Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.104402
Revised: April 8, 2025
Accepted: May 29, 2025
Published online: July 15, 2025
Processing time: 207 Days and 11 Hours
Pancreatic cancer (PC) remains one of the most lethal malignancies. While flap endonuclease-1 (FEN1) has been implicated in various cancers, its role in PC remains unclear.
To investigate the biological functions and mechanisms of FEN1 in PC prog
FEN1 expression and its prognostic significance were analyzed using Gene Expression Omnibus, The Cancer Genome Atlas, and Genotype-Tissue Expression databases. FEN1 was knocked down or overexpressed in PC cell lines using lentiviral vectors. Cell proliferation, migration, and invasion were assessed
FEN1 was significantly upregulated in PC tissues and correlated with poor prognosis. FEN1 promoted PC cell proliferation, migration, and invasion in vitro, as well as xenograft tumor growth in vivo. Mechanistically, FEN1 regulated epithelial-mesenchymal transition through the AKT/mTOR signaling pathway.
FEN1 functions as an oncogenic driver in PC progression via the AKT/mTOR signaling pathway, suggesting its potential as a therapeutic target.
Core Tip: This study elucidates the oncogenic role of flap endonuclease-1 (FEN1) in pancreatic cancer (PC), demonstrating its ability to promote cancer cell proliferation, migration, and invasion through modulation of the AKT/mTOR signaling pathway. Elevated FEN1 expression is closely associated with poor prognosis in PC patients, and FEN1 significantly enhances tumor growth both in vitro and in vivo. RNA sequencing, transcriptome analysis, and functional experiments further validate that the oncogenic effects of FEN1 are partially mediated by the activation of the AKT/mTOR signaling pathway, providing compelling evidence for FEN1 as a potential therapeutic target in PC.
- Citation: Xia Y, Guo N, Zhu CL, Gao JY, Da MX. Flap endonuclease-1 promotes pancreatic cancer progression via AKT/mTOR signaling pathway. World J Gastrointest Oncol 2025; 17(7): 104402
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/104402.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.104402
Pancreatic cancer (PC) represents one of the most lethal malignancies globally, with devastating mortality rates[1]. Despite significant advances in modern medicine, the five-year survival rate remains below 10%, with approximately 80% of patients diagnosed at advanced stages, precluding surgical intervention[2]. Current therapeutic strategies, including neoadjuvant therapy, immunotherapy, and molecular targeted approaches, have shown limited efficacy[3,4]. This underscores the urgent need to elucidate the molecular mechanisms driving PC progression.
Dysregulation of genome maintenance mechanisms plays a crucial role in PC progression, yet its therapeutic potential remains largely unexplored. Flap endonuclease-1 (FEN1), a member of the RAD2/XPG superfamily, serves as a central regulator of DNA replication and repair[5]. This highly conserved, multifunctional nuclease processes 5' flap structures during Okazaki fragment maturation, participates in long-patch base excision repair, and maintains genome stability through its 5'-3' exonuclease and gap endonuclease activities[6,7]. However, when these mechanisms are disrupted, genomic instability can arise, ultimately leading to cancer development. Emerging evidence indicates that FEN1 dysregulation is intimately linked to tumor development across multiple cancer types[8]. In breast cancer, FEN1 upregulation drives chemoresistance[9]; in hepatocellular carcinoma, FEN1 promotes tumor progression through P53 signaling suppression[10]; in cholangiocarcinoma, FEN1 enhances malignant phenotypes via Wnt/β-catenin pathway activation[11].
While previous studies have documented FEN1 overexpression in PC tissues[12], its mechanistic role and therapeutic potential remain undefined. Through comprehensive molecular and functional analyses, our study reveals the molecular mechanism by which FEN1 promotes PC progression through regulation of key signaling pathways, presenting a novel potential therapeutic target for PC treatment.
We analyzed FEN1 expression patterns using the GEPIA2 platform (http://gepia2.cancer-pku.cn), integrating data from The Cancer Genome Atlas and Genotype-Tissue Expression projects. Differential expression analysis was performed across PC and normal tissues. Additional validation was conducted using microarray datasets (GSE11838, GSE16515, and GSE62165) from the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/gds). Differential expression analysis was performed using the limma package (v4.4) in R, with visualization through ggplot2 (v3.5.1). Cancer Cell Line Encyclopedia (CCLE) (https: //sites.broadinstitute.org/ccle) data was analyzed using ggplot2 and ggpubr (v0.6.0) packages to assess FEN1 expression across PC cell lines.
Clinical specimens paired PC tissues and adjacent non-cancerous samples (n = 8 pairs) were obtained from Gansu Provincial Hospital, Lanzhou, China.
Tissues were flash-frozen in liquid nitrogen and stored at -80 °C. The study was approved by the Ethics Committee of Gansu Provincial Hospital (approval number: 2024-418), with informed consent obtained from all participants. Human experiments were performed in accordance with the Declaration of Helsinki.
Tissue sections were deparaffinized, rehydrated through a graded ethanol series, and subjected to antigen retrieval in citrate buffer (10 mmol/L, pH 6.0) under pressure. Sections were then incubated with a rabbit monoclonal anti-Ki67 antibody (ab15580, Abcam; 1:150 dilution), visualized using 3,3'-diaminobenzidine, and counterstained with hematoxylin. Negative controls were included in each experiment by substituting the primary antibody with phosphate-buffered saline (PBS).
For quantification, five random high-power fields (400 ×) were selected per section, and the percentage of Ki67-positive cells relative to the total number of cells was calculated. All sections were independently evaluated by two blinded researchers.
Human pancreatic cell lines (HPDE, AsPC-1, BxPC-3, CFPAC-1, PATU8988T, and PANC-1) were obtained from Shanghai Institute of Biological Sciences, China. Cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C with 5% CO2. Lentiviral constructs (GenePharma, Shanghai) were used to establish stable FEN1-modulated cell lines in PATU8988T and PANC-1 cells, with puromycin selection. Modification efficiency was validated by western blot and reverse transcription-quantitative PCR (RT-qPCR) analyses.
Quantitative Real-Time PCR Total RNA was isolated using RNAprep Cell Kit (NCM, Suzhou) and quality-assessed spectrophotometrically. cDNA was synthesized using RevertAid First Strand Kit (Thermo Scientific). qPCR was performed using SYBR Green Master (ROX) (Roche) on a 7500 Fast Real-Time PCR System. Primers for FEN1: Forward 5′-TGAAGGTCACTAAGCAGCACAATG-3′, reverse 5′-GCAGTCAGGTGTCGCATTAGC-3′; GAPDH: Forward 5′-CACCCACTCCTCCACCTTTGAC-3′, reverse 5′-GTCCACCACCCTGTTGCTGTAG-3′. Relative expression was calculated using the 2-ΔΔCT method, with GAPDH as internal control.
Transcriptome sequencing was performed by OE Biotech Co., Ltd. (Shanghai, China). Total RNA was isolated using TRIzol reagent and quality-assessed using a NanoDrop 2000 spectrophotometer and Agilent 2100 Bioanalyzer (RIN > 8.0 required). Libraries were constructed using the VAHTS Universal V6 RNA-seq Library Prep Kit and sequenced on a DNBSEQ-T7 platform, generating 150 bp paired-end reads (approximately 48M raw reads per sample). Raw reads were processed using fastp[13], aligned to the reference genome using HISAT2[14], and quantified for gene expression (FPKM)[15]. Differential expression analysis was performed using DESeq2[16] (q-value < 0.05, |fold change| > 2.0). Gene set enrichment analysis was conducted using GSEA[17] software, with pathway analyses performed using GO[18], KEGG[19], Reactome, and WikiPathways databases.
Cells were lysed in RIPA buffer containing protease inhibitors. Proteins were separated by 10% SDS-PAGE, transferred to PVDF membranes (0.45 μm), and blocked with 5% non-fat milk in TBST. Primary antibody incubation was performed overnight at 4 °C, followed by secondary antibody incubation for 1h at room temperature. Signals were visualized using ECL reagent (BL520A, Biosharp).
Male nude mice (4 to 5 weeks of age, n = 5 per group) were purchased from the Lanzhou Institute of Veterinary Medicine, Chinese Academy of Agricultural Sciences, and were housed in an SPF environment in the Lanzhou Veterinary Institute. The feed, bedding, cages, water bottles and drinking water of nude mice were autoclaved.
Nude mice (n = 5 per group) were subcutaneously injected in the axilla with 1 × 107 PATU8988T cells (shFEN1 or shNC). Tumor size was measured weekly from day 7 post-injection using a vernier caliper. On day 32, mice were anesthetized with sodium pentobarbital, injected with sodium fluorescein, and imaged using an in vivo imaging system. Tumors were harvested for immunohistochemical analysis of Ki67 expression.
The study is reported in accordance with ARRIVE guidelines. All animals were anesthetized and sedated with sodium pentobarbital (0.5% sodium solution of pentobarbital was intraperitoneally injected with 0.5 mL/100 g). Research investigators choose to euthanize mice by cervical dislocation. This animal study was approved by the Laboratory Animal Ethics Committee of Lanzhou Veterinary Research Institute (LVRIAEC-2024-042). All experiments were performed in accordance with relevant named guidelines and regulations. The study is reported in accordance with ARRIVE guidelines.
Cell proliferation was assessed using the BeyoClick EdU-594 kit (Beyotime™). Cells (1 × 105) were labeled with EdU (10 μM) for 2 hours, fixed, permeabilized, and visualized using a Zeiss microscope. For colony formation assays, cells were seeded at 800 cells per well in 6-well plates and cultured for 10 days. Colonies were then fixed with 4% paraformaldehyde for 15 minutes at room temperature and stained with crystal violet. Colony numbers were quantified using ImageJ software.
Transwell assays were performed using 8-μm pore chambers (CLS3422, Corning). For invasion assays, chambers were pre-coated with Matrigel. Cells (2 × 104) in serum-free medium were seeded in the upper chamber, with 20% FBS-containing medium in the lower chamber. After 36 hours, migrated/invaded cells were fixed, stained with crystal violet, and counted in three random fields.
Cell migration was assessed using a wound healing assay. Transfected cells were seeded in 6-well plates and grown to confluence. A wound was created using a sterile 200 μL pipette tip, followed by PBS washing and replacement with serum-free DMEM. Wound healing was monitored using an OLYMPUS microscope at 0 and 48 hours. Cell migration rates were quantified using ImageJ software by measuring wound areas at 0 and 48 hours. The migration rate was calculated as follows: [(wound area at 0 hour − wound area at 48 hours)/wound area at 0 hour] × 100%.
Data analysis was performed using ImageJ (v1.53t), GraphPad Prism (v9.5), and R (v3.2.0). Results represent at least three independent experiments and are presented as mean ± SD. Statistical significance was determined using student's t-test (two groups) or ANOVA (multiple groups), with P < 0.05 considered significant.
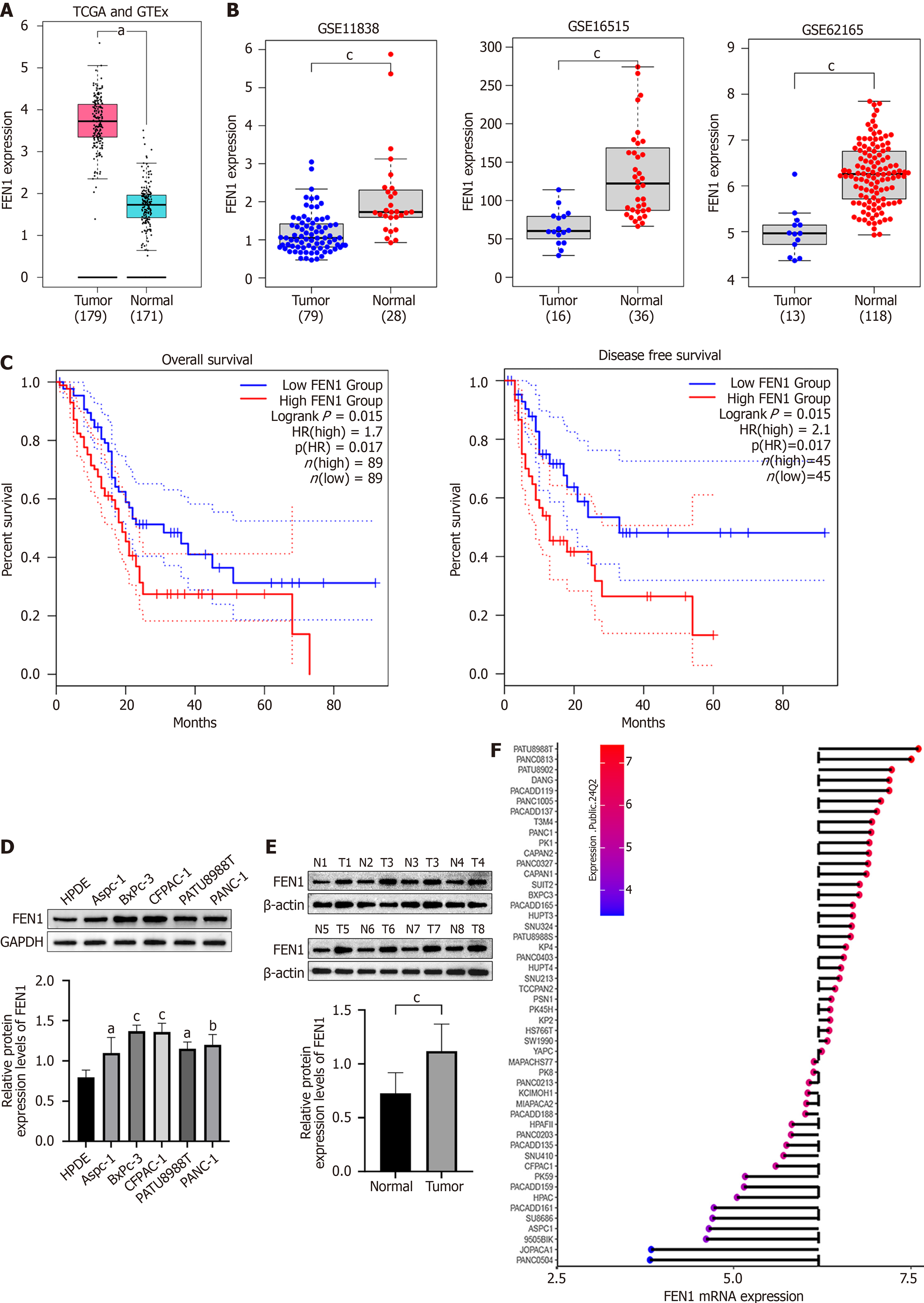
Analysis of the Gene Expression Profiling Interactive Analysis database revealed significant upregulation of FEN1 mRNA in PC tissues compared to normal tissues (Figure 1A). This finding was validated across multiple independent GEO datasets (GSE11838, GSE16515, and GSE62165; Figure 1B). Notably, high FEN1 expression significantly correlated with poor overall and disease-free survival (Figure 1C). Comparative analysis of FEN1 protein levels across PC cell lines (AsPC-1, BxPC-3, CFPAC-1, PATU8988T, and PANC-1) vs normal pancreatic ductal epithelial cells (HPDE) demonstrated consistent FEN1 upregulation in cancer cells (Figure 1D). This observation was further validated in paired tumor-normal tissue samples (n = 8 pairs; Figure 1E) and corroborated by CCLE data analysis (Figure 1F).
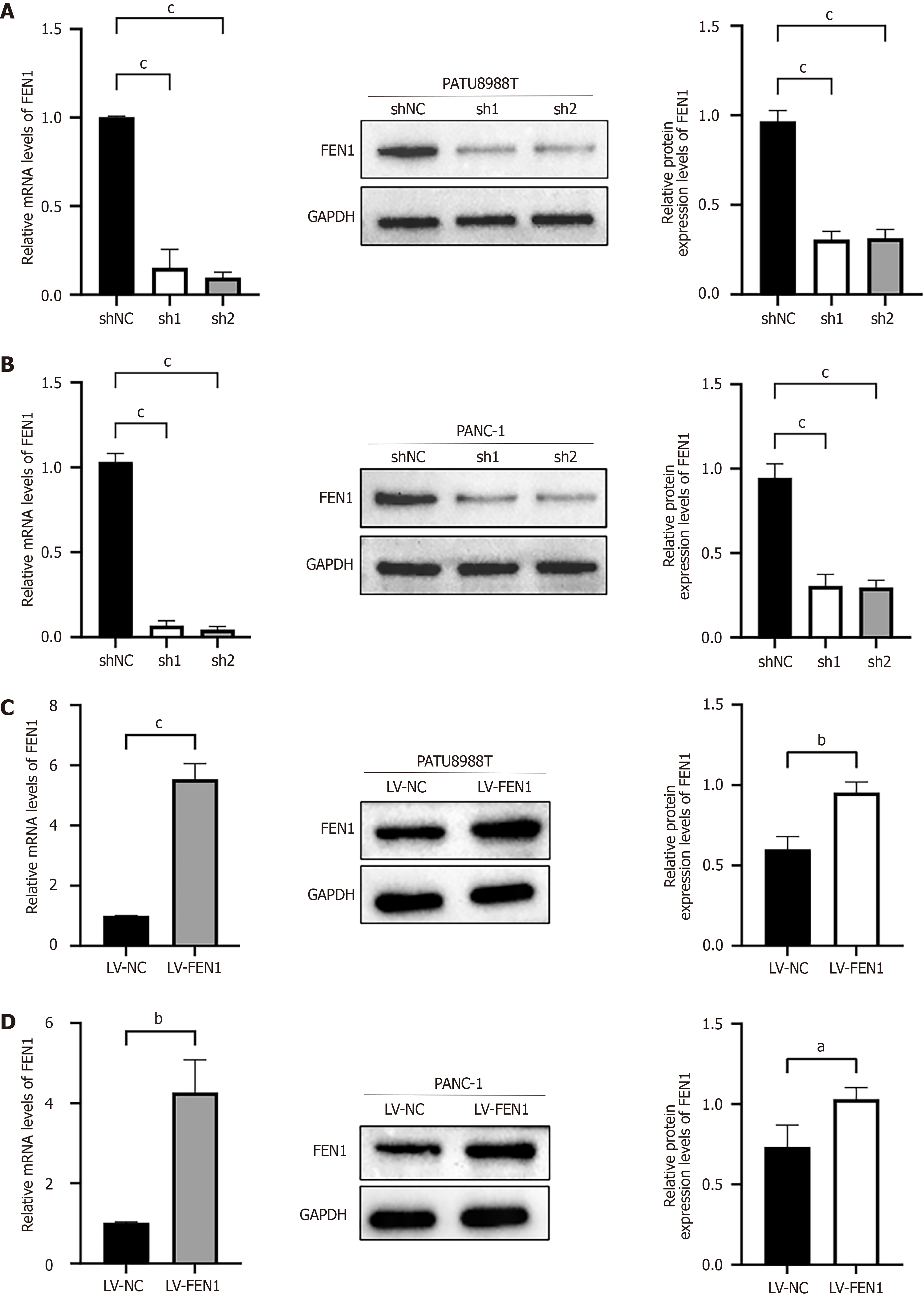
To elucidate the biological functions of FEN1, we conducted comprehensive analyses utilizing PATU8988T and PANC-1 PC cell lines, which exhibited relatively high endogenous FEN1 expression levels (Figure 1D). We established stable FEN1 knockdown and overexpression cell lines, with manipulation efficiency validated through western blot analysis and RT-qPCR (Figure 2).
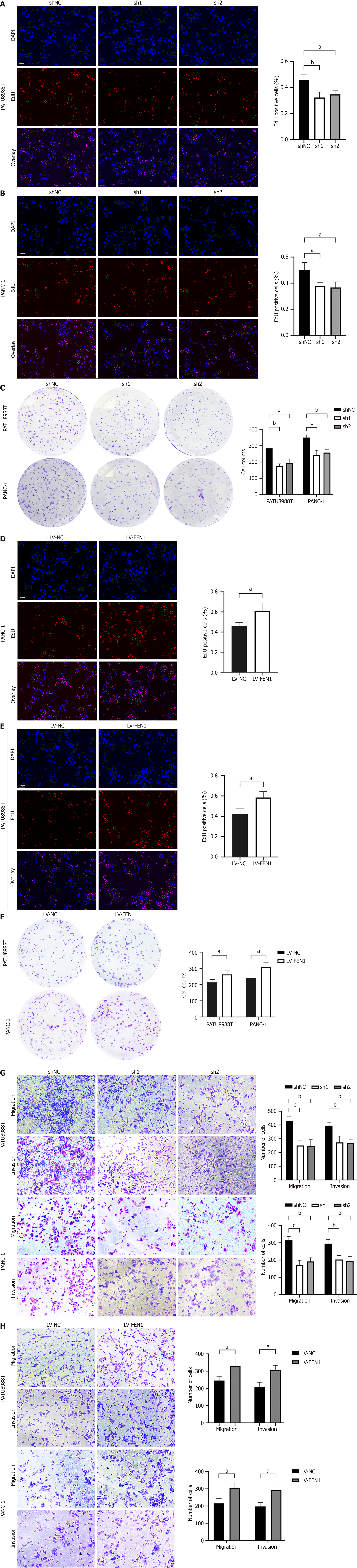
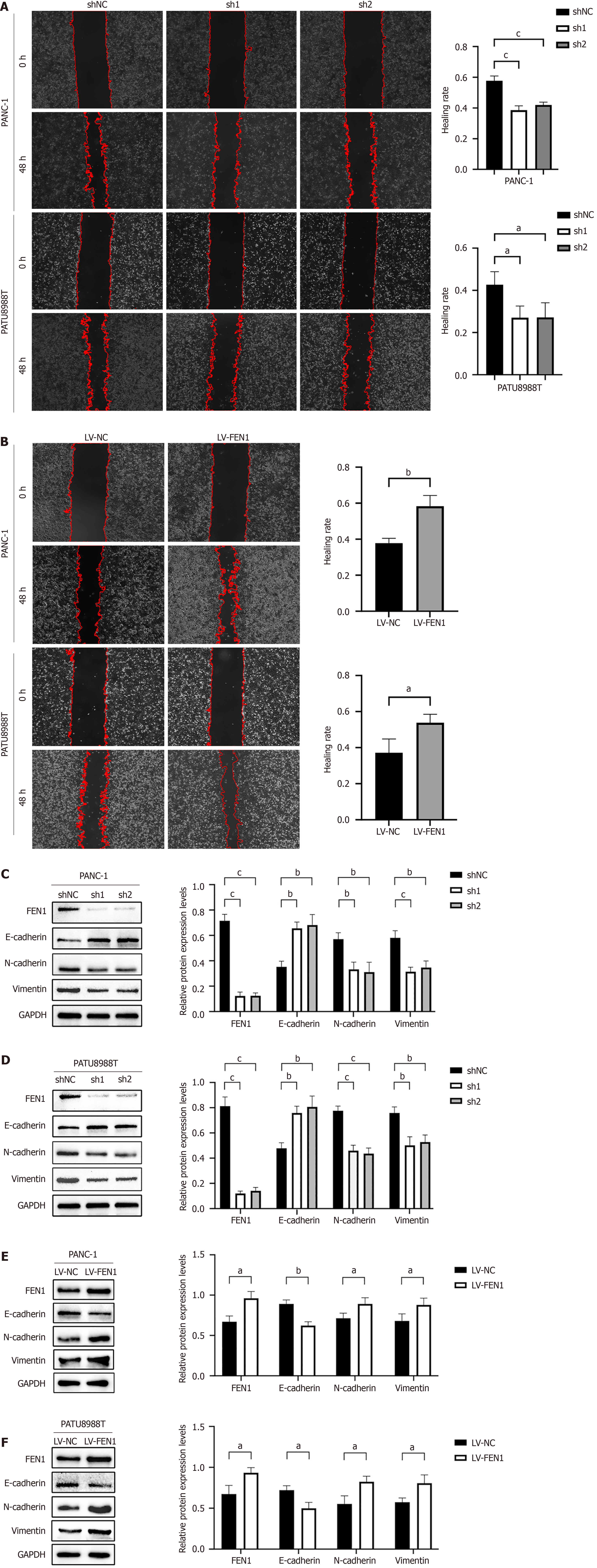
Our investigation revealed that FEN1 significantly enhances PC cell proliferation, migration, and invasion in vitro. Following FEN1 knockdown (sh1 and sh2), both PATU8988T and PANC-1 cells demonstrated markedly reduced proliferation rates and colony-forming capabilities compared to control cells (shNC) (Figure 3A-C). Conversely, FEN1 overexpression (LV-FEN1) resulted in significantly enhanced cellular proliferation and clonogenic potential (Figure 3D-F), suggesting that FEN1 augments the proliferative capacity of PC cells in vitro. Migration and invasion assays demonstrated that FEN1 overexpression substantially enhanced the metastatic potential of both PATU8988T and PANC-1 cells, while FEN1 knockdown exhibited opposing effects (Figure 3G and H). Wound healing assays revealed significantly reduced scratch widths in PATU8988T/PANC-1-LV-FEN1 groups, indicating enhanced migratory capacity. Conversely, sh1 and sh2 groups displayed significantly increased relative scratch widths compared to shNC controls, suggesting impaired migration following FEN1 silencing (Figure 4A and B). Western blot analysis of epithelial-mesenchymal transition (EMT) markers revealed that FEN1 overexpression significantly upregulated N-cadherin and Vimentin expression while downregulating E-cadherin, with FEN1 knockdown inducing reciprocal effects (Figure 4C-F).
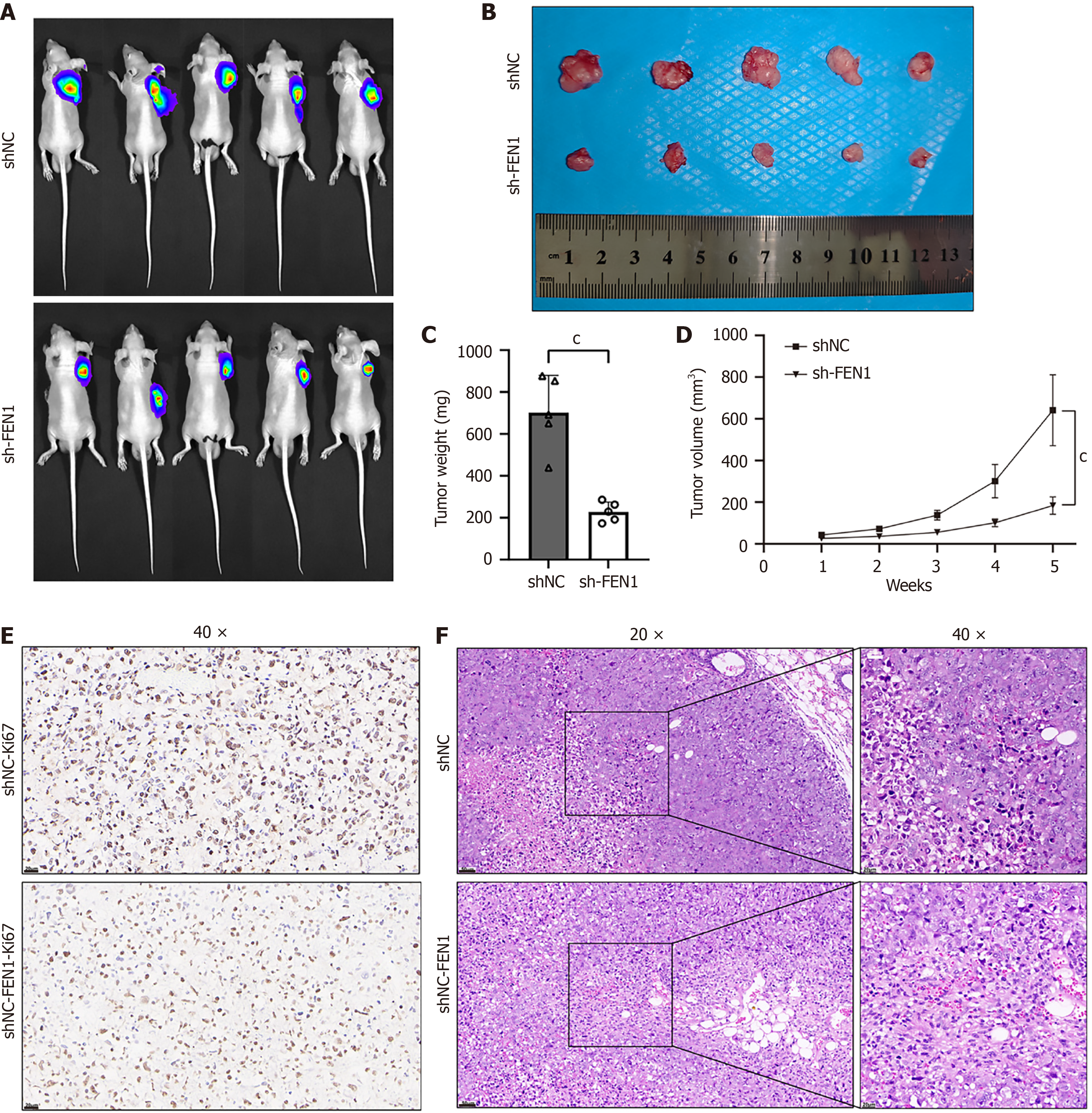
Subcutaneous tumors derived from PATU8988T+sh-FEN1 cells exhibited significantly suppressed growth compared to PATU8988T+shNC controls (Figure 5A-C). Tumor volume differences between groups became increasingly pronounced over time (Figure 5D) (P < 0.01). Immunohistochemical analysis revealed reduced Ki67 Levels in sh-FEN1 tumors compared to shNC controls (Figure 5E), with findings further corroborated by H&E staining (Figure 5F).
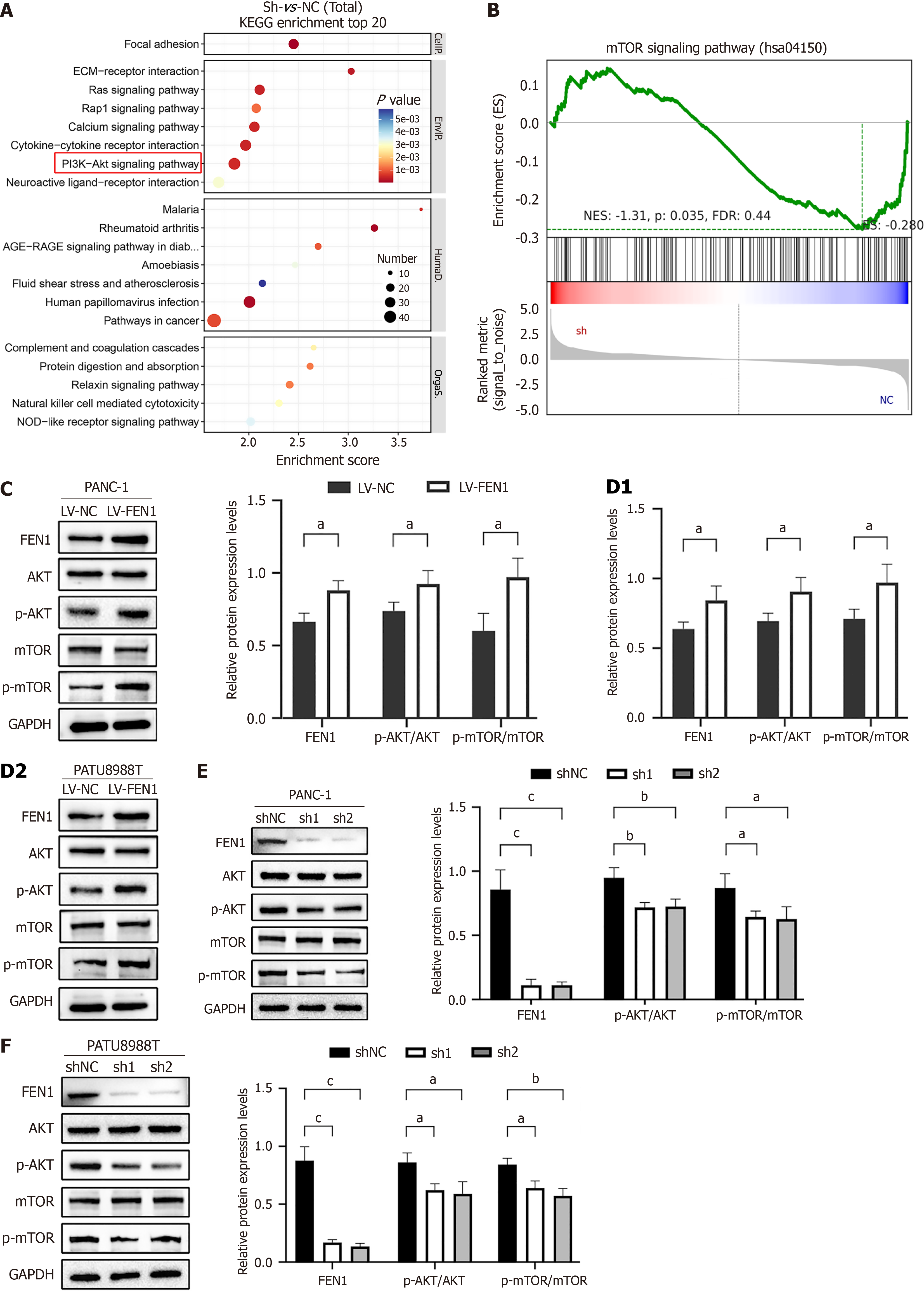
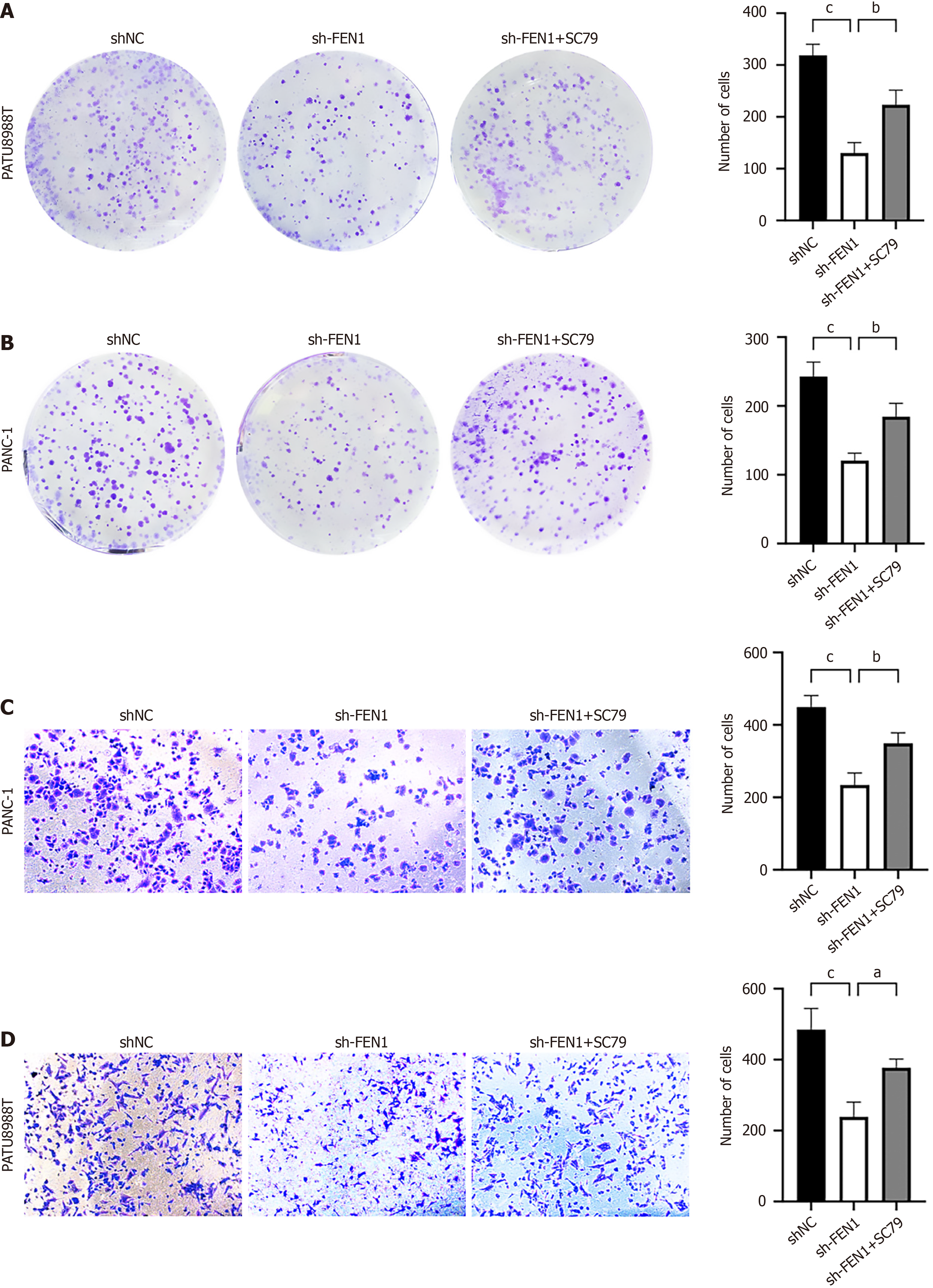
Mechanistic investigations through RNA sequencing revealed significant enrichment of FEN1-associated genes in the AKT/mTOR signaling pathway. This finding was further supported by KEGG and GSEA analyses (Figure 6A and B). Western blot analyses demonstrated that while total AKT and mTOR protein levels remained unchanged, FEN1 knockdown significantly reduced p-AKT and p-mTOR levels in both cell lines, with FEN1 overexpression inducing opposite effects (Figure 6C-F). Rescue experiments utilizing the AKT activator SC79 (8 μg/mL) partially restored the proliferative and migratory capabilities of FEN1-knockdown cells (Figure 7), substantiating the oncogenic role of FEN1 through AKT/mTOR signaling pathway activation in PC cells.
PC is among the most aggressive malignancies, frequently diagnosed at an advanced stage when therapeutic options are limited. Although surgical resection remains the only potentially curative treatment, recurrence rates are high, and the five-year survival rate is less than 10%[20], underscoring the urgent need for effective therapeutic strategies.
Through integrative bioinformatics analyses and statistical evaluation of clinical tissue samples, this study demonstrates that FEN1 is significantly overexpressed in PC tissues and is associated with adverse clinical outcomes. These findings are consistent with prior studies and extend the role of FEN1 as a prognostic biomarker across multiple cancers, including hepatocellular carcinoma[21] and gastric cancer[22]. Moreover, comprehensive in vitro and in vivo investigations reveal that FEN1 facilitates cell proliferation, migration, and invasion, primarily through modulation of the AKT/mTOR signaling pathway.
The role of FEN1 in maintaining genomic stability and promoting tumor progression has been widely investigated in various cancer types[23-26]. Previous studies have highlighted its involvement in diverse oncogenic processes, including cellular proliferation, migration, and resistance to therapy. Mechanistically, FEN1 has been shown to influence tamoxifen sensitivity in breast cancer via the ERα/cyclin D1/Rb axis, and to enhance the efficacy of radiotherapy and platinum-based chemotherapy in cervical cancer[9]. Furthermore, FEN1 is regulated by several microRNAs, notably miR-4324 and miR-140, which are closely linked to cancer progression[27].
In this study, a novel role of FEN1 in regulating EMT in PC is revealed-a key process in malignant transformation[28,29]. Transwell and wound-healing assays demonstrate that FEN1 overexpression upregulates mesenchymal markers (N-cadherin and Vimentin) while downregulating the epithelial marker E-cadherin; conversely, FEN1 inhibition yields the opposite effects. The AKT/mTOR pathway emerges as a critical downstream effector of FEN1 function. Experimental data show that FEN1 overexpression enhances p-AKT and p-mTOR expression in vitro, whereas FEN1 silencing suppresses this signaling axis. The role of this pathway was further confirmed through rescue experiments: treatment with the AKT activator SC79 (8 μg/mL) partially restored the proliferative and migratory capacity of FEN1-knockdown cells.
Growing interest in FEN1 as a therapeutic target has spurred the development of small-molecule inhibitors. Compounds such as N-hydroxyurea derivatives (e.g., Compound #8 and Compound #20) have exhibited potent antitumor activity across various cancer models[30]. SC13, a selective FEN1 inhibitor, disrupts DNA repair mechanisms, induces apoptosis, and enhances chemosensitivity in cervical cancer[31]. Notably, SC13 shows synergistic efficacy with paclitaxel, significantly reducing xenograft tumor burden while minimizing chemotherapy-associated toxicity[32,33]. In platinum-resistant ovarian cancer, FEN1 inhibitors (e.g., FEN1i) restore cisplatin sensitivity by preventing FEN1 nuclear translocation and impairing DNA repair capacity[34]. Given the association between high FEN1 expression and poor prognosis across cancers, screening for FEN1 overexpression or specific mutations (such as L209P) could help identify patients likely to benefit from FEN1-targeted therapies[35]. However, despite encouraging preclinical findings, the clinical translation of FEN1 inhibitors faces challenges, including suboptimal efficacy and pharmacokinetic limitations.
FEN1 contributes to tumorigenesis through diverse molecular mechanisms. It has been shown to enhance ERα transcriptional activity by facilitating its recruitment to transcriptional complexes, thereby promoting tumor cell proliferation[9]. Additionally, FEN1 suppresses the p53 signaling pathway by recruiting USP7, which inhibits MDM2 ubiquitination and degradation[10]. FEN1 mRNA stability is further enhanced by IGF2BP2, which directly binds to m6A-modified sites on FEN1 transcripts, thereby promoting tumor progression[36]. These mechanisms, in conjunction with its involvement in the AKT/mTOR pathway, support FEN1’s multifaceted role in PC.
Within the framework of PC pathogenesis, the activation of the AKT/mTOR signaling pathway by FEN1 suggests potential crosstalk with other oncogenic drivers, particularly KRAS, which is mutated in over 90% of PC cases[37]. The PI3K/AKT/mTOR axis is a well-established effector of KRAS-driven signaling in PC. The convergence of FEN1- and KRAS-mediated pathways at the AKT/mTOR signaling node implies a possible regulatory network underlying disease progression, although further experimental validation is warranted.
In conclusion, this study provides compelling evidence that FEN1 promotes PC progression via activation of the AKT/mTOR signaling pathway. The observed enhancement of tumor cell proliferation, migration, and invasion by FEN1 underscores its potential as a therapeutic target. These findings advance current understanding of PC biology and open new avenues for the development of FEN1-targeted therapies.
Nevertheless, several limitations must be acknowledged. First, the relatively small clinical sample size (n = 8 pairs) may limit the statistical power and generalizability of the findings. Future research should aim to validate these observations in larger, independent patient cohorts. Second, while this study establishes an association between FEN1 and the AKT/mTOR signaling pathway, the precise molecular mechanisms governing this interaction remain to be elucidated. Further investigations are needed to dissect these mechanisms and reinforce the translational relevance of the findings.
FEN1 functions as an oncogenic driver in PC progression via the AKT/mTOR signaling pathway, suggesting its potential as a therapeutic target.
| 1. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 863] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 2. | Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2129] [Cited by in RCA: 2182] [Article Influence: 145.5] [Reference Citation Analysis (5)] |
| 3. | Macedo FI, Ryon E, Maithel SK, Lee RM, Kooby DA, Fields RC, Hawkins WG, Williams G, Maduekwe U, Kim HJ, Ahmad SA, Patel SH, Abbott DE, Schwartz P, Weber SM, Scoggins CR, Martin RCG, Dudeja V, Franceschi D, Livingstone AS, Merchant NB. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg. 2019;270:400-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | Chinese Pancreatic Surgery Association, Chinese Society of Surgery, Chinese Medical Association. [Guidelines for the diagnosis and treatment of pancreatic cancer in China(2021)]. Zhonghua Wai Ke Za Zhi. 2021;59:561-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Guo E, Ishii Y, Mueller J, Srivatsan A, Gahman T, Putnam CD, Wang JYJ, Kolodner RD. FEN1 endonuclease as a therapeutic target for human cancers with defects in homologous recombination. Proc Natl Acad Sci U S A. 2020;117:19415-19424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Balakrishnan L, Bambara RA. Flap endonuclease 1. Annu Rev Biochem. 2013;82:119-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Varshney GK, Burgess SM. DNA-guided genome editing using structure-guided endonucleases. Genome Biol. 2016;17:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Zaher MS, Rashid F, Song B, Joudeh LI, Sobhy MA, Tehseen M, Hingorani MM, Hamdan SM. Missed cleavage opportunities by FEN1 lead to Okazaki fragment maturation via the long-flap pathway. Nucleic Acids Res. 2018;46:2956-2974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Xu L, Shen JM, Qu JL, Song N, Che XF, Hou KZ, Shi J, Zhao L, Shi S, Liu YP, Qu XJ, Teng YE. FEN1 is a prognostic biomarker for ER+ breast cancer and associated with tamoxifen resistance through the ERα/cyclin D1/Rb axis. Ann Transl Med. 2021;9:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Bian S, Ni W, Zhu M, Zhang X, Qiang Y, Zhang J, Ni Z, Shen Y, Qiu S, Song Q, Xiao M, Zheng W. Flap endonuclease 1 Facilitated Hepatocellular Carcinoma Progression by Enhancing USP7/MDM2-mediated P53 Inactivation. Int J Biol Sci. 2022;18:1022-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Yuwei X, Bingzi D, Zhaowei S, Yujie F, Wei Z, Kun L, Kui L, Jingyu C, Chengzhan Z. FEN1 promotes cancer progression of cholangiocarcinoma by regulating the Wnt/β-catenin signaling pathway. Dig Liver Dis. 2024;56:695-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Isohookana J, Haapasaari KM, Soini Y, Leppänen J, Karihtala P. Proteins of the retinoblastoma pathway, FEN1 and MGMT are novel potential prognostic biomarkers in pancreatic adenocarcinoma. Pathol Res Pract. 2018;214:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884-i890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4558] [Cited by in RCA: 17192] [Article Influence: 2456.0] [Reference Citation Analysis (0)] |
| 14. | Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9910] [Cited by in RCA: 16320] [Article Influence: 1483.6] [Reference Citation Analysis (0)] |
| 15. | Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol. 2011;12:R22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1015] [Cited by in RCA: 1217] [Article Influence: 81.1] [Reference Citation Analysis (0)] |
| 16. | Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34752] [Cited by in RCA: 64057] [Article Influence: 5823.4] [Reference Citation Analysis (1)] |
| 17. | Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545-15550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27252] [Cited by in RCA: 39994] [Article Influence: 1904.5] [Reference Citation Analysis (0)] |
| 18. | The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330-D338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2608] [Cited by in RCA: 3226] [Article Influence: 460.9] [Reference Citation Analysis (0)] |
| 19. | Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36:D480-D484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3661] [Cited by in RCA: 4660] [Article Influence: 245.3] [Reference Citation Analysis (0)] |
| 20. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6075] [Article Influence: 3037.5] [Reference Citation Analysis (4)] |
| 21. | Zhang Y, Liu X, Liu L, Chen J, Hu Q, Shen S, Zhou Y, Chen S, Xue C, Cui G, Yu Z. Upregulation of FEN1 Is Associated with the Tumor Progression and Prognosis of Hepatocellular Carcinoma. Dis Markers. 2020;2020:2514090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Zhao E, Zhou C, Chen S. Flap endonuclease 1 (FEN1) as a novel diagnostic and prognostic biomarker for gastric cancer. Clin Res Hepatol Gastroenterol. 2021;45:101455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Nikolova T, Christmann M, Kaina B. FEN1 is overexpressed in testis, lung and brain tumors. Anticancer Res. 2009;29:2453-2459. [PubMed] |
| 24. | Zheng L, Dai H, Hegde ML, Zhou M, Guo Z, Wu X, Wu J, Su L, Zhong X, Mitra S, Huang Q, Kernstine KH, Pfeifer GP, Shen B. Fen1 mutations that specifically disrupt its interaction with PCNA cause aneuploidy-associated cancer. Cell Res. 2011;21:1052-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Wang K, Xie C, Chen D. Flap endonuclease 1 is a promising candidate biomarker in gastric cancer and is involved in cell proliferation and apoptosis. Int J Mol Med. 2014;33:1268-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | He L, Zhang Y, Sun H, Jiang F, Yang H, Wu H, Zhou T, Hu S, Kathera CS, Wang X, Chen H, Li H, Shen B, Zhu Y, Guo Z. Targeting DNA Flap Endonuclease 1 to Impede Breast Cancer Progression. EBioMedicine. 2016;14:32-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Lu X, Liu R, Wang M, Kumar AK, Pan F, He L, Hu Z, Guo Z. MicroRNA-140 impedes DNA repair by targeting FEN1 and enhances chemotherapeutic response in breast cancer. Oncogene. 2020;39:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Campbell K, Casanova J. A common framework for EMT and collective cell migration. Development. 2016;143:4291-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 3571] [Article Influence: 396.8] [Reference Citation Analysis (0)] |
| 30. | Xu L, Qu JL, Song N, Zhang LY, Zeng X, Che XF, Hou KZ, Shi S, Feng ZY, Qu XJ, Liu YP, Teng YE. Biological and clinical significance of flap endonuclease1 in triplenegative breast cancer: Support of metastasis and a poor prognosis. Oncol Rep. 2020;44:2443-2454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Li JL, Wang JP, Chang H, Deng SM, Du JH, Wang XX, Hu HJ, Li DY, Xu XB, Guo WQ, Song YH, Guo Z, Sun MX, Wu YW, Liu SB. FEN1 inhibitor increases sensitivity of radiotherapy in cervical cancer cells. Cancer Med. 2019;8:7774-7780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | He L, Yang H, Zhou S, Zhu H, Mao H, Ma Z, Wu T, Kumar AK, Kathera C, Janardhan A, Pan F, Hu Z, Yang Y, Luo L, Guo Z. Synergistic antitumor effect of combined paclitaxel with FEN1 inhibitor in cervical cancer cells. DNA Repair (Amst). 2018;63:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Wu T, Zhu H, Zhang M, Sun Y, Yang Y, Gu L, Zhang J, Mu D, Wu C, Hu Z, Jiang L, Jia S, Zhang Y, He L, Pan FY, Guo Z. FEN1 inhibitor synergizes with low-dose camptothecin to induce increased cell killing via the mitochondria mediated apoptotic pathway. Gene Ther. 2022;29:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Yang F, Hu Z, Guo Z. Small-Molecule Inhibitors Targeting FEN1 for Cancer Therapy. Biomolecules. 2022;12:1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 35. | Sun H, He L, Wu H, Pan F, Wu X, Zhao J, Hu Z, Sekhar C, Li H, Zheng L, Chen H, Shen BH, Guo Z. The FEN1 L209P mutation interferes with long-patch base excision repair and induces cellular transformation. Oncogene. 2017;36:194-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Pu J, Wang J, Qin Z, Wang A, Zhang Y, Wu X, Wu Y, Li W, Xu Z, Lu Y, Tang Q, Wei H. IGF2BP2 Promotes Liver Cancer Growth Through an m6A-FEN1-Dependent Mechanism. Front Oncol. 2020;10:578816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 37. | Luo J. KRAS mutation in pancreatic cancer. Semin Oncol. 2021;48:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 254] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/