Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.105264
Revised: March 12, 2025
Accepted: April 21, 2025
Published online: July 15, 2025
Processing time: 179 Days and 6.3 Hours
Voltage-gated sodium channels (VGSCs, or Navs) are highly expressed in various tumors and play a critical role in tumor metastasis and invasion.
To identify Nav1.6-associated cancer genes through bioinformatics analysis and experimental validation, with the goal of determining the role of Nav1.6 in co
The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) data were analyzed using weighted correlation network analysis (WGCNA) and Venn analysis to identify Nav1.6-associated genes in CRC. siRNA, real-time PCR, and western blotting were employed to validate the Nav1.6-associated cancer genes and signaling pathways identified in CRC. Cell counting kit-8 and Transwell mi
The analysis of TCGA and GEO datasets, along with WGCNA, identified 575 differentially expressed genes as
These findings suggest that Nav1.6 promotes CRC cell proliferation and invasion which is related to the MAPK signaling pathway.
Core Tip: Voltage-gated sodium channels are prominently expressed in various tumors and play a crucial role in tumor metastasis and invasion. Our research identified 575 differentially expressed genes related to Nav1.6 in colorectal cancer (CRC), particularly enriched in MAPK signaling pathways. Tissue microarray revealed heightened Nav1.6 levels in CRC tissues. The cytoplasmic expression of Nav1.6 increases with T staging. Knockdown of SCN8A in colon tumor cells significantly decreased cell proliferation and invasion, along with downregulation of key proteins in the RAF-MAPK pathway. These findings suggest that Nav1.6 enhances CRC cell proliferation and invasion through modulation of the MAPK signaling pathway.
- Citation: Zhao LM, Hong WY, Xu JG, Lin SQ, Liu MS, Wang LH, Jiang XL, Sang M, Lv YB. Nav1.6 drives colorectal cancer proliferation and invasion through MAPK signaling pathway. World J Gastrointest Oncol 2025; 17(7): 105264
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/105264.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.105264
Colorectal cancer (CRC) is the third most common malignancy globally, with the second highest mortality rate[1]. Its incidence has steadily increased in recent years[1]. In 2023, it is estimated that approximately 153020 individuals will be diagnosed with CRC, resulting in 52550 deaths. This includes 19550 new cases and 3750 deaths among individuals under 50 years of age[1]. CRC metastasis refers to the spread of cancerous cells from the colon or rectum to distant organs. This complex process plays a pivotal role in CRC progression and significantly impacts patient prognosis and treatment outcomes[2]. Metastasis typically involves the detachment of cancer cells from the primary tumor, the invasion of adjacent tissues, and entry into the blood or lymphatic vessels, where they may establish secondary tumors in organs such as the liver, lungs, and peritoneum[2].
CRC is staged according to the tumor-node-metastasis classification, which takes into account the extent of the primary tumor (T), regional lymph node involvement (N), and the presence of distant metastasis (M). The "T" stage refers to the depth of invasion of the primary tumor into the surrounding tissues and organs. It is classified as follows: T1: The tumor invades the submucosa (the second layer of the colon wall). T2: The tumor invades the muscularis propria (the muscle layer). T3: The tumor invades through the muscularis propria into the subserosa or into the non-peritonealized pericolic or perirectal tissues. T4: The tumor invades the serosa (outermost layer of the colon) or adjacent structures, such as nearby organs or tissues[3].
The metastatic potential of CRC is influenced by various factors, including the molecular characteristics of the tumor, the tumor microenvironment, and the host’s immune response. A comprehensive understanding of the mechanisms driving CRC metastasis is essential for developing effective therapeutic strategies, particularly for advanced-stage disease, with the ultimate goal of improving patient survival outcomes[4,5].
Voltage-gated sodium channels (VGSCs or Navs) are large molecular complexes embedded in the cell membrane, composed of an α subunit that forms the ion-conducting pore and one or more associated β subunits[6]. The VGSC α subunit family includes nine isoforms-Nav1.1 through Nav1.9-encoded by the SCN1A-SCN11A genes[6]. VGSCs are predominantly expressed in excitable cells, such as neurons and cardiac myocytes, where they are essential for the initiation of action potentials and the transmission of electrical signals[7]. Specifically, Nav1.1, Nav1.2, and Nav1.3 are primarily expressed in the central nervous system; Nav1.4 is found in skeletal muscle; Nav1.5 in cardiac muscle; and Nav1.6, Nav1.7, Nav1.8, and Nav1.9 in the peripheral nervous system, including the enteric and sensory systems[6]. Recent studies have demonstrated that VGSCs are expressed in various cancer cells, including breast, cervical, colorectal, and prostate cancers, as well as in melanoma, neuroblastoma, and non-small cell lung cancer[8]. The aberrant overexpression of VGSC α subunits in cancer cells has been implicated in promoting tumor cell migration and invasion[9]. Notably, Nav1.1, Nav1.2, Nav1.3, Nav1.4, and Nav1.9 show heightened expression in ovarian cancer, non-small cell lung cancer, and prostate cancer[8]. The Nav1.7 α subunit has also been shown to facilitate the progression of gastric cancer through the MACC1-mediated upregulation of NHE1[10]. Furthermore, the expression of Nav1.5 is closely associated with poor prognosis in several cancers, including breast cancer[11], non-small cell lung cancer[12], ovarian cancer[13], and prostate cancer[14].
Nav1.6, encoded by the SCN8A gene, is expressed in a variety of solid tumors, including breast cancer, cervical cancer, non-small cell lung cancer, ovarian cancer, and prostate cancer[8]. The elevated expression of Nav1.6 has been shown to enhance the metastasis and invasiveness of cervical cancer cells, and the specific inhibition of Nav1.6 currents significantly reduces their invasive potential[15]. In our previous study, we demonstrated for the first time that Nav1.6 is aberrantly overexpressed in more than 50% of CRC tumor tissues[16]. Furthermore, the high expression of Nav1.6 correlated positively with vascular invasion and lymph node metastasis in patients with CRC[16]. These findings suggest that Nav1.6 may contribute to CRC metastasis; however, the underlying mechanisms remain to be fully elucidated. A deeper understanding of the role of Nav1.6 in CRC lymph node metastasis and the molecular pathways through which elevated Nav1.6 expression promotes this process is essential for advancing our comprehension of CRC metastasis.
With advancements in sequencing technologies, large-scale gene co-expression analysis has become a cornerstone in understanding disease mechanisms and has become increasingly pivotal in life sciences research. This study aimed to investigate how Nav1.6 potentially promotes lymph node metastasis by leveraging data from The Cancer Genome Atlas (TCGA) database. The analysis revealed a positive correlation between elevated Nav1.6 expression and the CRC T stage. This finding was further validated through tumor tissue microarray (TMA) analysis of CRC patients. Using weighted correlation network analysis (WGCNA) of TCGA data, we identified several differentially expressed genes (DEGs) associated with Nav1.6, including members of the MAPK pathway. Further examination of CRC single-cell sequencing data from the Gene Expression Omnibus (GEO) database identified epithelial cells as the predominant cell type in tumor tissues, where Nav1.6, MAP3K10, and other tumor-related genes were prominently co-expressed. The siRNA-mediated knockdown of Nav1.6 in colorectal cells confirmed these findings. Collectively, these results provide novel insight into the molecular mechanisms by which Nav1.6 may contribute to colorectal carcinogenesis. They not only underscore the role of VGSCs in tumor metastasis but also open new avenues for exploring the molecular basis of CRC metastasis.
The microarray datasets utilized in this study were obtained from the NCBI GEO (http://www.ncbi.nlm.nih.gov/geo/) and the TCGA database. Human CRC single-cell sequencing data (GSE201348) were selected for analysis. For each dataset, the differential expression analysis of tumor tissue versus normal tissue was performed using the DESeq2 program to generate a list of DEGs. A threshold of P < 0.05 and a fold change of > 2 were applied. Several web-based tools were employed to identify SCN8A-related gene signatures, including Cluster Heatmap, Principal Component Analysis, GO/KEGG analysis, Venn Diagram Generator, Co-expression Correlation Analysis, and Protein-Protein Interaction Network (https://cloud.oebiotech.com), to identify Nav1.6-related DEGs.
Single-cell transcriptomic data from GSE201348 were analyzed using the Seurat package in R (version 4.2.2). Batch effects across samples were mitigated using the batch-balanced K-nearest neighbors (BBKNN) method from the BBKNN package. Non-linear dimensionality reduction was performed using uniform manifold approximation and projection. Cell types were classified through unsupervised clustering based on the expression of canonical marker genes. The expression levels of specific genes across cell clusters were visualized with bubble plots, where the size of each bubble represented the proportion of cells expressing the gene, and the color indicated the scaled average expression level.
WGCNA was conducted using the WGCNA package in R[17,18]. An unsigned co-expression network was constructed based on an adjacency matrix, and genes with highly similar co-expression patterns were grouped using average linkage hierarchical clustering of the topological overlap. The Dynamic Hybrid Tree Cut algorithm was applied to segment the hierarchical clustering tree, with modules defined as the branches resulting from tree cutting. Moderately large and distinct modules were identified by setting the minimum module size to 30 genes and applying a merging height threshold of 0.25. The resulting modules were assigned random color labels. Each module was characterized by its first principal component, termed the module eigengene, which represents the scaled (standardized) expression profile of the module. Correlations between module eigengenes and clinical phenotypes, such as estimated glomerular filtration rate and interstitial fibrosis, were then analyzed.
The study enrolled patients diagnosed with stage II-III colon cancer (American Joint Committee on Cancer 8th edition), with tumor lesions greater than 2 cm in size, who had not received radiotherapy or chemotherapy prior to laparoscopic surgery. A total of 90 patients aged 34-81 years participated, including 61 males and 29 females. Among these, 19 were classified as having stage II, 69 as having stage III, and two as having stage I. Lymph node metastasis was present in 55 cases and absent in 25 cases, while perineural invasion was observed in 22 cases and absent in 68 cases. Following surgery, specimens were promptly collected for in vitro analysis. A 0.5 cm × 0.5 cm segment of CRC tissue, isolated from the luminal surface of the intestine, was used for further examination. A 0.5 cm × 0.5 cm section of intestinal wall tissue (primarily the mucosa and submucosa), located 5 cm from the tumor margin, served as the normal tissue control. All clinically obtained samples were stored at -86 °C within 2 minutes of isolation. The remaining tumor tissue was preserved in 4% formalin for subsequent hematoxylin and eosin (H&E) staining.
A TMA was constructed using 1 mm2 tissue cores from 90 patients, utilizing a TMArrayer (Pathology Devices). Areas with high tumor content were selected for inclusion in the TMA block based on H&E-stained sections from the entire tissue block, ensuring the presence of CRC cells in each sample. TMA sections, 4 µm thick, were cut using a microtome (Leica RM2125 RTS), mounted on SuperFrost™ Plus slides (Thermo Fisher Scientific), and air-dried overnight at room temperature. Nav1.6 staining was performed following previously established protocols[16]. After staining, the slides were scanned using a Vectra 3.0 scanner, and images were visualized with Phenochart v.1.0.8 (AKOYA Bioscience). Optimal scanning protocols were determined by adjusting the exposure times for each L cube at × 10 and × 20 magnifications. Whole-slide scans were conducted using a × 10 objective lens, while high-resolution images of individual core tissue regions were captured with a × 20 objective lens. TMA core images were analyzed using HALO v2.2 software (Indica Labs). The random forest tissue classifier was trained on multiple tissue areas to identify tissue and slide regions. Positivity for each marker was assessed based on staining intensity, ensuring a minimum 10-fold signal-to-noise ratio, staining patterns that are consistent with the manufacturer’s specifications and previous literature, and comparable staining with control tissues. The analysis settings specific to each staining category were optimized by adjusting the thresholds, and all samples were analyzed.
Cut the paraffin embedded tissue sample into 3-5 μm thickness using a slicer and adhere it to a glass slide. According to the manufacturer's instructions, use TSA fluorescence kit (Melady)® Biosciences is used for target protein labeling. These slices underwent a one-hour baking process at 60 °C, followed by dewaxing in xylene and graded ethanol series (100%, 95%, 80%, and 70%), and rinsing in ddH2O. Use a microwave oven to perform thermally induced antigen repair in EDTA buffer at pH 9.0. Quench endogenous peroxidase activity with 3% hydrogen peroxide, then seal the slices with a blocking solution. After 20 minutes, shake off the blocking solution and incubate the slices with primary antibody overnight at 4 °C. The slices were incubated with HRP secondary antibodies suitable for the species at room temperature for 20 minutes, washed off with PBS, and then incubated with appropriate fluorescent dyes for TSA staining. The first antibodies we use are CD31 (Abcam, ab231436, 1:50), KI67 (Abcam, ab92742, 1:500), DCX (Santa Cruz Biotechnology Company, SC-271390, 1:100). After completing TSA staining of the first antibody, repeat the process for the second antibody. After staining all targets, wash the slices with PBS and counterstain with DAPI. Finally, install the slice using a suitable mounting medium, perform full slide scanning imaging using a multi-channel fluorescence scanner (3DHISTECH, Pannoramic MIDI), and view the image using SlideViewer software.
All cell lines were obtained from the Shanghai Institute of Cell Biology. CaCo2 cells were cultured in MEM supplemented with 10% FBS and 1% penicillin-streptomycin; DLD-1 cells were cultured in 1640 supplemented with 10% FBS and 1% penicillin-streptomycin; and HT-29 cells were cultured in McCoy’s 5A medium supplemented with 10% FBS and 1% penicillin-streptomycin. All cell culture reagents were purchased from Gibco (Thermo Fisher Scientific). Cells were transfected with the si-hNav1.6 plasmid (GenePharma, China) using Lipofectamine 3000 (Thermo Fisher Scientific) diluted in Opti-MEM (Gibco) to knock down the expression of Nav1.6. The plasmid and transfection reagent were diluted separately, incubated for 5 minutes, mixed, and allowed to stand for 20 minutes at room temperature. The transfection mixture was then added to DLD-1 cells seeded in 6-well plates and incubated for 5 hours. Afterward, the medium was replaced with a complete growth medium. Cells were collected for further analysis after 24 hours.
Total RNA was extracted using Trizol (Vazyme) following the manufacturer’s instructions. Next, 1 μg of RNA was reverse-transcribed into complementary DNA (cDNA) using HiScript III RT SuperMix for quantitative PCR (qPCR) (Vazyme). qPCR was performed on an ABI 7500 System (Invitrogen Life Technologies) using human-specific primers (Table 1). The PCR cycling conditions were as follows: Initial denaturation at 95 °C for 30 seconds, followed by 40 cycles of denaturation at 95 °C for 5 seconds, and annealing/extension at 60 °C for 30 seconds. A melting curve analysis was conducted using SYBR Green (Vazyme) to confirm the specificity of the PCR products. The samples were analyzed in duplicate to ensure the reliability of the qRT-PCR data, and the results were based on the average of the duplicate values for each sample. Relative gene expression was calculated using the 2-ΔΔCt method, using GAPDH as the internal control and untreated samples from control mice as the calibrator. PCR data were normalized to the mean values of the control group to minimize variability. The primers used are listed in Table 1.
| Primer name | Sequences (5’-3’) |
| hGAPDH-F | GTCTCCTCTGACTTCAACAGCG |
| hGAPDH-R | ACCACCCTGTTGCTGTAGCCAA |
| hNav1.6-F | GGATTGAGACCATGTGGGACTG |
| hNav1.6-R | ATCTGTGGCAGCCAGGTTGTCT |
After transfection, cells were harvested by centrifugation, washed with ice-cold 1 × PBS, and lysed using radioimmunoprecipitation assay (RIPA) buffer (Beyotime). The lysed proteins in the supernatant were collected following centrifugation and mixed with 5 × SDS loading buffer (Beyotime). The mixture was then boiled and loaded onto SDS-polyacrylamide gels. Protein concentrations were quantified using a BCA assay (Beyotime) to ensure equal loading. Proteins were separated by electrophoresis, transferred to PVDF membranes, and blocked with 10% BSA. The membranes were incubated overnight at 4 °C with primary antibodies against Nav1.6 (Abcam ab65166), MAP3K10 (Bioworld BS61034), phospho-c-Raf (Cell Signaling Technology 9427), ERK1/2 (Abcam ab184699), MEK1/2 (Abcam ab178876), Raf1 (Abcam ab137435), MEK1/2 (phospho-S221) (Abcam ab278564), GAPDH (Abcam ab8245), and β-actin (AiFang Biological AF10678). After washing, the membranes were incubated with HRP-conjugated secondary antibodies and subjected to chemiluminescent detection. Image analysis for quantification was performed using Image Lab software, with back
Cell invasion ability was assessed using Transwell assays with 24-well chambers containing 8 μm polycarbonate membranes (Millipore, MA, United States). The filters were pre-coated with 500 ng/mL Matrigel solution (BD Biosciences, United States) for invasion assays and incubated for 4 hours at 37 °C. The lower chamber was placed in a 24-well plate containing 500 μL of medium with 10% FBS, and 1 × 105 cells in 200 μL of serum-free medium were seeded into the upper chamber. After 18 hours of incubation at 37 °C, non-invading cells on the upper membrane surface were gently removed by scraping. The invading cells on the membrane were fixed with methanol, stained with 0.5% crystal violet (Beyotime), and photographed. Invasive cells were counted in fewer than five random 200 × microscopic fields per well using a Nikon Eclipse Ti inverted research microscope. A cell migration assay was conducted in parallel under the same conditions, except that the chambers were not pre-coated with Matrigel.
Cells (1 × 105) were seeded in 6-well plates. After 16 hours, the complete medium was replaced with a low-serum medium (2%). A uniform wound was created in each well by scratching the surface with a 10 μL pipette tip once the cells reached 90% confluence. The cells were gently washed twice with PBS to remove any detached cells, and a serum-free medium was added. Multiple reference marks were made at the center of the denuded surface to ensure consistency in the wound area. Scratch wounds were photographed using an inverted microscope at 0, 12, and 24 hours. Image analysis was performed using AxioVision Rel. 4.8 software to assess the migratory capacity of the cancer cells. The experiment was repeated three times to ensure reproducibility.
DLD-1 cells (5 × 103 cells/well) were seeded in a 96-well plate and incubated with complete medium containing the specified concentration of CNB at 37 °C for 12, 24, and 48 hours. Cell viability was assessed using the cell counting kit-8 (CCK-8) assay (TargetMOL, C0005), following the manufacturer's instructions. The optical density at 450 nm was measured using a microplate reader (Thermo Fisher).
The Student's t-test, one-way ANOVA, and two-way ANOVA without correction were used to assess statistical significance between groups (aP < 0.05, bP < 0.01, cP < 0.001, and dP < 0.0001). Data are presented as mean ± SEM. All analyses were conducted using GraphPad Prism 10. The statistical review of the study was performed by a biomedical statistician.
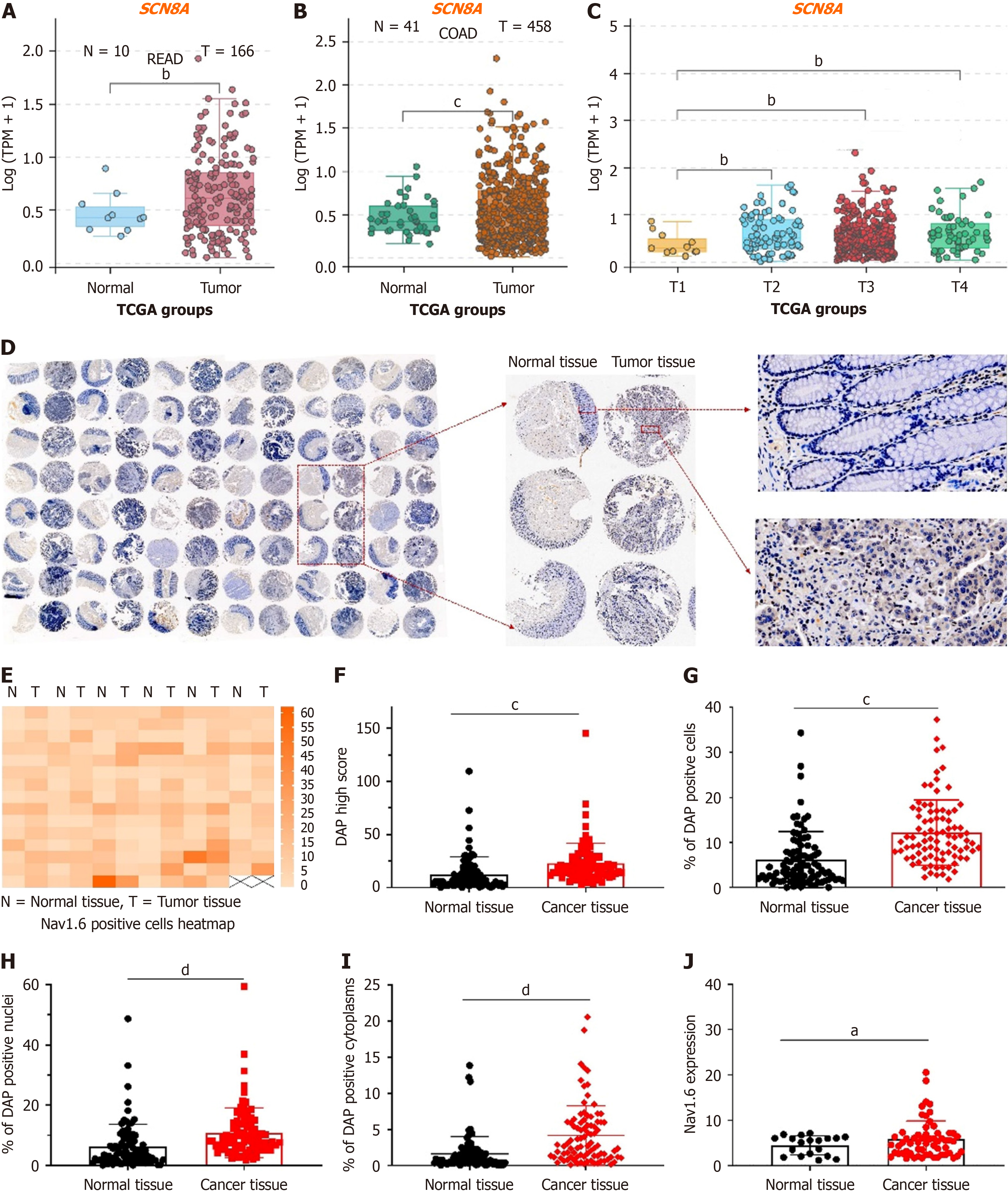
In our previous study, we analyzed the mRNA expression of VGSCs in CRC tissue using collected CRC samples and found that SCN8A was significantly overexpressed in both tumor tissues and metastatic lymph nodes, with a strong correlation with the lymph node stage[18]. We extracted transcriptomic sequencing data for rectal adenocarcinoma (READ) and colon adenocarcinoma (COAD) from the TCGA database and analyzed SCN8A expression to further explore the relationship between Nav1.6 expression and tumor metastasis in CRC. Our results showed a significant upregulation of SCN8A in both READ (Figure 1A) and COAD tumor tissues (Figure 1B). Additionally, SCN8A expression increased with advancing T stages (Figure 1C), further supporting our earlier findings that the aberrant overexpression of Nav1.6 may be linked to CRC metastasis.
We performed TMA (immunohistochemical staining) analysis of surgical resection samples from 90 CRC patients to validate these findings, which included adjacent normal tissues and tumor tissues (2 T1, 19 T2, and 69 T3). CRC typically progresses through several stages, reflecting the extent of tumor growth and spread within the colon or rectum as well as to other parts of the body. This study focused on stages T1, T2, and T3 of stage II. The results showed significantly elevated Nav1.6 expression in tumor tissues (Figure 1D). Subsequent analysis using HALO TMA analysis software included heatmap generation, H-SCORE analysis, and quantification of the proportion of positive cells, as well as positive nuclear and cytoplasmic staining. These analyses revealed a notably higher proportion of Nav1.6-positive cells in tumor tissues compared to normal colorectal tissues (Figure 1D). Importantly, Nav1.6 expression was significantly elevated in both the cytoplasm and nucleus of CRC tissues (Figure 1E-I), suggesting not only overexpression but also the aberrant subcellular localization of Nav1.6. Further analysis showed a positive association between cytoplasmic Nav1.6 expression and local T staging (Figure 1J), confirming the results from the TCGA database analysis and highlighting the link between cytoplasmic Nav1.6 expression and local staging in CRC.
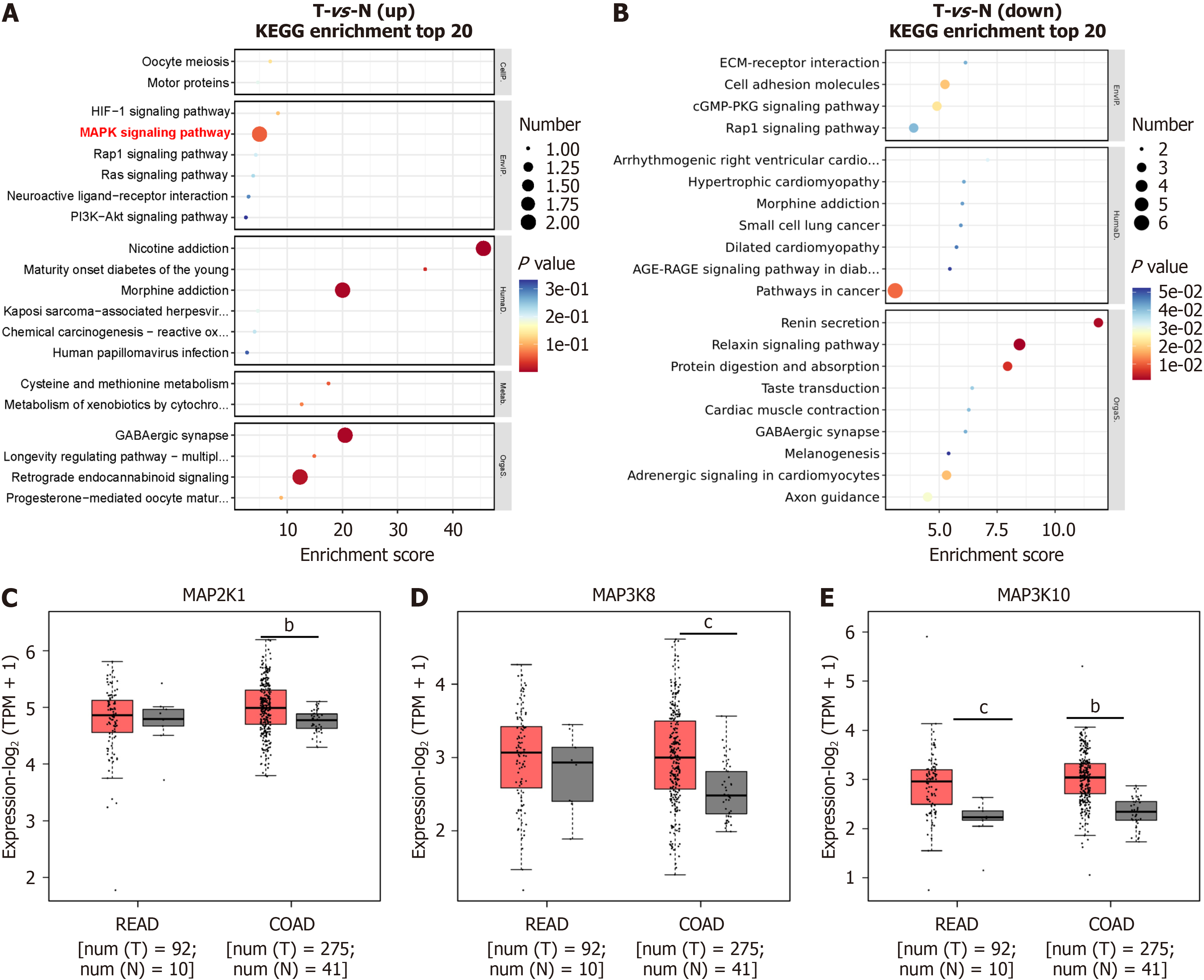
We performed WGCNA on DEGs (fold change ≥ 1.5) derived from the RNA-seq data of CRC tumor and normal samples in TCGA to identify Nav1.6-related tumor genes in CRC. A total of 575 DEGs, comprising 230 upregulated genes and 345 downregulated genes, were selected from the intersection of SCN8A-correlated genes in COAD, SCN8A-correlated genes in READ, and DEGs from tumors versus normal comparisons. These genes were used to construct a co-expression network (Figure 2A and B). Genes with low expression variability (standard deviation ≤ 0.5) were excluded, resulting in 293 genes for further analysis. A soft-thresholding power was applied to the network topology to assess scale inde
We performed KEGG enrichment analysis on the 167 T stage-related genes identified through WGCNA to further identify Nav1.6-related functional genes. The bubble chart from the KEGG analysis revealed that the upregulated genes were predominantly involved in MAPK signaling, nicotine addiction, and morphine addiction (Figure 3A and B). In contrast, the downregulated genes were mainly associated with pathways in cancer, renin secretion, and protein di
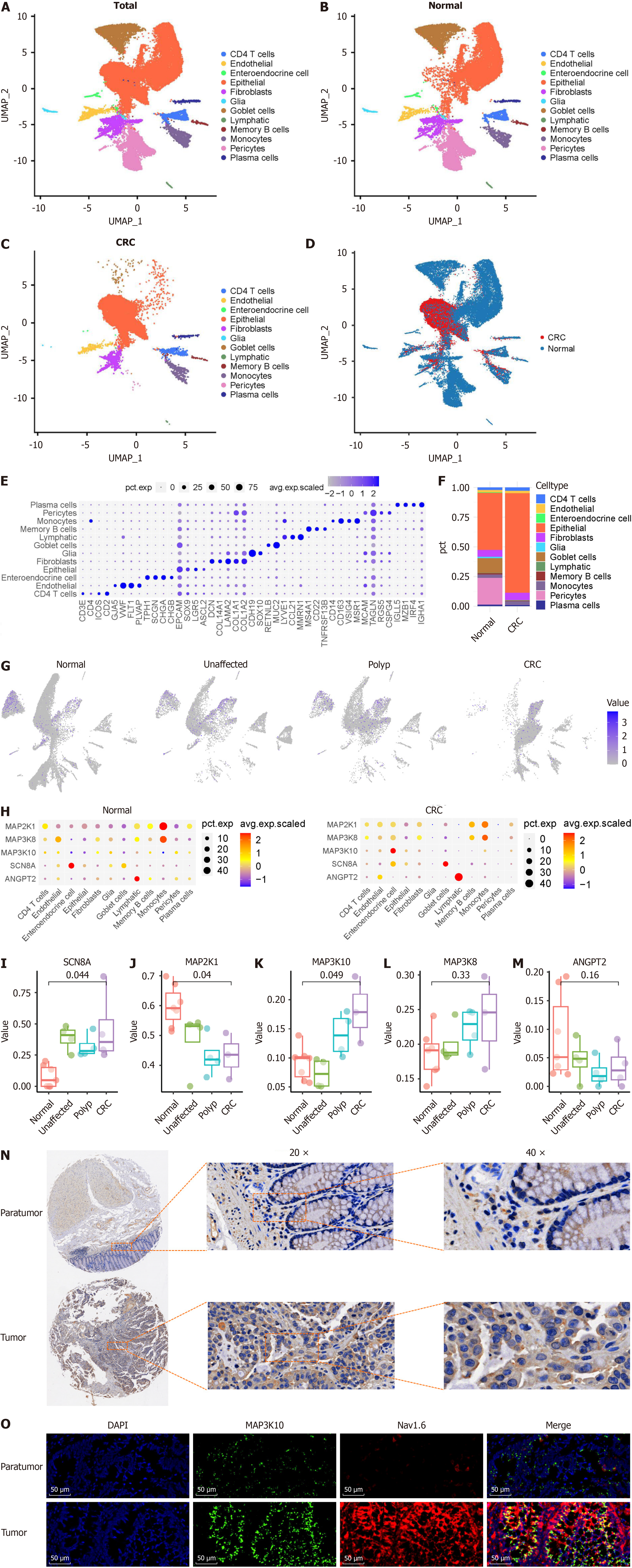
We analyzed CRC single-cell sequencing data to assess the expression and distribution of SCN8A and SCN8A-related functional genes across different cell types during CRC progression and predict the potential molecular mechanisms underlying SCN8A involvement in CRC. The analysis was performed using the GEO database GSE201348, which in
We further examined the expression of SCN8A and functional, T-stage-related genes across different cell types. Figure 4G shows that SCN8A was predominantly expressed in goblet and epithelial cells in healthy colon tissue. However, as CRC progressed, goblet cells gradually disappeared, and the proportion of epithelial cells increased, leading to SCN8A being predominantly expressed in epithelial cells in CRC. In contrast, SCN8A-related MAPK genes were widely expressed across various cell types, with genes such as MAP2K1 and MAP3K8 showing higher average expression levels and a greater proportion of positive monocytes (Figure 4H).
Next, we analyzed the difference in MAPK gene expression most strongly correlated with SCN8A expression in epithelial cells. As shown in Figure 4I-M, SCN8A and MAP3K10 were significantly upregulated as CRC progressed, whereas MAP2K1 and ANGPT2 were downregulated. The decreased expression of these genes, which are positively co-expressed with SCN8A at the tissue level, suggests that changes in gene expression within CRC tissues may arise from alterations in the expression and distribution of these genes across various cell types (Figure 4I-M). Among the selected genes, only MAP3K10 showed a trend toward upregulated expression during CRC progression, similar to that of Nav1.6. Therefore, we further investigated the expression of MAP3K10 in CRC using TMA analysis. The results indicated that MAP3K10 expression was significantly higher in CRC tissues compared to that in adjacent non-tumor tissues (Figure 4N). In addition, immunofluorescence showed that MAP3K10 had significant co-localization with Nav1.6 in tumor which suggested that MAP3K10 could interact with Nav1.6 in CRC tissues (Figure 4O).
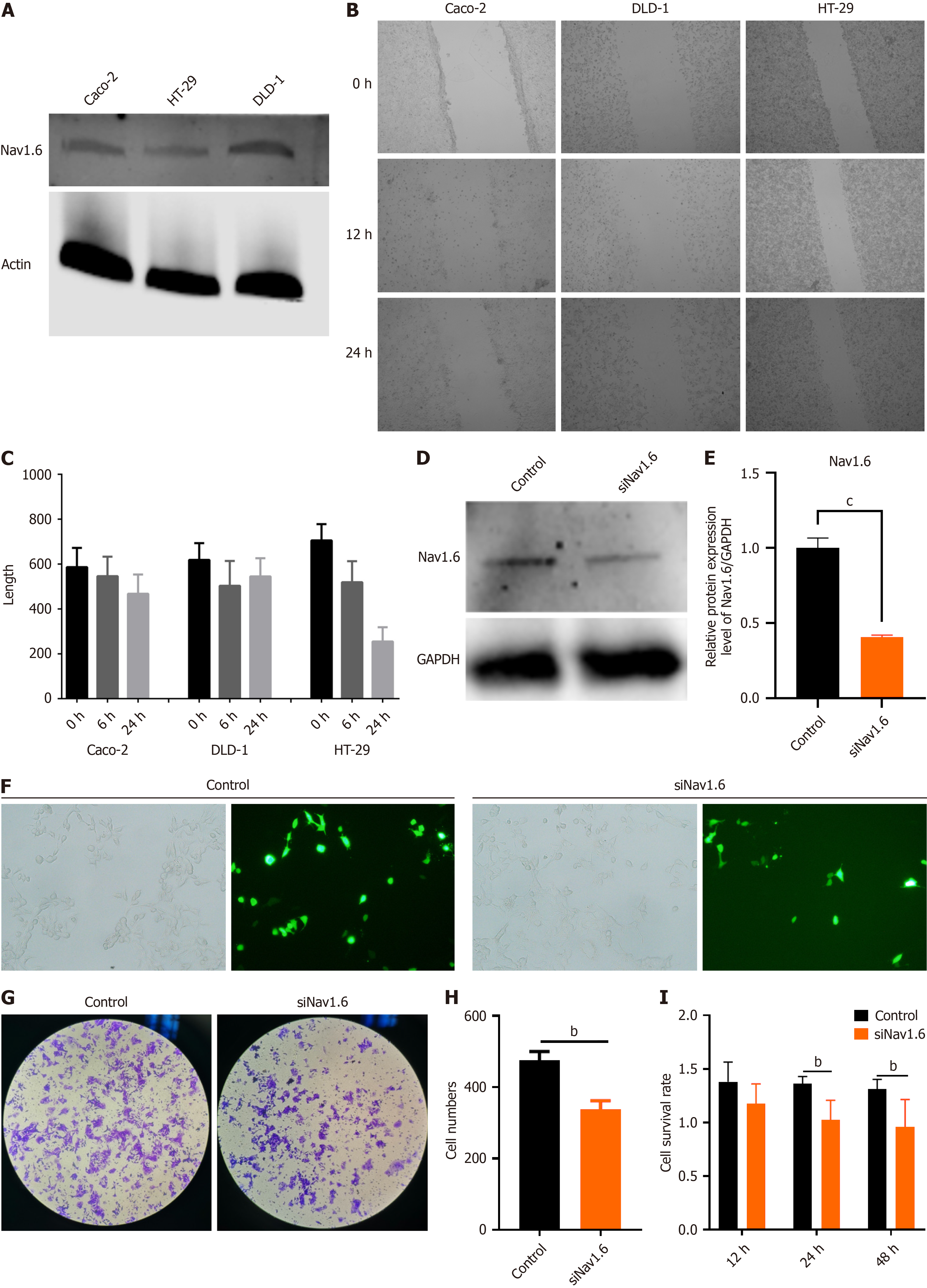
We established an SCN8A knockdown model using a colon cancer cell line to validate the correlation between SCN8A expression and MAPK signaling-related genes identified in TCGA and GEO databases and elucidate the molecular role of Nav1.6 in CRC metastasis. Initially, Nav1.6 expression was assessed in CaCo2, DLD-1, and HT-29 CRC cell lines. Western blot analysis revealed that Nav1.6 expression was highest in DLD-1 cells (Figure 5A). However, the scratch assay showed that the expression of Nav1.6 in different CRC cell lines did not significantly correlate with the wound repair ability of tumor cells (Figure 5B and C). Based on these results, DLD-1 cells were selected for the Nav1.6 knockdown experiments, where siRNA-mediated interference reduced Nav1.6 expression by approximately 50% (Figure 5D and E). Fluorescence photos of cells after transfection showed that the transfection efficiency of SiNav1.6 was around 50% (Figure 5F). Subsequently, the Transwell migration assays demonstrated a significant decrease in cell migration 24 h after SCN8A knockdown in DLD-1 cells (Figure 5G and H). CCK-8 assays were performed to assess the effect of Nav1.6 on cell viability, which showed that the viability of DLD-1 cells significantly decreased 24 and 48 hours post-SCN8A knockdown (Figure 5I). These findings suggest that Nav1.6 may be involved in the proliferation and metastasis of CRC cells.
We performed RNA-sequencing (RNA-seq) analysis on DLD-1 cells before and after SCN8A knockdown to further investigate whether Nav1.6 contributes to the proliferation and invasion of CRC cells by regulating the MAPK signaling pathway. As shown in the volcano plot (Figure 6A), SCN8A knockdown resulted in the downregulation of 77 genes and the upregulation of 71 genes (|log2 fold change| > 1, P < 0.05). Among the top 20 DEGs, MAP3K10 and RAF1 were significantly downregulated after SCN8A knockdown, which corroborates the findings from TCGA data analysis (Figure 6B). This suggests that the MAPK signaling pathway may play a role in regulating CRC cell proliferation and invasion through Nav1.6. Given that MAP3K10 is associated with both the ERK signaling and TGF-β pathways, we analyzed alterations in the MAPK pathway before and after Nav1.6 knockdown. Western blot analysis revealed a marked decrease in the expression of MAP3K10, as well as in the ratio of p-ERK1/2 to total ERK1/2 and p-c-Raf to total c-Raf, in DLD-1 cells following Nav1.6 knockdown compared to the control group (Figure 6C-F). Additionally, the levels of MEK1/2 were reduced in DLD-1 cells after siNav1.6 treatment (Figure 6G). These findings suggest that Nav1.6 plays a role in CRC metastasis by regulating the MAPK signaling pathway.
CRC is the leading cause of cancer-related morbidity and mortality worldwide. Understanding the molecular me
We first confirmed the elevated expression of SCN8A in CRC through transcriptomic data analysis of TCGA data. Both READ and COAD samples exhibited significantly higher levels of SCN8A expression in tumor tissues compared to that in normal tissues, consistent with our previous findings in CRC and metastatic lymph nodes. We observed that SCN8A expression increased with advancing CRC T stages, a result that was further validated by TMA analysis in a cohort of 90 CRC patients. The positive correlation between SCN8A expression and local T stage strongly suggests that Nav1.6 is involved in tumor progression, particularly in the early stages of metastasis, a key determinant of CRC prognosis.
The aberrant subcellular localization of Nav1.6, with significant elevation in both the cytoplasm and nucleus of CRC tissues, adds a novel aspect to its role in CRC. The cytoplasmic expression of Nav1.6 was correlated with local T staging, providing further evidence that it may contribute to CRC progression[16]. The shift in localization from normal colon tissue (where Nav1.6 is primarily expressed in goblet and epithelial cells) to CRC tissue (where it is predominantly expressed in epithelial cells) suggests that Nav1.6 may play an evolving role in tumorigenesis as CRC progresses.
We conducted WGCNA and identified several SCN8A-associated DEGs in CRC tissues to further explore the molecular pathways involved. These genes were clustered into distinct modules, with one module positively correlated with advancing T stages and another negatively correlated. KEGG pathway analysis of these DEGs revealed that the upre
Single-cell RNA-seq data further supported this hypothesis. In healthy colon tissue, SCN8A was mainly expressed in goblet and epithelial cells. However, as CRC progressed, the proportion of goblet cells decreased, and SCN8A expression became more localized in epithelial cells, reinforcing the idea that Nav1.6 is primarily involved in epithelial cell proliferation during CRC progression. Moreover, MAPK-related genes such as MAP3K10 were expressed at higher levels in epithelial cells, highlighting the potential link between SCN8A and MAPK signaling in CRC.
Functional experiments in CRC cell lines, specifically DLD-1 cells, confirmed the role of Nav1.6 in regulating CRC cell migration and survival. SCN8A knockdown resulted in a significant reduction in cell migration and viability, which was accompanied by the downregulation of MAPK signaling components, including MAP3K10, c-RAF, and ERK1/2. These findings suggest that Nav1.6 may facilitate CRC progression by modulating the MAPK pathway, with potential implications for therapeutic targeting.
While these findings strongly suggest a role for Nav1.6 in CRC metastasis, some limitations of our study should be acknowledged. First, the transfection efficiency in our knockdown experiments was not optimal, with around 50% knockdown efficiency. This could have impacted the strength of the observed phenotypic effects, and further op
Our study provides compelling evidence that Nav1.6 is overexpressed in CRC tissues and is positively correlated with tumor progression. We propose that Nav1.6 contributes to CRC metastasis, at least in part, by regulating the MAPK signaling pathway. Given its critical role in tumor progression, Nav1.6 could serve as a potential biomarker and therapeutic target for CRC. However, additional studies are necessary to fully elucidate its functional role and therapeutic potential.
We express our deepest gratitude to all of the doctors and nurses at the Department of Gastrointestinal Surgery at Quzhou People's Hospital for their kind support.
| 1. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1846] [Reference Citation Analysis (5)] |
| 2. | Cañellas-Socias A, Sancho E, Batlle E. Mechanisms of metastatic colorectal cancer. Nat Rev Gastroenterol Hepatol. 2024;21:609-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 125] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 3. | Lea D, Håland S, Hagland HR, Søreide K. Accuracy of TNM staging in colorectal cancer: a review of current culprits, the modern role of morphology and stepping-stones for improvements in the molecular era. Scand J Gastroenterol. 2014;49:1153-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Abedizadeh R, Majidi F, Khorasani HR, Abedi H, Sabour D. Colorectal cancer: a comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 2024;43:729-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 133] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 5. | Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2020;22:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 6. | de Lera Ruiz M, Kraus RL. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J Med Chem. 2015;58:7093-7118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 7. | Goodwin G, McMahon SB. The physiological function of different voltage-gated sodium channels in pain. Nat Rev Neurosci. 2021;22:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 8. | Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels (Austin). 2012;6:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Bian Y, Tuo J, He L, Li W, Li S, Chu H, Zhao Y. Voltage-gated sodium channels in cancer and their specific inhibitors. Pathol Res Pract. 2023;251:154909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Xia J, Huang N, Huang H, Sun L, Dong S, Su J, Zhang J, Wang L, Lin L, Shi M, Bin J, Liao Y, Li N, Liao W. Voltage-gated sodium channel Nav 1.7 promotes gastric cancer progression through MACC1-mediated upregulation of NHE1. Int J Cancer. 2016;139:2553-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, Brackenbury WJ, Theodorou D, Koyutürk M, Kaya H, Battaloglu E, De Bella MT, Slade MJ, Tolhurst R, Palmieri C, Jiang J, Latchman DS, Coombes RC, Djamgoz MB. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381-5389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 380] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 12. | Roger S, Rollin J, Barascu A, Besson P, Raynal PI, Iochmann S, Lei M, Bougnoux P, Gruel Y, Le Guennec JY. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol. 2007;39:774-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Liu J, Liu D, Liu JJ, Zhao C, Yao S, Hong L. [Corrigendum] Blocking the Nav1.5 channel using eicosapentaenoic acid reduces migration and proliferation of ovarian cancer cells. Int J Oncol. 2020;57:1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Shan B, Dong M, Tang H, Wang N, Zhang J, Yan C, Jiao X, Zhang H, Wang C. Voltage-gated sodium channels were differentially expressed in human normal prostate, benign prostatic hyperplasia and prostate cancer cells. Oncol Lett. 2014;8:345-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Lopez-Charcas O, Espinosa AM, Alfaro A, Herrera-Carrillo Z, Ramirez-Cordero BE, Cortes-Reynosa P, Perez Salazar E, Berumen J, Gomora JC. The invasiveness of human cervical cancer associated to the function of Na(V)1.6 channels is mediated by MMP-2 activity. Sci Rep. 2018;8:12995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Lin S, Lv Y, Xu J, Mao X, Chen Z, Lu W. Over-expression of Nav1.6 channels is associated with lymph node metastases in colorectal cancer. World J Surg Oncol. 2019;17:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10254] [Cited by in RCA: 18058] [Article Influence: 1003.2] [Reference Citation Analysis (0)] |
| 18. | Farhadian M, Rafat SA, Panahi B, Mayack C. Weighted gene co-expression network analysis identifies modules and functionally enriched pathways in the lactation process. Sci Rep. 2021;11:2367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi J, John A, Lim YC, Kibria KMK, Mohiuddin AKM, Ming LC, Goh KW, Hadi MA. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers (Basel). 2022;14:1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 549] [Article Influence: 137.3] [Reference Citation Analysis (1)] |
| 20. | Koveitypour Z, Panahi F, Vakilian M, Peymani M, Seyed Forootan F, Nasr Esfahani MH, Ghaedi K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019;9:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/