Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.105286
Revised: May 11, 2025
Accepted: June 16, 2025
Published online: July 15, 2025
Processing time: 87 Days and 4 Hours
Surgical treatment for primary liver cancer can effectively reduce infection risks. Accurate prediction is crucial for timely intervention, particularly to reduce the risk of infection.
To explore the predictive and prognostic value of the nutritional risk index (NRI) in hepatitis B virus (HBV)-related liver cancer.
Ninety-six patients with HBV-related primary liver cancer who underwent surgery at our hospital between May 2022 and May 2024 were included. Patients were classified into infection and non-infection groups, and the NRI was compared. The infection group was further divided into mild and severe infection groups and then into survival and deceased groups, and the NRI was compared. Postoperative follow-up lasted 6 months. The predictive value of NRI for surgical site infections (SSIs), severity of infections, and prognostic assessment was analyzed.
Compared with patients with mild infection, those with severe infections had a significantly lower NRI (P < 0.05). Compared with patients with mild infections, those with severe infections had a significantly higher NRI (P < 0.05). The NRI was significantly lower in the good prognosis group than in the poor prognosis group (P < 0.05). Receiver operating characteristic curve analysis showed that the areas under the curve for NRI in predicting SSIs, infection severity, and patient prognosis were 0.984, 0.986, and 0.949, respectively.
The NRI can accurately predict postoperative SSIs in patients with HBV-related primary liver cancer and plays a role in predicting the severity of infections and in prognostic assessment.
Core Tip: The nutritional risk index is closely related to postoperative surgical site infections in patients with hepatitis B virus-related primary liver cancer and can be used for infection prediction and prognostic assessment.
- Citation: Zhai XZ, Dong MY, Ding YF, Luo TT. Nutritional risk index in hepatitis B virus-related liver cancer: Infection prediction and prognosis. World J Gastrointest Oncol 2025; 17(7): 105286
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/105286.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.105286
Primary liver cancer, also known as liver cancer, is a malignant tumor with high incidence and mortality rates. A statistical report from the National Cancer Center of China estimated 4.8747 million new cancer cases in 2022 in the country, with liver cancer ranked fourth, followed by lung, colorectal, and thyroid cancers[1]. Liver cancer ranked as the second leading cause among new cancer-related deaths, after lung cancer. Hepatitis B virus (HBV) infection is the main cause of the disease, with related markers detected in the serum of over 90% of patients. Aflatoxin exposure and water pollution can increase the risk of diseases. The disease typically develops insidiously and is usually discovered during follow-up or physical examination. The main clinical symptoms include liver pain, fatigue, appetite loss, and weight loss. In advanced stages, the disease can metastasize to other parts of the body, causing related symptoms and leading to death in severe cases.
Surgical resection is the preferred treatment option for liver cancer. Radical or palliative surgery can be performed based on the age, condition, and physical status to control symptoms and delay disease progression. Although surgery can improve survival rates and extend life, it can lead to a series of complications, with surgical site infections (SSIs) being relatively common. A previous study reported a 1.6% incidence of SSIs within 30 days of surgery among 17353 patients who underwent gastrointestinal surgery[2]. Compared with patients undergoing other types of surgery, patients with cancer have lower immunity and resistance, making them more susceptible to SSIs postoperatively. The incidence of SSIs following hepatectomy in patients with colorectal liver metastasis is 13%[3]. This infection type is an external surgical infection caused by the entry and proliferation of microorganisms from the surrounding environment into the surgical incision through body tissues such as the skin, leading to symptoms such as redness, swelling, fever, inflammation, and pain. This can lead to secondary harm to patients, prolonging hospital stays and increasing medical costs. Preoperative malnutrition can increase the risk of postoperative site infections. Therefore, assessing the preoperative nutritional status of patients is crucial to accurately predict postoperative infections and assess patient prognosis.
The nutritional risk index (NRI) was developed by the parenteral nutrition study assistance group of the Veterans’ Association of the United States. It is commonly used to evaluate the effectiveness of preoperative parenteral nutritional support in patients undergoing thoracic and abdominal surgery. The NRI assesses nutritional risk based on blood hemoglobin levels and changes in body weight with high sensitivity and specificity. The related parameters are easy to obtain and simple to apply[4]. Clinically, this indicator is often used to reflect the nutritional status of patients with cancer and can predict patient outcomes. This study aimed to analyze the predictive value of the NRI for postoperative SSIs and its prognostic value in patients with HBV-related primary liver cancer.
Ninety-six patients, including 52 men and 44 women, with HBV-related primary liver cancer who were treated between May 2022 and May 2024 were included. The age, disease duration, and body mass index (BMI) of the patients ranged from 28 to 75 years (mean: 52.16 ± 7.32 years), 6 to 15 months (mean: 10.78 ± 1.54 months), and 17 to 24 kg/m² (mean: 21.18 ± 0.75 kg/m²), respectively. The liver cancer types included 65, 21, and 10 cases of hepatocellular carcinoma, cholangiocellular carcinoma, and mixed cell carcinoma, respectively. The inclusion criteria were as follows: (1) Diagnosis of primary liver cancer confirmed based on surgical pathology; (2) Age > 18 years; (3) Surgery performed by the same surgeon; and (4) Complete data available for research support. The exclusion criteria were as follows: (1) Patients with severe heart, brain, or kidney diseases; (2) Patients with other types of malignant tumors; (3) Patients receiving conservative treatment; (4) Patients with preoperative infections; and (5) Patients who could not complete follow-up.
NRI: The NRI was calculated as follows: [1.489 serum albumin concentration (g/L)] + [41.7 (current weight/previous weight)]. To measure hemoglobin levels, 5 mL of preoperative fasting venous blood was collected and centrifuged using a medical centrifuge (TD-3A, record No. 20150081, Changchun Boyan Scientific Instrument Co., Ltd.) at 3500 rpm for 5 minutes, with a rotational radius of 12 cm. The upper serum was then collected, and hemoglobin levels were determined using a hemoglobin analyzer (URIT-12, registration certificate no. 20142220026, Guilin Youli Medical Electronics Co., Ltd.). The stable weight recorded at least 6 months prior to disease onset was defined as the previous weight. If the current weight/previous weight was > 1, it was recorded as 100%. An NRI > 100 indicated no malnutrition, with lower values reflecting a higher risk of malnutrition.
Grouping criteria: The patients were classified into infection and non-infection groups based on the diagnosis according to the definition proposed by the Centers for Disease Control and Prevention in 1999[5]. Infections occurring in the incision, deep surgical organs, or body cavities during the perioperative period were considered SSIs.
Severity of infection: Patients with SSIs were divided into mild and severe groups based on the severity of the infection. Mild infection was defined as superficial incision infection occurring within 30 days postoperatively, involving only the surface skin and subcutaneous tissue, and meeting at least one of the following criteria: (1) Superficial incision with purulent discharge; (2) Bacterial growth confirmed by culture of the discharge; (3) Presence of pain, tenderness, redness, heat, or swelling; and (4) Infection diagnosed by a surgeon. Severe infections included deep incisions and organ or space infections. Deep incision infection was defined as an infection occurring within 30 days postoperatively, involving the fascia and muscle layer, and meeting one of the following criteria: (1) Purulent discharge from the deep incision; (2) Spontaneous dehiscence of the incision or opening by a physician, with a temperature > 38 °C or local pain/tenderness; (3) Clinical/surgical/pathological/imaging diagnosis showing an abscess in the deep incision; and (4) Infection diagnosed by a surgeon. Notably, patients with combined superficial infections were also classified as having deep infections. Organ/space infection was defined as an infection occurring within 30 days postoperatively in the organs or spaces related to the surgical procedure and meeting one of the following criteria: (1) Purulent discharge from a drain located in the organ or space; (2) Pathogenic bacteria grown from the culture of organ/space tissue/fluid; (3) Surgical/pathological/imaging diagnosis showing an abscess in the organ or space; and (4) Infection diagnosed by a surgeon.
Prognostic grouping: Patients in the infection group were followed up for 6 months and divided into deceased and survived groups based on their prognosis.
The NRI was compared between the infection and non-infection groups, followed by a comparison between patients with mild and severe infections. Additionally, the NRI was compared between survivors and deceased patients. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive value of the NRI for the surgical site and severe infections, as well as its value in assessing the prognosis of infected patients.
Statistical analyses were performed using SPSS 22.0. For normally distributed measurement data, results were expressed as mean ± SD, and the t-test was used. The predictive and evaluative values were assessed using ROC curve analysis. P < 0.05 was considered statistically significant.
Among the 96 patients, 61 and 35 were included in the infection and non-infection groups, respectively. The NRI in the infection group was significantly lower than that in the non-infection group (P = 0.000; Table 1).
| Group | Cases | Nutritional risk index |
| Infection group | 61 | 85.36 ± 2.44 |
| Non-infection group | 35 | 92.27 ± 1.21 |
| t value | 15.661 | |
| P value | 0.000 |
Of the 61 patients with postoperative SSI, 36 had mild and 25 had severe infection. The NRI was significantly lower in patients with severe infection than in those with mild infection (P = 0.000; Table 2).
| Group | Cases | Nutritional risk index |
| Mild infection | 36 | 89.18 ± 1.56 |
| Severe infection | 25 | 83.72 ± 2.18 |
| t value | 11.413 | |
| P value | 0.000 |
Of the 61 patients with postoperative site infection, 40 survived and 21 died. The difference in NRI between the two groups was significant (P = 0.000; Table 3).
| Group | Cases | Nutritional risk index |
| Survivors | 40 | 88.72 ± 2.32 |
| Deceased | 21 | 83.48 ± 1.96 |
| t value | 8.820 | |
| P value | 0.000 |
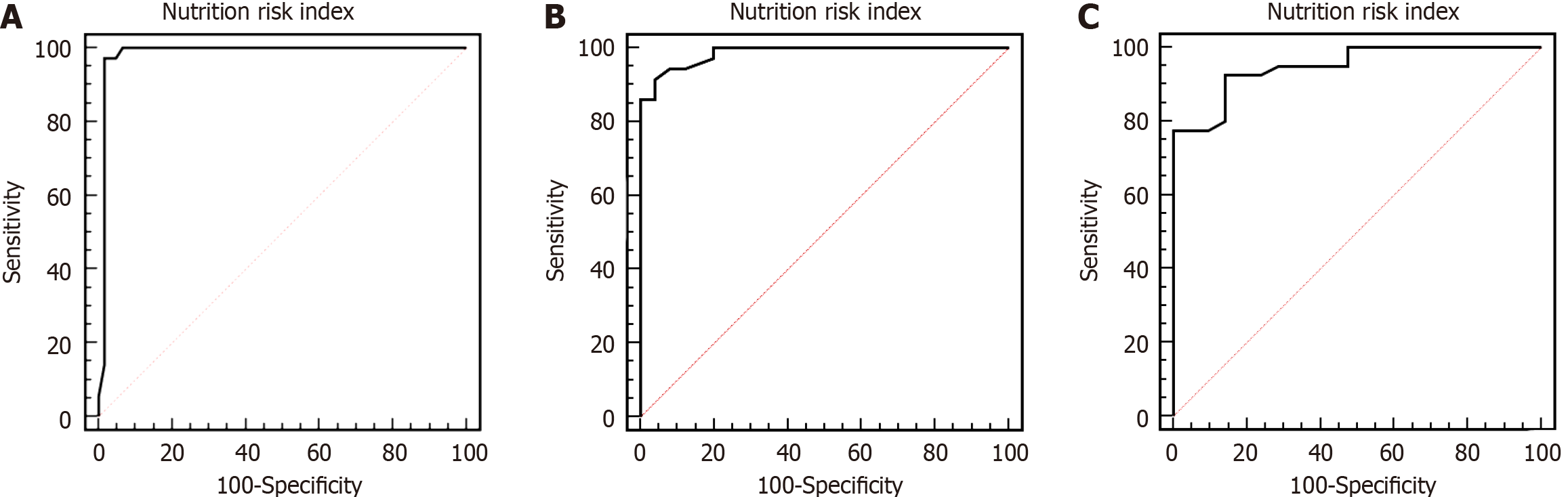
The areas under the curve for the NRI predicting postoperative SSI, severity of infection, and prognostic assessment were 0.984, 0.986, and 0.949, respectively (Tables 4, 5 and 6; Figure 1). An NRI < 89.6 predicted postoperative SSI with 90.17% sensitivity and 95.14% specificity. An NRI < 86.5 predicted severity of infection with 92.00% sensitivity and 94.44% specificity. An NRI < 85.6 predicted death with 92.50% sensitivity and 85.71% specificity.
| Group | Cases | Nutritional risk index (mean ± SD) | Group | Cases | Nutritional risk index |
| Nutritional risk index | 61 | 85.36 ± 2.44 | Infection group | 61 | 85.36 ± 2.44 |
| Group | Cases | Nutritional risk index | Group | Cases | Nutritional risk index |
| Nutritional risk index | 0.986 | 0.916-1.000 | 91.67 | 96.00 | 0.8767 |
| Group | Cases | Nutritional risk index | Group | Cases | Nutritional risk index |
| Nutritional risk index | 0.949 | 0.860-0.989 | 92.50 | 85.71 | 0.7821 |
Surgery is a common therapeutic approach in Western medicine and is performed by experienced surgeons using specialized instruments and advanced techniques to remove diseased tissue to alleviate symptoms and cure diseases. However, surgical treatment may also lead to complications such as SSIs. SSIs, also known as postoperative wound infections, pose a significant burden on the healthcare system. SSIs are most commonly observed in surgeries involving skin incisions, typically occurring within 30 days postoperatively. The incidence of SSIs varies, but they are among the most common types of hospital-acquired infections. SSIs prolong hospital stays and increase medical costs, posing serious threats to patient safety in severe cases[6,7].
SSIs are primarily influenced by factors related to both the patient and surgical procedure. Advanced age, obesity, prolonged preoperative hospital stay, the presence of underlying diseases, inadequate preoperative preparation, extended surgical duration, and incomplete postoperative management can increase the risk of SSIs. A study by Gupta et al[8] involving 6169 patients with hip fractures who underwent primary hemiarthroplasty reported an SSI incidence rate of 1.3%. Furthermore, BMI, preoperative functional status, surgical time, and wound dehiscence were identified as the related factors, with BMI ≥ 35 kg/m² and surgical time ≥ 120 minutes serving as independent risk factors. A study by Gomaa et al[9] reported that 828 (5.34%) of the 15502 women who underwent cesarean delivery developed SSIs. Specifically, lack of prenatal care, obesity, hypertension, diabetes, labor duration ≥ 24 hours, and blood loss > 1000 mL were identified as significant risk factors.
HBV-related primary liver cancer is the most common form of primary liver cancer caused by HBV infection. This DNA virus can activate proto-oncogenes or inactivate tumor suppressor genes, thereby promoting the development of primary liver cancer. Patients affected by other diseases exhibit decreased immune capabilities and poor resistance to diseases, rendering them more susceptible to SSIs following surgical treatment. In a study by Hasegawa et al[10] involving 324 patients with liver cancer who underwent hepatectomy, univariate and multivariate analyses revealed ascites bacterial infection, lack of preoperative oral management, and severe liver fibrosis as the risk factors for SSIs. Malnutrition is a significant contributor to postoperative SSIs in patients with malignant tumors. A previous study involving adult patients who underwent open right hemicolectomy and anastomosis reported an association between vitamin D deficiency and an increased incidence of postoperative surgical wound infections, suggesting that poor preoperative nutritional status may increase the risk of SSIs[11].
Currently, various scales or tools, including the NRS2002 nutritional risk screening score sheet, subjective global assessment, and the malnutrition universal screening tool, are available for nutritional risk screening. These tools can be used to assess the nutritional status of patients based on different items. However, each of these tools has certain limitations. The NRS2002 nutritional risk screening score sheet requires the patient to be alert and primarily reflects the overall patient's nutritional status, offering only a general assessment of acute nutritional changes. The clinical reports on the universal malnutrition screening tool are limited, and its sensitivity remains unclear[12,13]. Unlike the aforementioned nutritional risk screening methods, the NRI requires only three parameters—hemoglobin level, current weight, and usual weight—for assessment, making it simple to use. Weight change is the most direct reflection of a patient's nutritional status, with weight loss indicating a risk of malnutrition, which is closely associated with nutritional status and can lead to a decrease in hemoglobin levels. Guo et al[14] reported that the NRI could predict the prognosis of patients undergoing malignant tumor surgery. Compared with conventional nutritional indicators, the area under the curve for predicting patient prognosis was higher, highlighting it as a new indicator for prognostic assessment in patients with oligometastatic prostate cancer following cytoreductive radical prostatectomy. In a study by Kim et al[15] involving patients with gastric cancer who underwent radical gastrectomy, a multivariate Cox regression analysis revealed a significant association between the preoperative NRI and overall survival rate of patients. Furthermore, patients with a high NRI had a longer survival period, highlighting the important role of NRI in predicting mortality prognosis.
The present study is the first to analyze the predictive value of preoperative NRI for postoperative site infections and its prognostic value for patients with HBV-related primary liver cancer. Our findings revealed that the NRI of patients in the infection group was lower, and that of patients with severe infections was lower than that of patients with mild infections (P < 0.05). Further ROC curve analysis revealed that the sensitivity and specificity of the NRI for predicting SSIs and severe infections were both above 90%, confirming its predictive value. SSIs are a type of healthcare-associated infection, mostly caused by contamination during the surgical procedure but also influenced by patient characteristics. Patients with primary liver cancer experience liver cell damage, resulting in reduced food intake, reduced absorption, and high consumption. Additionally, nausea and vomiting due to the diseases can decrease the appetite. Moreover, tumor growth requires large amounts of nutrients, further increasing the risk of malnutrition. Surgical resection of the lesion can affect the functioning of the digestive system. The body enters a hypermetabolic state in response to tissue trauma from surgery, increasing the demand for energy and trace elements. Even with the adequate nutrition intake, protein and trace element deficiencies may still occur, leading to a decrease in the immune cell numbers, impaired immune regulation, decreased immune cell activity, and compromised bactericidal, phagocytic, and secretory cell functions, affecting the normal immune system function. The immune system acts as a barrier against infections. It can recognize foreign pathogens, including bacteria and viruses, activate effector cells, and eliminate pathogenic bacteria. The immune system also removes damaged cells and metabolic waste to maintain homeostasis. Once immune function declines, the body's defense mechanisms are compromised, impairing its ability to effectively combat the invasion of pathogenic bacteria, thereby increasing the risk of infection. Patients with malignant tumors have abnormal metabolism with a decline in digestive tract function, affecting the body's ability to absorb and assimilate nutrients, thereby inducing and exacerbating malnutrition. As nutritional risk increases, nutrient deficiencies in the patient become more pronounced, leading to more significant impairments in immune function. This can further exacerbate the immune dysfunction and increase the severity of SSIs. Therefore, the NRI of patients with HBV-related primary liver cancer can be used not only to predict postoperative SSIs but also to predict severe infections.
This study further analyzed the value of the NRI in evaluating the prognosis of patients with SSIs. The NRI of patients in the survival group was higher, and the sensitivity and specificity of this indicator in evaluating death were 92.50% and 85.71%, respectively, confirming its utility in evaluating patient prognosis. Insufficient intake of protein and vitamins and a lack of trace elements in patients with primary liver cancer increase the risk of postoperative SSIs and prolong the healing time of surgical incisions. Moreover, the poor nutritional status can exacerbate immune function damage, leading to other related complications, increasing the complexity and severity of the disease, and further increasing the risk of death. Shi et al[16] analyzed the impact of NRI on the prognosis of patients with non-small cell lung cancer and reported the area under the ROC curve of 0.770 for predicting the overall survival. These findings suggest that the NRI can be used to evaluate the prognosis of patients with malignant tumors. In a study by De la Garza Ramos et al[17] involving patients with metastatic spinal disease who underwent tumor surgery, multivariate regression analysis demonstrated the predictive value of the NRI for the 90-day and 12-month mortality, verifying the relationship between nutritional status and mortality of patients with tumors. Karabulut et al[18] used the NRI to evaluate the nutritional status of patients with metastatic gastric adenocarcinoma and reported a significant association between malnutrition and decreased overall survival. Furthermore, severe malnutrition was associated with a shorter median overall survival, verifying the value of this indicator for evaluating patient prognosis.
This study also has some limitations. First, it was a single-center study with a small sample size, which limits the generalizability of the results. Second, this was a retrospective study, and relevant parameters may have been influenced by the patient's condition at that time, potentially introducing bias. Third, only patients with HBV-related primary liver cancer were included, and the predictive and evaluative value of the NRI indicator for SSIs and prognosis of patients with other primary liver cancer types was not verified. Future research should include more patients based on established inclusion and exclusion criteria. Furthermore, prospective, multicenter, randomized controlled studies across different hospitals should be conducted to explore the predictive and evaluative value of the NRI for SSIs and the prognosis of patients with liver cancer of various etiologies.
The NRI is a valuable tool for predicting postoperative SSIs and their severity in patients with HBV-related primary liver cancer. Our study showed that patients with lower NRI levels were at higher risk of developing infections and experiencing worse prognoses. Owing to its high sensitivity and specificity, the NRI offers a reliable and efficient way to assess patients' nutritional status and predict infection risk. However, our conclusions are based on a single-center, small-sample, retrospective study, and further validation through prospective, multicenter, randomized controlled trials is essential.
| 1. | Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 1226] [Article Influence: 613.0] [Reference Citation Analysis (0)] |
| 2. | Wang S, Liu W, Zhan L, He Y, Xu J. A commentary on 'Prediction models of surgical site infection after gastrointestinal surgery: a nationwide prospective cohort study'. Int J Surg. 2024;110:2473-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | Furukawa K, Onda S, Taniai T, Hamura R, Yanagaki M, Tsunematsu M, Haruki K, Yasuda J, Sakamoto T, Gocho T, Ikegami T. Risk Factors and Overcoming Strategies of Surgical Site Infection After Hepatectomy for Colorectal Liver Metastases. Anticancer Res. 2021;41:5651-5656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Li G, He L, Sun H. Nutritional risk index predicts the prognosis of gastric cancer patients with pyloric stenosis who received preoperative parenteral nutrition. Oncol Lett. 2023;26:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Bauer JM, Welling SE, Bettinger B. Can we automate spine fusion surgical site infection data capture? Spine Deform. 2023;11:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | De Simone B, Sartelli M, Coccolini F, Ball CG, Brambillasca P, Chiarugi M, Campanile FC, Nita G, Corbella D, Leppaniemi A, Boschini E, Moore EE, Biffl W, Peitzmann A, Kluger Y, Sugrue M, Fraga G, Di Saverio S, Weber D, Sakakushev B, Chiara O, Abu-Zidan FM, Ten Broek R, Kirkpatrick AW, Wani I, Coimbra R, Baiocchi GL, Kelly MD, Ansaloni L, Catena F. Intraoperative surgical site infection control and prevention: a position paper and future addendum to WSES intra-abdominal infections guidelines. World J Emerg Surg. 2020;15:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Rahmawati S, Setyawati A, Tahir T. The experience of infection prevention and control nurse (IPCN) in conducting post-discharge surveillance (PDS) of surgical site infections (SSI): A qualitative study. Infect Dis Health. 2024;29:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Gupta A, Shin J, Oliver D, Vives M, Lin S. Incidence and risk factors for surgical site infection (SSI) after primary hip hemiarthroplasty: an analysis of the ACS-NSQIP hip fracture procedure targeted database. Arthroplasty. 2023;5:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Gomaa K, Abdelraheim AR, El Gelany S, Khalifa EM, Yousef AM, Hassan H. Incidence, risk factors and management of post cesarean section surgical site infection (SSI) in a tertiary hospital in Egypt: a five year retrospective study. BMC Pregnancy Childbirth. 2021;21:634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Hasegawa T, Takeda D, Tanaka M, Amano R, Saito I, Kakei Y, Kimoto A, Fukumoto T, Akashi M. Effects of preoperative dental examination and oral hygiene instruction on surgical site infection after hepatectomy: a retrospective study. Support Care Cancer. 2021;29:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Ametejani M, Masoudi N, Homapour F, Rezaei S, Moosavi SA, Kafili E, Heidarlou AJ. Association between Pre-Operative 25-Hydroxy Vitamin D Deficiency and Surgical Site Infection after Right Hemicolectomy Surgery. Surg Infect (Larchmt). 2022;23:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | García-Fuente I, Corral-Gudino L, Gabella-Martín M, Olivet-de-la-Fuente VE, Pérez-Nieto J, Miramontes-González P. How to detect non-institutionalized older patients at risk of malnutrition during their hospitalization? Comparison of 8 screening tools for malnutrition or nutritional risk. Rev Clin Esp (Barc). 2024;224:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Theilla M, Rattanachaiwong S, Kagan I, Rigler M, Bendavid I, Singer P. Validation of GLIM malnutrition criteria for diagnosis of malnutrition in ICU patients: An observational study. Clin Nutr. 2021;40:3578-3584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Guo Y, Wang R, Wu P, Zhang W, Mao S, Wu Y, Liu J, Ma W, Zheng Z, Zhang J, Yao X, Liu Y. Preoperative Nutritional Risk Index Predicts Recurrence of Oligometastatic Prostate Cancer in Patients Undergoing Cytoreductive Radical Prostatectomy. Nutr Cancer. 2021;73:1440-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Kim KW, Lee K, Lee JB, Park T, Khang S, Jeong H, Ko CS, Yook JH, Kim BS, Lee IS. Preoperative nutritional risk index and postoperative one-year skeletal muscle loss can predict the prognosis of patients with gastric adenocarcinoma: a registry-based study. BMC Cancer. 2021;21:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Shi Z, Zheng D, Tang X, Du Y. Correlation of immune inflammatory indices and nutritional risk index with prognosis in patients with non-small cell lung cancer. Am J Transl Res. 2023;15:4100-4109. [PubMed] |
| 17. | De la Garza Ramos R, Ryvlin J, Hamad MK, Fourman MS, Gelfand Y, Murthy SG, Shin JH, Yassari R. Predictive value of six nutrition biomarkers in oncological spine surgery: a performance assessment for prediction of mortality and wound infection. J Neurosurg Spine. 2023;39:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Karabulut S, Dogan I, Usul Afsar C, Karabulut M, Ak N, Duran A, Tastekin D. Does nutritional status affect treatment tolerability, chemotherapy response and survival in metastatic gastric cancer patients? Results of a prospective multicenter study in Turkey. J Oncol Pharm Pract. 2022;28:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/