TO THE EDITOR
Pancreatic cancer is notorious for its aggressive nature, poor prognosis, and limited treatment options. Despite advances in medical technology, the 5-year survival rate remains dismally low due to rapid disease progression, late diagnosis, and resistance to conventional therapies[1,2]. A key feature of pancreatic cancer pathology is the tumor microenvironment (TME), primarily consisting of a dense stromal network, which includes cancer-associated fibroblasts (CAFs), endothelial cells, and pancreatic stellate cells, as well as acellular components such as collagen and extracellular matrix proteins[3,4]. Among these, CAFs are particularly important due to their dynamic interactions with pancreatic cancer cells, which facilitate tumor growth, metastasis, and resistance to chemotherapy[5]. Recent research underscores the complex role of cellular senescence in cancer. While senescence can inhibit unchecked cell growth, senescent cells, particularly CAFs, can acquire a senescence-associated secretory phenotype (SASP). This phenotype is characterized by the release of inflammatory cytokines, chemokines, and proteases, which can paradoxically facilitate tumor progression. Specifically, senescent CAFs create a pro-tumorigenic environment that enhances cancer cell proliferation, invasion, and metastatic potential[6-8]. Therefore, targeting senescent CAFs presents a novel therapeutic avenue for mitigating the aggressiveness of pancreatic cancer. Resveratrol, a natural polyphenolic compound found in grapes and other plants, has garnered attention for its diverse biological activities[9]. The study by Jiang et al[10] investigates resveratrol’s promising role in pancreatic cancer treatment by targeting senescent CAFs. Their research reveals that resveratrol not only reduces the number of senescent CAFs but also diminishes the expression of SASP factors, thereby impeding the pro-tumorigenic activities of these cells. This editorial discusses the implications of Jiang et al’s findings, exploring how resveratrol’s multifaceted effects on the TME, combined with its direct anti-cancer actions, position it as a potential adjunctive therapy in the fight against pancreatic cancer[10]. Through a comprehensive analysis of Jiang et al’s methodologies and key findings, we can appreciate the innovative approaches being explored to target the TME and improve therapeutic outcomes for pancreatic cancer patients[10].
RESVERATROL’S ROLE IN THE PANCREATIC TME
The pancreatic cancer TME is notably dense, with stromal components accounting for up to 90% of the tumor mass. This stroma includes cellular elements such as CAFs, endothelial cells, and pancreatic stellate cells, as well as acellular components such as collagen and extracellular matrix proteins[3,11]. Among these, CAFs are particularly important as they interact dynamically with pancreatic cancer cells, thereby promoting tumorigenesis, metastasis, and chemoresistance[12,13]. Cellular senescence, a state of permanent cell cycle arrest, typically serves as an obstacle to cancer development[14]. Nevertheless, senescent cells can develop a SASP, which paradoxically aids tumor progression by releasing pro-inflammatory cytokines, chemokines, and proteases[15]. Senescent CAFs have been shown to foster a pro-tumorigenic environment, enhancing cancer cell proliferation, invasion, and metastatic potential[16,17]. Resveratrol, a polyphenol found in grapes and other plants, is known for its anti-inflammatory, antioxidant, cardioprotective, and neuroprotective properties[18-20]. Recently, its anti-cancer properties have been recognized, with studies demonstrating its ability to induce growth arrest, apoptosis, and autophagy in various cancer cell types[21-23]. Notably, resveratrol nanoparticles have been shown to exert inhibitory effects on CAFs, thereby disrupting the supportive stromal network essential for tumor progression[24]. In addition to its effects on the TME, resveratrol directly impacts pancreatic cancer cells by inducing apoptosis and inhibiting cell proliferation. Resveratrol triggers apoptotic pathways by modulating the expression of pro-apoptotic and anti-apoptotic proteins, leading to mitochondrial dysfunction and caspase activation[25,26]. By modulating the TME, inducing apoptosis, and inhibiting proliferation and metastasis, resveratrol represents a promising therapeutic agent for pancreatic cancer.
METHODOLOGIES AND KEY FINDINGS
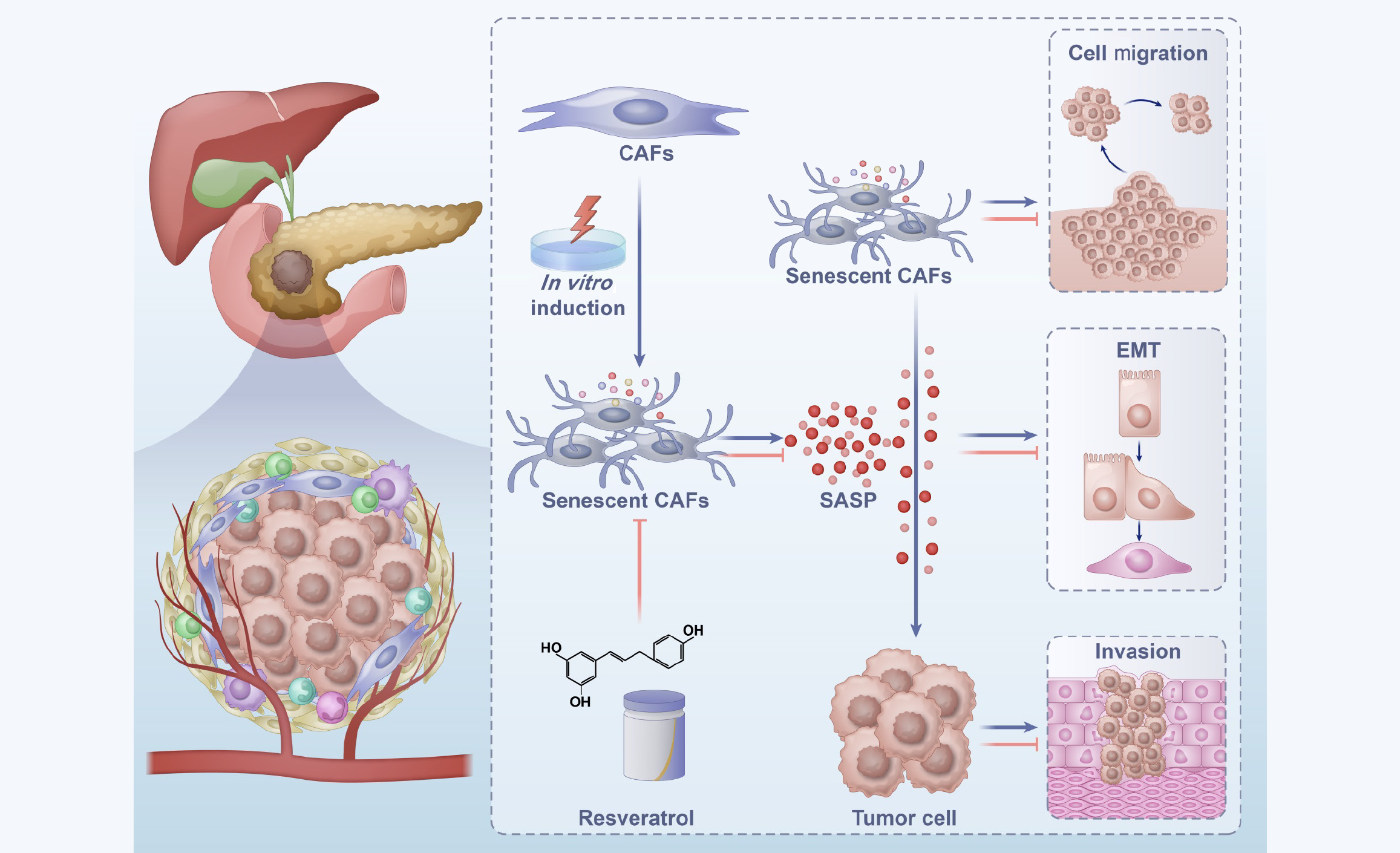
The study by Jiang et al[10], published in the World Journal of Gastrointestinal Oncology, investigates the potential of resveratrol to inhibit pancreatic cancer proliferation and metastasis by depleting senescent tumor-associated fibroblasts. They used a comprehensive experimental approach to elucidate the role of resveratrol in modulating the pancreatic cancer microenvironment. Through immunofluorescence staining, they detected a significant presence of senescent CAFs within pancreatic tumor tissues, marked by the coexpression of α-SMA and p16. In vitro experiments demonstrated that senescence in CAFs could be induced by treatment with a combination of IL-1α, IL-1β, and TNF-α, resulting in growth arrest and increased expression of senescence markers p16 and p21. Resveratrol treatment significantly diminished the population of senescent CAFs and lowered the levels of SASP factors. The study emphasized the role of senescent CAFs in promoting the growth, migration, and invasion of pancreatic cancer cells. Notably, resveratrol mitigated these effects by reducing the number of senescent CAFs and suppressing the epithelial-mesenchymal transition in cancer cells, a critical process in cancer metastasis. Resveratrol’s ability to restore lamin B1 expression and reduce SASP factor secretion underscores its potential to modulate the TME. Jiang et al’s findings suggest a promising therapeutic avenue for pancreatic cancer by targeting the TME, particularly senescent CAFs, to inhibit tumor progression and overcome resistance to conventional therapies (Figure 1)[10]. With its multifaceted biological activities and ability to modulate the TME, resveratrol emerges as a potential adjunctive therapy in the management of pancreatic cancer. Previous studies have reported that resveratrol participates in modulating key signaling pathways, including NF-κB and p38 MAPK[27,28]. Further exploration of how resveratrol regulates these pathways in the context of cellular senescence is crucial, as it may provide important insights into its therapeutic potential. Moreover, the identification of biomarkers predictive of response to resveratrol treatment will be key in guiding personalized treatment approaches.
Figure 1 The role of cancer-associated fibroblasts in pancreatic cancer progression and the modulatory effects of resveratrol.
This diagram illustrates the dynamic role of cancer-associated fibroblasts (CAFs) in pancreatic cancer progression and highlights the potential of resveratrol in targeting senescent CAFs to disrupt these pro-tumorigenic activities. CAFs: Cancer-associated fibroblasts; SASP: Senescence-associated secretory phenotype; EMT: Epithelial-mesenchymal transition.
CHALLENGES AND FUTURE DIRECTIONS
Resveratrol’s clinical application is limited by its low bioavailability and rapid metabolism. To overcome these challenges, several approaches have been explored, including the use of nanoformulations, co-administration of bioenhancers, and modification of its structure[29,30]. Future research should prioritize optimizing these approaches to improve the pharmacokinetic profile of resveratrol and enhance its therapeutic efficacy. A few clinical trials have investigated the safety and pharmacokinetics of resveratrol in cancer patients. These studies have demonstrated that resveratrol is well-tolerated and can achieve biologically relevant concentrations in tissues[31,32]. To translate the preclinical findings into clinical practice effectively, well-designed randomized controlled trials are paramount. Such trials should evaluate the safety and therapeutic efficacy of resveratrol, both as a monotherapy and in combination with existing standard-of-care treatments. In addition, adaptive trial designs could expedite the identification of optimal dosages, delivery methods, and biomarkers of response by allowing for real-time modification of study protocols based on interim analyses. This approach can help refine patient selection, improve therapeutic outcomes, and reduce the time and cost associated with conventional trial designs. Given the complexity of pancreatic cancer and the multifaceted role of the TME, combination therapies targeting multiple components of the TME may offer superior therapeutic benefits. Combining resveratrol with other therapeutic agents, such as chemotherapeutic drugs, immune checkpoint inhibitors, or other TME-targeting agents, could enhance its anti-cancer effects and help overcome resistance mechanisms[33,34]. Future studies should explore the synergistic effects of resveratrol in combination with other therapies and evaluate their potential in clinical settings. The identification of biomarkers predictive of response to resveratrol treatment is crucial for the successful implementation of resveratrol-based therapies. Biomarkers reflecting the presence and activity of senescent CAFs, the SASP, and other components of the TME could help identify patients who are most likely to benefit from resveratrol treatment. Future research should focus on discovering and validating these biomarkers to guide personalized treatment approaches. Although substantial progress has been made in understanding the mechanisms that underlie the anti-cancer effects of resveratrol, additional studies are required to thoroughly clarify its mode of action. Advanced technologies, such as spatial transcriptomics, single-cell RNA sequencing, and proteomics, can provide a comprehensive understanding of the molecular and cellular effects of resveratrol on senescent CAFs and the TME. These insights could reveal new therapeutic targets and support the development of more effective resveratrol-based therapies.
DISCUSSION
Resveratrol holds significant promise as an adjunctive therapy for pancreatic cancer, offering a potential new treatment option in combination with conventional treatments. However, several critical factors must be considered in translating these promising preclinical findings into practical clinical applications. The efficacy of resveratrol in clinical settings will largely depend on overcoming its inherent limitations, such as its low bioavailability and rapid metabolism. To maximize its therapeutic potential, researchers must explore optimized dosing strategies and delivery methods. Several approaches have been proposed to improve the pharmacokinetic profile of resveratrol. For instance, nanoformulations such as resveratrol-loaded nanoparticles or liposomes have been developed to enhance bioavailability, improve tissue penetration, and prolong circulating half-life[35,36]. These formulations not only enable targeted delivery to the tumor site but also reduce systemic side effects. Another potential strategy is the co-administration of resveratrol with bioenhancers, such as piperine, which has been shown to increase resveratrol absorption[37]. Clinical studies investigating the safety, tolerability, and pharmacokinetics of these novel formulations are crucial for determining the optimal dosage and administration routes, such as oral, intravenous, or intra-tumoral delivery. As clinical trials investigating the safety and efficacy of resveratrol continue, the integration of resveratrol into clinical practice will require a detailed and nuanced approach. The identification of biomarkers predictive of response to resveratrol, along with optimized dosing strategies and combination therapy regimens, will be pivotal in translating preclinical findings into real-world clinical benefits.
Resveratrol shows significant promise as a therapeutic agent for inhibiting pancreatic cancer proliferation and metastasis by targeting senescent CAFs. By inducing apoptosis in senescent CAFs, inhibiting the SASP, and modulating the TME, resveratrol can disrupt the pro-tumorigenic interactions between cancer cells and the TME, thereby hindering tumor progression and metastasis. While preclinical research has shown promising results, additional clinical trials are necessary to confirm these outcomes and assess resveratrol’s therapeutic potential in patients with pancreatic cancer. Overcoming challenges related to bioavailability, optimizing combination therapies, developing reliable biomarkers, and gaining deeper mechanistic insights will be crucial for successfully translating resveratrol-based therapies into clinical practice. Recent studies in lung cancer have shown that immune cells within the TME, such as T cells, can become dysfunctional due to antigenic exhaustion, leading to therapy resistance, a phenomenon linked to LUAD’s immune microenvironment heterogeneity[38,39]. Similarly, research in breast cancer emphasizes the role of hypoxia-related genes in modulating the TME to influence prognosis and therapeutic outcomes[40]. These studies mirror the findings of Jiang et al[10], who demonstrate that resveratrol can modulate the TME by targeting senescent CAFs, reducing their tumor-promoting effects and promoting therapeutic efficacy. Other pivotal studies have also explored targeting CAFs to enhance the efficacy of pancreatic cancer therapies. For instance, research by Li et al[41] demonstrated that the depletion of CAFs by genetic and pharmacological methods reduced tumor growth and enhanced chemotherapeutic response. Similarly, the work by Liu et al[42] investigated the inhibition of the TME by targeting CAF-secreted factors, showing promising results in reducing metastasis and promoting apoptosis in cancer cells. While these studies underscore the importance of CAFs in pancreatic cancer progression, Jiang et al’s focus on senescent CAFs and the modulation of SASP factors distinguishes their work by offering a targeted approach to modulating the tumor-supportive stroma, potentially enhancing both the efficacy and specificity of treatment[10]. In summary, the research by Jiang et al[10] underscores the potential of resveratrol as a multifaceted therapeutic agent in the fight against pancreatic cancer. Resveratrol targets senescent CAFs within the TME, effectively reducing tumor growth and metastasis while enhancing the efficacy of current therapies. The strengths of the methodologies used in this study include the comprehensive use of molecular markers to characterize senescent CAFs and the direct assessment of resveratrol’s effects on these cells. The study’s focus on the TME and senescent CAFs highlights an important, yet underexplored, aspect of pancreatic cancer biology. However, the limitations of the models, such as the reliance on cell lines and the absence of a complete immune context, reduce the immediate applicability of these findings to clinical settings. Therefore, while the findings are promising, further validation in more clinically relevant models is necessary to assess the true therapeutic potential of resveratrol for pancreatic cancer. In summary, this study paves the way for further exploring resveratrol and similar compounds in the context of pancreatic cancer therapy, highlighting the need for a comprehensive approach to cancer treatment that includes modulating the TME.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade A
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Xu SS S-Editor: Qu XL L-Editor: A P-Editor: Zhao S