Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.105723
Revised: May 1, 2025
Accepted: June 5, 2025
Published online: July 15, 2025
Processing time: 159 Days and 23.4 Hours
Neutrophil extracellular traps (NETs) are associated with an immunosuppressive tumor microenvironment and may influence the efficacy of immune-based the
To investigate the prognostic and predictive value of NET density in LAGC patients undergoing NACI.
We enrolled 31 LAGC patients treated with NACI. NET density was assessed through dual immunofluorescence staining of citrullinated histone H3 and myeloperoxidase in pretreatment biopsy and post-treatment surgical specimens. Patients were stratified into high and low pre-NACI NET groups based on median NET density. Pathological complete response (pCR) and overall response rates were evaluated in relation to NET density. Logistic regression analyses were performed to identify independent predictors of treatment outcomes. Dynamic changes in NET density during NACI were also analyzed.
Patients with low pre-NACI NET density demonstrated significantly higher rates of pCR (40% vs 6%, P = 0.037) and overall response (53% vs 12%, P = 0.023) compared to those with high NET density. Low pre-NACI NET density and higher programmed death protein ligand 1 expression were identified as independent protective factors for achieving pCR and better response rates. NACI increased NET density; however, this increase was primarily observed in non-pCR and nonresponder groups. Patients in the pCR and responder groups showed stable NET density before and after treatment. Higher post-NACI NET density was associated with poorer respond to NACI. High post-NACI NET density was associated with increased infiltration of immunosuppressive FOXP3+ T regulatory cells (P = 0.025) and CD68+ macrophages (P = 0.038).
Pre-NACI NET density serves as a prognostic and predictive biomarker for NACI efficacy in LAGC patients. Low pretreatment NET density is associated with favorable outcomes, while increased post-treatment NET density correlates with poorer response. Targeting NET formation may represent a novel therapeutic strategy to enhance NACI efficacy in LAGC.
Core Tip: This study identified neutrophil extracellular traps (NETs) as a novel prognostic and predictive biomarker for neoadjuvant chemotherapy combined with immunotherapy (NACI) in locally advanced gastric cancer (LAGC). Low pretreatment NET density correlated with higher pathological complete response and overall response rates, while increased post-treatment NET density was associated with poorer outcomes. These findings suggest that targeting NET formation could enhance NACI efficacy, offering a potential therapeutic strategy for LAGC patients.
- Citation: Lv Y, Li WX, Sun L, Xin LP, Li T, Zhong WT, Zhu HY, An R, Liu AJ, Chen L. Neutrophil extracellular traps predict sensitivity to neoadjuvant chemotherapy combined with immunotherapy in locally advanced gastric cancer. World J Gastrointest Oncol 2025; 17(7): 105723
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/105723.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.105723
Gastric cancer (GC) remains a significant global health burden and ranks as one of the leading causes of cancer-related mortality[1]. Despite advances in diagnosis and treatment, the prognosis of locally advanced GC (LAGC) remains poor, primarily because of the high rates of recurrence and metastasis following surgery[2]. Neoadjuvant chemotherapy (NAC) has been established as a standard strategy to downstage tumors and improve resectability, with the additional benefit of eliminating micrometastatic disease[2,3]. More recently, the advent of immunotherapy, particularly immune checkpoint inhibitors (ICIs), has revolutionized cancer treatment and shown promise in enhancing the efficacy of traditional therapies. NAC combined with immunotherapy (NACI) is emerging as a promising treatment modality for LAGC, to improve long-term survival outcomes[4]. However, the heterogeneity of treatment responses underscores the need for robust biomarkers to predict efficacy and guide personalized therapeutic strategies[5,6].
Neutrophil extracellular traps (NETs) are web-like structures composed of chromatin and granular proteins released by activated neutrophils[7]. Originally identified as a host defense mechanism against pathogens, NETs have since been implicated in cancer progression and metastasis[8]. Within the tumor microenvironment (TME), NETs promote tumor growth, facilitate immune evasion, and contribute to the establishment of an immunosuppressive milieu[9]. Increasing evidence suggests that NETs may interfere with the efficacy of immune-based therapies, including ICIs, by suppressing antitumor immune responses and fostering an immunosuppressive TME[9]. However, the role of NETs in modulating the response to NACI in LAGC remains largely unexplored. NETs are not merely passive scaffolds but active regulators of the TME. They facilitate tumor progression through multiple mechanisms: (1) Physical barrier formation: NETs-derived DNA-histone complexes physically shield tumor cells from cytotoxic T lymphocytes and natural killer cells; (2) Immunosuppressive signaling: Proteases embedded in NETs, such as NE and myeloperoxidase (MPO), cleave chemokines critical for T-cell recruitment (e.g., CXCL9/10), thereby impairing antitumor immunity[10]; and (3) Checkpoint ligand presentation: Recent studies have demonstrated that NETs carry immune checkpoint molecules like PD-L1, directly inhibiting T-cell activation[11]. For instance, some researchers revealed that tumor-associated platelet particles (TRAPs) induce PD-L1-decorated NETs, which suppress CD8+ T-cell function via PD-1/PD-L1 axis activation[11]. These findings underscore the dual role of NETs as both structural and functional mediators of immune evasion.
This study investigated the predictive significance of NETs in LAGC patients undergoing NACI. By assessing the density of NETs in pre-NACI biopsies and post-NACI surgical specimens, we aimed to elucidate their relationship with treatment response and pathological complete response (pCR). Additionally, we explored dynamic changes in NET density during NACI and their potential impact on treatment efficacy.
This retrospective study was conducted on gastric adenocarcinoma patients who underwent radical resection at the 7th Medical Center of the Chinese PLA General Hospital. Clinical stage II or III (T3-4 any N or T ≤ 2 N+) tumors according to the 8th edition of the American Joint Committee on Cancer (AJCC) TNM classification for GC were defined as LAGC[12]. Enhanced computed tomography of the abdomen and magnetic resonance imaging of the gastric region were used to confirm the TNM stage.
The inclusion criteria were: (1) Diagnosis of GC by preoperative biopsy; (2) Surgical cohort had postoperative pathological staging of LAGC, and the neoadjuvant therapy cohort was diagnosed with LAGC by preoperative imaging data and biopsy; (3) Complete medical and follow-up records; and (4) Controlled comorbidities. The exclusion criteria were: (1) Incomplete neoadjuvant therapy; (2) Comorbidities with severe infections, autoimmune diseases, and other life-threatening conditions; (3) Death within 30 days after surgery; (4) Gastric tumors from other origins; and (5) History of other cancers. Using these criteria, 31 LAGC patients who received NACI from December 2023 to December 2024 were enrolled. This study was conducted under the approval of the Institutional Ethics Committee.
The chemotherapy regimen included SOX [S-1 (tegafur) and oxaliplatin] or paclitaxel. Immunotherapy options included nivolumab, camrelizumab, penpulimab, toripalimab, or zimberelimab. After two treatment cycles, imaging evaluation (enhanced abdominal CT) was performed to assess tumor response. Responses were categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). For patients with CR, PR, or SD, the existing regimen was continued for an additional two cycles, followed by a final imaging evaluation. For patients with PD, the treatment regimen was discontinued.
Tumor regression grade (TRG) was evaluated in biopsy samples following the AJCC criteria, using a four-tier grading scale: 0, complete regression with no detectable viable tumor cells; 1, near-complete regression with only isolated cells or small clusters of tumor cells; 2, partial regression characterized by residual tumor being outweighed by fibrosis; and 3, minimal or absent regression with extensive residual malignancy and little to no fibrotic response. Patients were divided into two groups: Responders (TRG 0 or 1) and nonresponders (TRG 2 or 3). TRG 0 was specifically defined as pCR, while TRG 1-3 were grouped under incomplete pathological remission (i-pCR).
Immunohistochemistry was conducted on 4-μm-thick formalin-fixed paraffin-embedded sections that were dewaxed, hydrated, and underwent antigen retrieval in EDTA, using anti-CD68 macrophages (Abcam, ab213363), and anti-FOXP3 T regulatory (Treg) cells (Abcam, ab20034). After blocking with 5% bovine serum albumin for 30 minutes, the sections were incubated overnight at 4 °C with primary antibody. On the next day, the sections were incubated with secondary antibody for 1 hour at room temperature and diaminobenzidine for 3 minutes followed by hematoxylin staining. All sections were dehydrated and scanned using Leica CS2 and analyzed using Aperio Image Scope.
Quantification of Treg Density Using QuPath: Treg cell density in tumor regions was evaluated. CD68 expression was graded based on staining intensity (negative, weak, moderate, or strong) and the percentage of positive cells was determined. The scoring system categorized samples as follows: Low expression (0 or 1): ≤ 20% positive cells (moderate/strong intensity) or ≤ 100% positive cells (weak intensity)[13]. High expression (2 or 3): ≥ 21% positive cells with moderate/strong intensity. Samples were classified into low (0 or 1) and high (2 or 3) expression groups to analyze the correlation with tumor characteristics.
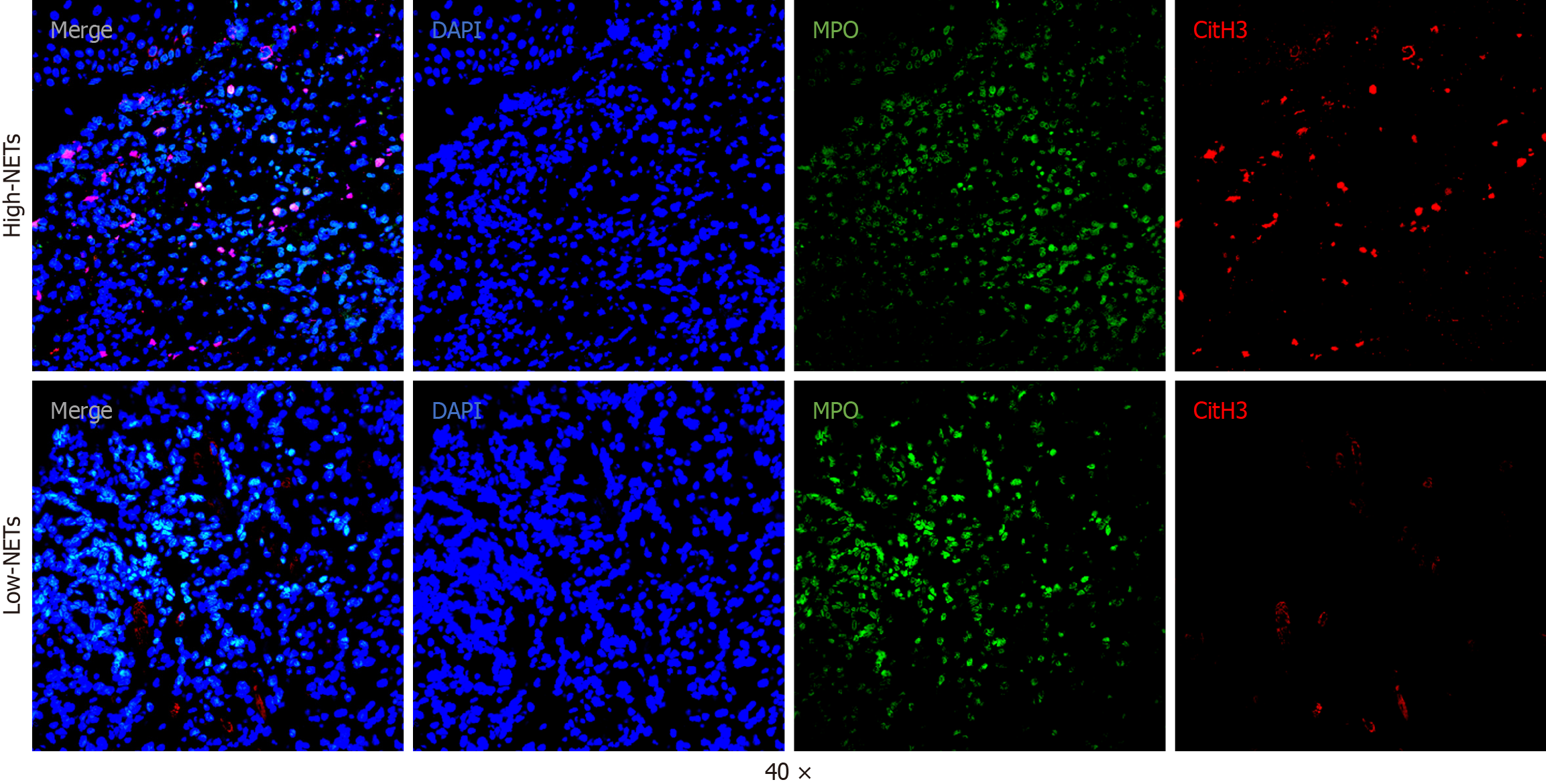
Tumor samples were fixed in formalin, embedded in paraffin, and sectioned at a thickness of 4 μm under the supervision of a pathologist. The tissue slides underwent deparaffinization, rehydration, and antigen retrieval using EDTA buffer. Primary antibodies targeting citrullinated histone H3 (CitH3, 1:1000, ab5103, Abcam) and myeloperoxidase (MPO, 1:1000, AF3667, Abcam) were applied, followed by overnight incubation at 4 °C. Subsequently, fluorophore-conjugated secondary antibodies were added and incubated for 1 h. After washing, nuclei were counterstained, and the slides were visualized using an Olympus Fluoview FV3000 confocal microscope.
Tumor areas were first determined by hematoxylin and eosin staining. Immunofluorescent imaging of these regions was performed with a confocal microscope (Olympus Fluoview FV3000). The images were analyzed using QuPath (v0.4.1) and ImageJ (v1.52c). NETs were recognized based on the colocalization of MPO and CitH3 signals. To quantify NETs, five random fields per slide were selected, and average NET density was calculated. The density was expressed as the percentage of NETs per DAPI-stained nuclei using the following formula: (NET count/DAPI count) × 100.
R version 4.2.1 was used in this study. The χ2 test and Fisher's exact test were used to analyze categorical variables, and the Mann-Whitney U test was used to analyze continuous variables. We used Kaplan-Meier analysis, univariate and multivariate analysis to examine risk factors for disease-free survival. We used logistic multivariate analysis to identify variables associated with TRG. All statistical methods used two-sided analysis, and P < 0.05 was considered statistically significant.
We enrolled 31 patients with LAGC who underwent NACI (Table 1). The cohort included 13 patients aged ≥ 65 years and 18 aged < 65 years. There were 11 female and 20 male patients. Clinical staging revealed seven patients classified as cT3, 7 as cT4a, and 17 as cT4b. For lymph node involvement, one patient was classified as cN1, 11 as cN2, and 19 as cN3. Nine tumors were in the lower stomach, 15 in the middle, and seven in the upper part. Preoperative biopsy grading indicated one patient with a G1 tumor, 10 with G2 tumors, and 20 with G3 tumors. The median PD-L1 score was 7 (interquartile range: 2.5-9.5), and the neutrophil-to-lymphocyte ratio (NLR) was 3 ± 1.38. In terms of immunotherapy regimens, 15 patients received nivolumab, 12 received tislelizumab, and three each received sepranolimab, camrelizumab, toripalimab, or penpulimab. For chemotherapy, 29 patients received the SOX regimen, while two were switched to paclitaxel because of adverse events. Twenty-four patients underwent surgery, while seven did not because of tumor progression.
| Characteristics | Total (n = 31) | High-NETs (n = 16) | Low-NETs (n = 15) | P value |
| Sex | 0.537 | |||
| Female | 11 (35) | 7 (44) | 4 (27) | |
| Male | 20 (65) | 9 (56) | 11 (73) | |
| Age | 0.378 | |||
| < 65 | 18 (58) | 11 (69) | 7 (47) | |
| ≥ 65 | 13 (42) | 5 (31) | 8 (53) | |
| Location | 0.426 | |||
| Lower third | 9 (29) | 5 (31) | 4 (27) | |
| Middle third | 15 (48) | 9 (56) | 6 (40) | |
| Upper third | 7 (23) | 2 (12) | 5 (33) | |
| cT | 0.505 | |||
| cT3 | 7 (23) | 4 (25) | 3 (20) | |
| cT4a | 7 (23) | 5 (31) | 2 (13) | |
| cT4b | 17 (55) | 7 (44) | 10 (67) | |
| cN | 0.014 | |||
| cN1 | 1 (3) | 1 (6) | 0 (0) | |
| cN2 | 11 (35) | 2 (12) | 9 (60) | |
| cN3 | 19 (61) | 13 (81) | 6 (40) | |
| Tumor grade | 0.34 | |||
| G1 | 1 (3) | 0 (0) | 1 (7) | |
| G2 | 10 (32) | 4 (25) | 6 (40) | |
| G3 | 20 (65) | 12 (75) | 8 (53) | |
| PD-L1 scores, median (Q1, Q3) | 7 (2.5, 9.5) | 3.5 (1, 7) | 9 (5, 16) | 0.004 |
| NLR, mean ± SD | 3 ± 1.38 | 3.16 ± 1.53 | 2.84 ± 1.24 | 0.532 |
| PD1 | 0.131 | |||
| Nivolumab | 15 (48) | 5 (31) | 10 (67) | |
| Tislelizumab | 12 (39) | 8 (50) | 4 (27) | |
| Camrelizumab | 1 (3) | 1 (6) | 0 (0) | |
| Penpulimab | 1 (3) | 1 (6) | 0 (0) | |
| Toripalimab | 1 (3) | 1 (6) | 0 (0) | |
| Zimberelimab | 1 (3) | 0 (0) | 1 (7) | |
| Chemotherapy | 0.484 | |||
| Paclitaxel | 2 (6) | 2 (12) | 0 (0) | |
| SOX | 29 (94) | 14 (88) | 15 (100) |
To investigate the relationship between NET density and NACI sensitivity in LAGC, we performed dual immunofluorescence analysis of CitH3 and MPO on pre-NACI biopsy tissues from all 31 patients and post-NACI surgical specimens from 24 patients. Representative images of NETs are shown in Figure 1. Patients were stratified into high and low pre-NACI NETs groups based on median density. Table 1 summarizes the clinical features of these groups. The high pre-NACI NETs group exhibited higher cN stages and lower PD-L1 scores than the low pre-NACI NETs group. However, no significant differences were observed in other clinical features, including age, gender, tumor location, cT stage, tumor grade, NLR, and treatment regimens.
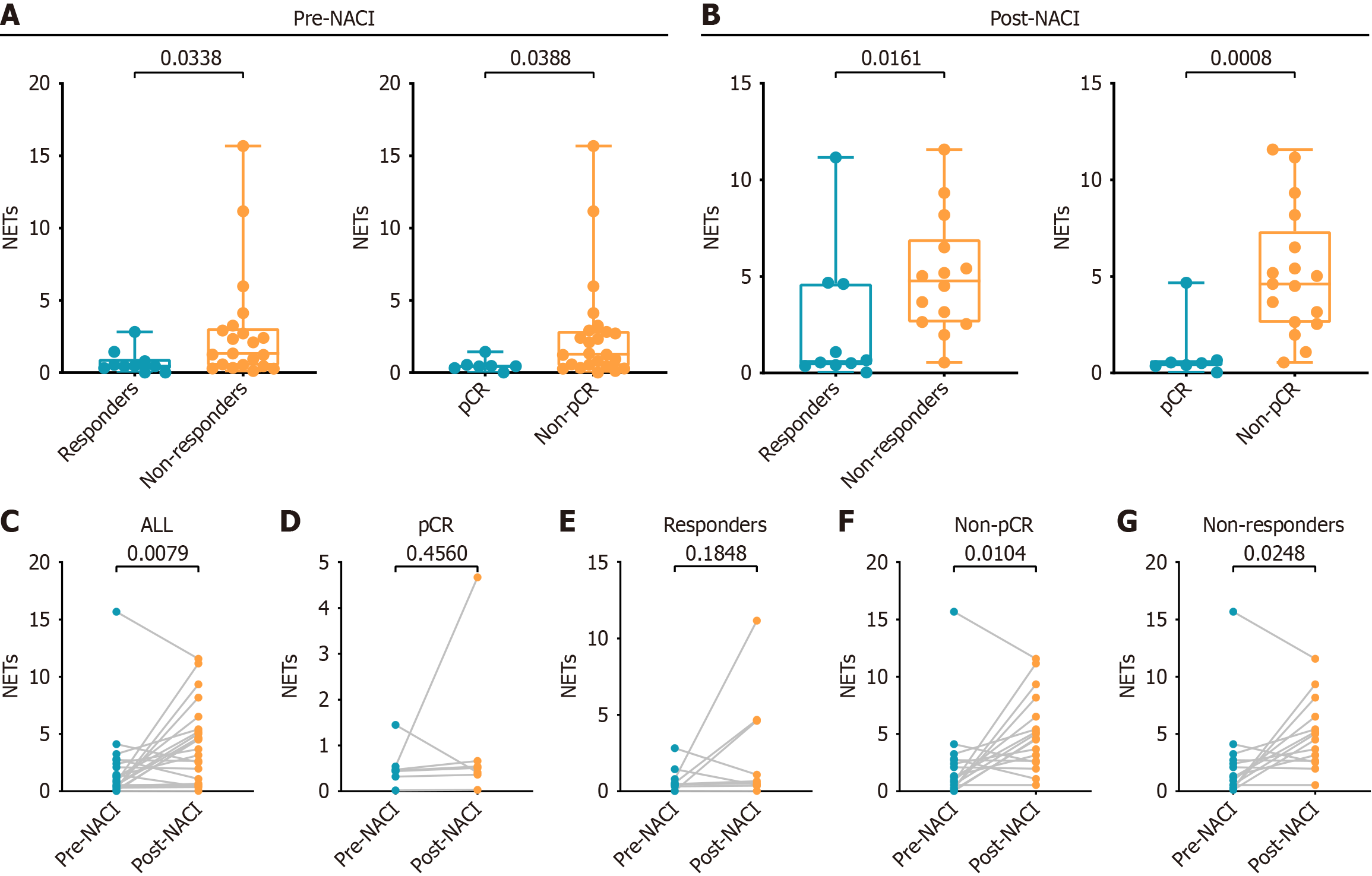
We next assessed the impact of pre-NACI NET density on pCR and response to NACI. The low pre-NACI NETs group demonstrated a significantly higher pCR rate (40% vs 6%, P = 0.037) and response rate (53% vs 12%, P = 0.023) compared to the high pre-NACI NETs group (Table 2). Post-NACI NET density was also evaluated. The pCR group had lower post-NACI NET density than the no-pCR group (0.450 vs 1.290, P = 0.0388) (Figure 2A). Similarly, the response group showed lower post-NACI NETs density than the no-response group (0.460 vs 1.330, P = 0.0338) (Figure 2A).
| Characteristics | High NETs (n = 16) | Low NETs (n = 15) | P value |
| pCR | 0.037 | ||
| no-pCR | 15 (94) | 9 (60) | |
| pCR | 1 (6) | 6 (40) | |
| Respond | 0.023 | ||
| No | 14 (88) | 7 (47) | |
| Yes | 2 (12) | 8 (53) |
Univariate logistic regression analysis identified low pre-NACI NET density [hazard ratio (HR) = 0.10, 95% confidence interval (CI): 0.01-0.97, P = 0.047] and higher PD-L1 scores (HR = 0.87, 95%CI: 0.77-0.99, P = 0.028) as significant factors for achieving pCR with NACI (Table 3). Multivariate analysis confirmed these findings, showing that low pre-NACI NET density (HR = 0.07, 95%CI: 0.0-0.96, P = 0.047) and higher PD-L1 scores (HR = 0.85, 95%CI: 0.73-0.99, P = 0.041) were independent protective factors for pCR (Table 3). Furthermore, both univariate and multivariate logistic regression analyses consistently demonstrated that low pre-NACI NET density (HR = 0.13, 95%CI: 0.02-0.75, P = 0.023) was an independent protective factor associated with better treatment response to NACI (Supplementary Table 1).
| Variables | Univariate analyses | Multivariate analyses | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | ||||
| Female | 1 [Reference] | NA | ||
| Male | 1.50 (0.27-8.38) | 0.644 | ||
| Age | ||||
| < 65 | 1 [Reference] | NA | ||
| ≥ 65 | 2.12 (0.34-13.13) | 0.421 | ||
| Tumor location | ||||
| Lower third | 1 [Reference] | NA | ||
| Middle third | 5.20 (0.71-37.90) | 0.104 | ||
| Upper third | 4.80 (0.40-58.01) | 0.217 | ||
| cT stage | ||||
| cT3 | 1 [Reference] | NA | ||
| cT4a | 1.00 (0.05-19.96) | 1.000 | ||
| cT4b | 0.40 (0.04-4.24) | 0.447 | ||
| cN stage | ||||
| cN1 | 1 [Reference] | NA | ||
| cN2 | 0.00 (0.00-Inf) | 0.995 | ||
| cN3 | 0.00 (0.00-Inf) | 0.995 | ||
| PD-L1 scores | 0.87 (0.77-0.99) | 0.028 | 0.85 (0.73-0.99) | 0.041 |
| Tumor grade | ||||
| G1-2 | 1 [Reference] | NA | ||
| G3 | 1.50 (0.27-8.38) | 0.644 | ||
| Immunotherapy | ||||
| Nivolumab | 1 [Reference] | NA | ||
| Tislelizumab | 1.82 (0.27-12.17) | 0.538 | ||
| Others | 1.09 (0.09-13.78) | 0.946 | ||
| NLR | ||||
| High | 1 [Reference] | NA | ||
| Low | 0.33 (0.03-3.26) | 0.345 | ||
| Pre-NACI NET | ||||
| High | 1 [Reference] | NA | 1 [Reference] | NA |
| Low | 0.10 (0.01-0.97) | 0.047 | 0.07 (0.00-0.96) | 0.047 |
We evaluated the relationship between post-NACI NET density and sensitivity after NACI. The pCR group had significantly lower post-NACI NET density compared to the no-pCR group (0.510 vs 4.610, P = 0.0008) (Figure 2B). Similarly, the response group had a lower post-NACI NETs density compared to the no-response group (0.600 vs 4.765, P = 0.0161) (Figure 2B).
We explored the dynamic changes in NET density during NACI. NACI significantly increased NET density (mean difference = 2.138, P = 0.0091) (Figure 2C). Subgroup analysis revealed no significant pre- to post-NACI NET changes in the pCR group (mean difference = 0.4986, P = 0.4560) (Figure 2D) or responders (mean difference = 0.1848, P = 0.1848) (Figure 2E). However, significant increases were observed in the no-pCR group (mean difference = 2.814, P = 0.0104) (Figure 2F) and nonresponders (mean difference = 2.473, P = 0.0248) (Figure 2G).
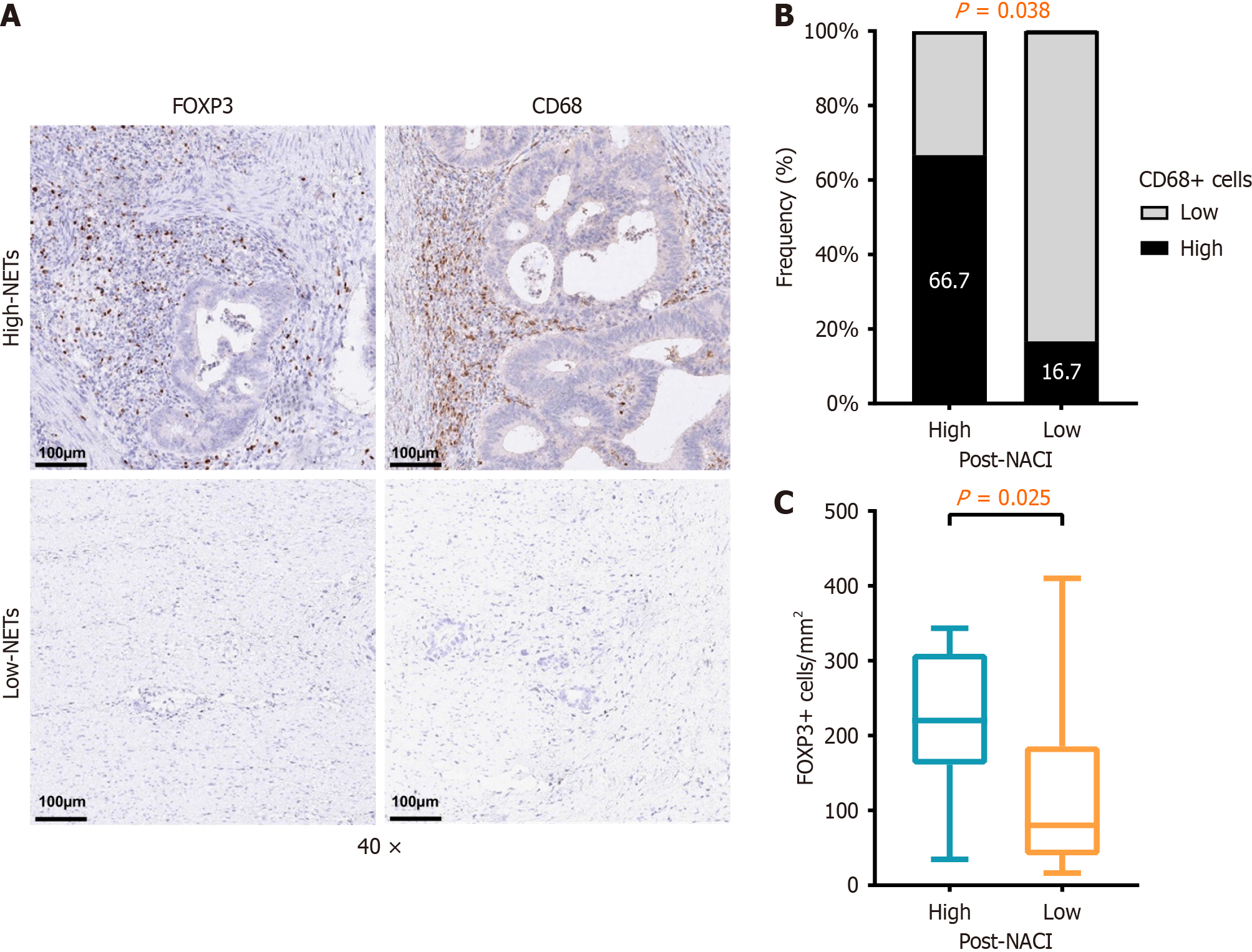
We performed immunohistochemistry to quantify the density of immunosuppressive cells, such as FOXP3+ Treg cells and CD68+ macrophages cells, on tumor tissue (Figure 3A). Our results showed that FOXP3+ Treg cells (P = 0.025), and CD68+ macrophages (P = 0.038) were significantly enriched in the high post-NACI NETs group compared to the low post-NACI NETs (Figure 3B and C).
We investigated the role of NETs in the context of NACI for LAGC. Pre-NACI NET density was associated with both treatment response and pathological outcomes, and changes in NET density during treatment may provide insights into NACI sensitivity.
Patients with low pre-NACI NET density achieved markedly higher rates of pCR and response compared to those with high pre-NACI NETs density. Logistic regression analysis further identified low pre-NACI NET density as an independent protective factor for achieving pCR and favorable treatment responses. These results align with previous research suggesting that excessive NET formation can contribute to an immunosuppressive TME, which diminishes the efficacy of immune-based therapies[14,15]. Specifically, NETs are implicated in promoting tumor progression through mechanisms such as angiogenesis, immune evasion, and metastasis facilitation[16]. Thus, low pre-NACI NET density may indicate a less immunosuppressive TME, enhancing the antitumor effects of NACI.
NACI treatment increased NET density; however, subgroup analyses revealed this increase was predominantly observed in non-pCR and nonresponder groups. Conversely, NET density remained stable in pCR and responder groups before and after NACI. This suggests that failure to suppress NETs during treatment may reduce therapeutic efficacy. Persistent neutrophil activation and NET formation likely maintain an immunosuppressive TME, undermining the benefits of NACI. Shinde-Jadhav et al[17] have demonstrated that radiotherapy can stimulate NET formation in bladder cancer, with elevated NET levels being closely associated with radiation resistance. Tumor-derived high mobility group box (HMGB)1 released after radiotherapy promotes NET formation through Toll-like receptor 4 activation, underscoring the bidirectional relationship between treatment modalities and NETs[17]. These findings highlight the potential utility of targeting NETs as a strategy to improve NACI efficacy.
Our study also observed that patients in the high pre-NACI NETs group had lower PD-L1 scores, suggesting reduced tumor immunogenicity. Higher PD-L1 expression was independently associated with achieving pCR, consistent with its established role as a predictive biomarker for ICI efficacy[18,19]. These findings point to a complex interplay between NETs and PD-L1 expression, where NETs may influence immune escape mechanisms within the TME. Future investigations should explore the molecular pathways linking NETs to PD-L1 signaling to better understand their combined impact on tumor immunity. Zhou et al[11] revealed that in lung metastasis of breast cancer, TRAPs activate the TLR4/Myd88/ERK/p38 signaling axis by releasing HMGB1, inducing the formation of NETs in human and mouse neutrophils, and PD-L1 carried by TRAPs drives the formation of a premetastatic immunosuppressive microenvironment by inhibiting T-cell function.
Our observation that high NET density correlated with reduced PD-L1 scores aligns with findings by Au et al[20], where NET-rich tumors exhibited compensatory PD-L1 downregulation due to T-cell exclusion. Additionally, the association between rising NETs and resistance mirrors clinical data from Li et al[21], where serum NET biomarkers predicted immunotherapy failure in GC.
Our findings agree with emerging evidence that NETs dynamically interact with tumor and immune cells. We observed that high post-NACI NET density was significantly associated with increased infiltration of immunosuppressive cells, particularly FOXP3+ Treg cells and CD68+ macrophages. Several mechanisms may underlie this association. First, NET-derived HMGB1 activates the TLR4/MyD88 pathway in dendritic cells (DCs), impairing their antigen-presenting capacity and subsequent T-cell priming[22,23]. Second, citrullinated histones within NETs directly induce apoptosis of CD8+ T cells via caspase-3 activation[20]. Third, NETs serve as reservoirs for proteases (e.g., matrix metalloproteinase-9) that degrade extracellular matrix components, facilitating metastatic niche formation while excluding effector immune cells. This multifaceted immunosuppression likely explains why high pre-NACI NET density correlates with reduced PD-L1 scores in our cohort- a phenomenon potentially reflecting compensatory downregulation of PD-L1 in highly NETs-rich, T-cell-excluded tumors[24].
The findings of this study suggest that NET density could serve as a valuable biomarker for stratifying LAGC patients undergoing NACI. Pretreatment assessment of NET density may help identify patients unlikely to respond to NACI, guiding alternative or combination therapeutic strategies. Additionally, monitoring NET dynamics during treatment could provide real-time insights into therapeutic efficacy. Developing interventions to modulate NETs, such as inhibitors targeting neutrophil activation or NET formation, could enhance treatment outcomes for LAGC patients. Han et al[25] found that exogenous irisin significantly inhibits the formation of NETs through the integrin αVβ5/P38/MAPK signaling axis, which may be a key regulatory target for NET production.
Despite these promising findings, our study had several limitations. First, the small sample size limits generalization of the results. The study included 31 patients who met the inclusion criteria and voluntarily participated. Due to financial constraints and other practical considerations, only this number of patients were enrolled at this stage. While a larger cohort would provide additional statistical power, the current sample size remains sufficient to generate meaningful insights. Larger, multicenter studies are needed to validate the utility of NET density as a predictive biomarker in LAGC. Second, while significant associations were observed, the underlying mechanisms remain speculative. Experimental studies are warranted to establish a causal link between NETs and NACI sensitivity. Third, potential confounders, including comorbidities or variations in neutrophil function, were not accounted for, which may have influenced NETs formation and related outcomes.
The absence of mature survival data currently precludes definitive conclusions about the long-term prognostic value of NET density. Extended follow-up studies are warranted to validate the association between NET dynamics and survival outcomes. Emerging evidence suggests that NETs may recruit or activate immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and Treg cells. NET-derived components (e.g., histones and proteases) can activate the TLR9/NF-κB pathway in myeloid cells, promoting MDSC expansion and immunosuppressive functions. Additionally, NET-associated HMGB1 has been shown to enhance MDSC-mediated T-cell suppression via STAT3 signaling[26]. NET-derived DNA and histones may stimulate DCs to secrete transforming growth factor-β and interleukin-10, which are known to drive Treg cell differentiation[27]. In pancreatic cancer, NETs were found to upregulate PD-L1 on DCs, indirectly fostering Treg cell infiltration.
This study underscores the prognostic and predictive value of NET density in LAGC patients undergoing NACI. A low pre-NACI NET density was associated with favorable pathological and clinical responses, while an increase in post-NACI NET density was linked to poorer outcomes. These findings highlight the potential of targeting NETs as a novel therapeutic strategy to improve the efficacy of NACI in LAGC.
The authors acknowledge gratitude to all the staff who participated in this study.
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3304] [Article Influence: 550.7] [Reference Citation Analysis (6)] |
| 2. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, Farjah F, Gerdes H, Gibson MK, Hochwald S, Hofstetter WL, Ilson DH, Keswani RN, Kim S, Kleinberg LR, Klempner SJ, Lacy J, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Outlaw D, Park H, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian N, Pluchino LA. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:167-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 1163] [Article Influence: 290.8] [Reference Citation Analysis (0)] |
| 3. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21:4012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 971] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 4. | Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 5. | Ji Z, Peng Z, Gong J, Zhang X, Li J, Lu M, Lu Z, Shen L. Hyperprogression after immunotherapy in patients with malignant tumors of digestive system. BMC Cancer. 2019;19:705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Di Bartolomeo M, Morano F, Raimondi A, Miceli R, Corallo S, Tamborini E, Perrone F, Antista M, Niger M, Pellegrinelli A, Randon G, Pagani F, Martinetti A, Fucà G, Pietrantonio F; ITACA-S study group. Prognostic and Predictive Value of Microsatellite Instability, Inflammatory Reaction and PD-L1 in Gastric Cancer Patients Treated with Either Adjuvant 5-FU/LV or Sequential FOLFIRI Followed by Cisplatin and Docetaxel: A Translational Analysis from the ITACA-S Trial. Oncologist. 2020;25:e460-e468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Adrover JM, McDowell SAC, He XY, Quail DF, Egeblad M. NETworking with cancer: The bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell. 2023;41:505-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 214] [Reference Citation Analysis (0)] |
| 8. | Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front Immunol. 2020;11:1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 9. | Fang Q, Stehr AM, Naschberger E, Knopf J, Herrmann M, Stürzl M. No NETs no TIME: Crosstalk between neutrophil extracellular traps and the tumor immune microenvironment. Front Immunol. 2022;13:1075260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Zhong W, Wang Q, Shen X, Lv Y, Sun L, An R, Zhu H, Cai H, Chen G, Liu A, Du J. Neutrophil Extracellular Trap is Surrogate Biomarker for Prognosis and Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. J Inflamm Res. 2023;16:6443-6455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Zhou X, Wu C, Wang X, Pan N, Sun X, Chen B, Zheng S, Wei Y, Chen J, Wu Y, Zhu F, Chen J, Chen H, Wang LX. Tumor cell-released autophagosomes (TRAPs) induce PD-L1-decorated NETs that suppress T-cell function to promote breast cancer pulmonary metastasis. J Immunother Cancer. 2024;12:e009082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Rausei S, Boni L, Rovera F, Dionigi G. Locally advanced gastric cancer: a new definition to standardise. J Clin Pathol. 2013;66:164-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Lohr M, Edlund K, Botling J, Hammad S, Hellwig B, Othman A, Berglund A, Lambe M, Holmberg L, Ekman S, Bergqvist M, Pontén F, Cadenas C, Marchan R, Hengstler JG, Rahnenführer J, Micke P. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013;333:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Xin H, Lai Q, Zhou Y, He J, Song Y, Liao M, Sun J, Li M, Zhang M, Liang W, Bai Y, Zhang Y, Zhou Y. Noninvasive evaluation of neutrophil extracellular traps signature predicts clinical outcomes and immunotherapy response in hepatocellular carcinoma. Front Immunol. 2023;14:1134521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 15. | Han AX, Long BY, Li CY, Huang DD, Xiong EQ, Li FJ, Wu GL, Liu Q, Yang GB, Hu HY. Machine learning framework develops neutrophil extracellular traps model for clinical outcome and immunotherapy response in lung adenocarcinoma. Apoptosis. 2024;29:1090-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Xia J, Zhang Z, Huang Y, Wang Y, Liu G. Regulation of neutrophil extracellular traps in cancer. Int J Cancer. 2024;154:773-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Shinde-Jadhav S, Mansure JJ, Rayes RF, Marcq G, Ayoub M, Skowronski R, Kool R, Bourdeau F, Brimo F, Spicer J, Kassouf W. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun. 2021;12:2776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 18. | Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi D, Yu D, Gao P, Chen C, Wei M, Zhou W, Wang J, Zhao Z, Dai X, Xu Q, Zhang X, Huang M, Huang K, Wang J, Li J, Sheng L, Liu L. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 170] [Article Influence: 56.7] [Reference Citation Analysis (1)] |
| 19. | Yeong J, Lum HYJ, Teo CB, Tan BKJ, Chan YH, Tay RYK, Choo JR, Jeyasekharan AD, Miow QH, Loo LH, Yong WP, Sundar R. Choice of PD-L1 immunohistochemistry assay influences clinical eligibility for gastric cancer immunotherapy. Gastric Cancer. 2022;25:741-750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 20. | Au L, Hatipoglu E, Robert de Massy M, Litchfield K, Beattie G, Rowan A, Schnidrig D, Thompson R, Byrne F, Horswell S, Fotiadis N, Hazell S, Nicol D, Shepherd STC, Fendler A, Mason R, Del Rosario L, Edmonds K, Lingard K, Sarker S, Mangwende M, Carlyle E, Attig J, Joshi K, Uddin I, Becker PD, Sunderland MW, Akarca A, Puccio I, Yang WW, Lund T, Dhillon K, Vasquez MD, Ghorani E, Xu H, Spencer C, López JI, Green A, Mahadeva U, Borg E, Mitchison M, Moore DA, Proctor I, Falzon M, Pickering L, Furness AJS, Reading JL, Salgado R, Marafioti T, Jamal-Hanjani M; PEACE Consortium, Kassiotis G, Chain B, Larkin J, Swanton C, Quezada SA, Turajlic S; TRACERx Renal Consortium. Determinants of anti-PD-1 response and resistance in clear cell renal cell carcinoma. Cancer Cell. 2021;39:1497-1518.e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 21. | Li Q, Chen W, Li Q, Mao J, Chen X. A novel neutrophil extracellular trap signature to predict prognosis and immunotherapy response in head and neck squamous cell carcinoma. Front Immunol. 2022;13:1019967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 22. | Liu Z, Falo LD Jr, You Z. Knockdown of HMGB1 in tumor cells attenuates their ability to induce regulatory T cells and uncovers naturally acquired CD8 T cell-dependent antitumor immunity. J Immunol. 2011;187:118-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Li J, Xia Y, Sun B, Zheng N, Li Y, Pang X, Yang F, Zhao X, Ji Z, Yu H, Chen F, Zhang X, Zhao B, Jin J, Yang S, Cheng Z. Neutrophil extracellular traps induced by the hypoxic microenvironment in gastric cancer augment tumour growth. Cell Commun Signal. 2023;21:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 24. | Kaltenmeier C, Yazdani HO, Morder K, Geller DA, Simmons RL, Tohme S. Neutrophil Extracellular Traps Promote T Cell Exhaustion in the Tumor Microenvironment. Front Immunol. 2021;12:785222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 25. | Han F, Ding ZF, Shi XL, Zhu QT, Shen QH, Xu XM, Zhang JX, Gong WJ, Xiao WM, Wang D, Chen WW, Hu LH, Lu GT. Irisin inhibits neutrophil extracellular traps formation and protects against acute pancreatitis in mice. Redox Biol. 2023;64:102787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 26. | Chen X, Bao S, Liu M, Han Z, Tan J, Zhu Q, Huang X, Tian X. Inhibition of HMGB1 improves experimental mice colitis by mediating NETs and macrophage polarization. Cytokine. 2024;176:156537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 27. | Xia X, Zhang Z, Zhu C, Ni B, Wang S, Yang S, Yu F, Zhao E, Li Q, Zhao G. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13:1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/