G, Ding Q, Luo Q, Cai R, Lv P, Song T, Xiong S. Efficacy and Safety of Banxia XieXin Decoction, a Blended Traditional Chinese Medicine, as Monotherapy for Patients With Advanced Hepatocellular Carcinoma. Integr Cancer Ther. 2020;19:1534735420942587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Zhou K, Xie M, Liu Y, Zheng L, Pu J, Wang C. Virtual screening and network pharmacology-based synergistic coagulation mechanism identification of multiple components contained in compound Kushen Injection against hepatocellular carcinoma. J Ayurveda Integr Med. 2024;15:101055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Wang KX, Chen YP, Lu AP, Du GH, Qin XM, Guan DG, Gao L. A metabolic data-driven systems pharmacology strategy for decoding and validating the mechanism of Compound Kushen Injection against HCC. J Ethnopharmacol. 2021;274:114043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Gao L, Wang KX, Zhou YZ, Fang JS, Qin XM, Du GH. Uncovering the anticancer mechanism of Compound Kushen Injection against HCC by integrating quantitative analysis, network analysis and experimental validation. Sci Rep. 2018;8:624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Sun Q, Ma W, Gao Y, Zheng W, Zhang B, Peng Y. Meta-analysis: therapeutic effect of transcatheter arterial chemoembolization combined with compound kushen injection in hepatocellular carcinoma. Afr J Tradit Complement Altern Med. 2012;9:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Shen H, Qu Z, Harata-Lee Y, Aung TN, Cui J, Wang W, Kortschak RD, Adelson DL. Understanding the Mechanistic Contribution of Herbal Extracts in Compound Kushen Injection With Transcriptome Analysis. Front Oncol. 2019;9:632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Yao M, Yang JL, Wang L, Yao DF. [Carcinoembryonic type specific markers and liver cancer immunotherapy]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Wang KX, Du GH, Qin XM, Gao L. Compound Kushen Injection intervenes metabolic reprogramming and epithelial-mesenchymal transition of HCC via regulating β-catenin/c-Myc signaling. Phytomedicine. 2021;93:153781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Yu Q, Wang Y, Dong L, He Y, Liu R, Yang Q, Cao Y, Wang Y, Jia A, Bi Y, Liu G. Regulations of Glycolytic Activities on Macrophages Functions in Tumor and Infectious Inflammation. Front Cell Infect Microbiol. 2020;10:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Wang H, Hu H, Rong H, Zhao X. Effects of compound Kushen injection on pathology and angiogenesis of tumor tissues. Oncol Lett. 2019;17:2278-2282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/