Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.99304
Revised: November 24, 2024
Accepted: January 2, 2025
Published online: March 15, 2025
Processing time: 210 Days and 2.6 Hours
Gastrointestinal stromal tumors (GISTs) are caused by mutations in the KIT and platelet derived growth factor receptor alpha genes in approximately 90% of cases. A minority of wild-type GISTs are associated with neurofibromatosis type 1 (NF1), an autosomal dominant genetic disease resulting from pathogenic muta
A 51-year-old woman presented to the emergency department with complaints of dizziness, fatigue, chest tightness, and dark stools. Initial examination revealed a red blood cell count of 1.99 × 1012/L and a hemoglobin level of 43 g/L. She un
Given the diversity of clinical symptoms associated with NF1 and the complexity of NF1-related GISTs, surgical resection with complete tumor removal remains the preferred treatment option. However, the absence of a stan
Core Tip: Gastrointestinal stromal tumor (GIST) is one of the rare complications of neurofibromatosis type 1 (NF1). In NF1-related GISTs, tumors are more common in the small intestine (90%) and are rarely found in the stomach. The condition of NF1-related GIST is complex, and there is no standard treatment plan for postoperative adjuvant therapy. Postoperative regorafenib treatment was administered in this patient, and no recurrence was noted at the one-year follow-up.
- Citation: Bai GY, Shan KS, Li CS, Wang XH, Feng MY, Gao Y. Gastric gastrointestinal stromal tumor in a patient with neurofibromatosis type I presenting with anemia: A case report. World J Gastrointest Oncol 2025; 17(3): 99304
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/99304.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.99304
Neurofibromatosis encompasses a group of tumor syndromes characterized by genetic heterogeneity, leading to tumors in the central and peripheral nervous systems and causing various types of systemic damage. This autosomal dominant genetic disease results from genetic defects. The global incidence is approximately 1 in 3000 to 1 in 4000 individuals. Neurofibromatosis type 1 (NF1) is the most prevalent type, accounting for approximately 96% of cases, whereas NF2 represents around 3%. Schwannomas are even rarer[1,2]. NF1 is caused by pathogenic mutations in the NF1 gene, which encodes neurofibromin. Characteristic symptoms of NF1 include café-au-lait macules (CALMs) and neurofibromas, while gastrointestinal stromal tumors (GISTs) are rare complications. Among NF1-related GISTs, 90% occur in the small intestine, with gastric occurrences being extremely rare. These tumors are complex, making complete surgical resection challenging. There is currently no standardized protocol for adjuvant therapy post-surgery, necessitating vigilant clinical attention.
The 51-year-old female patient had been suffering from anemia for the past two years and tested positive for fecal occult blood one year ago.
Over the last two years, the patient frequently experienced episodes of sudden dizziness and fatigue. Recently, she experienced symptoms including dizziness, weakness, chest tightness, and the passage of black stools.
The patient had multiple CALMs and skin nodules distributed across the head, face, trunk, and limbs, with both the quantity and size progressively escalating over the preceding 30 years. A biopsy conducted three decades ago on a nodular mass located on the upper limb yielded findings consistent with neurofibroma. Additionally, the patient repor
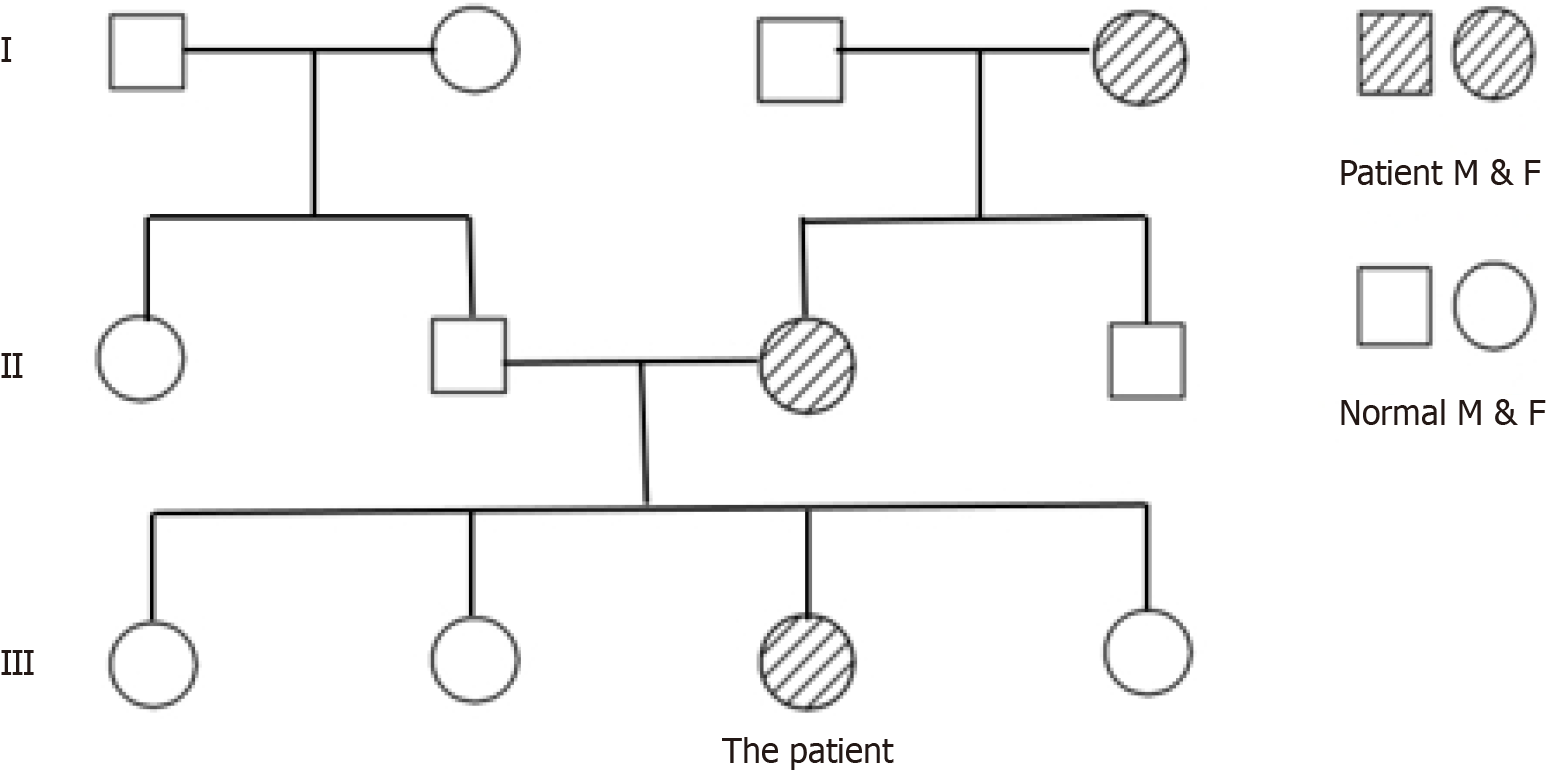
The patient is unmarried and has no children. Both her maternal grandmother and mother have a history of neurofi
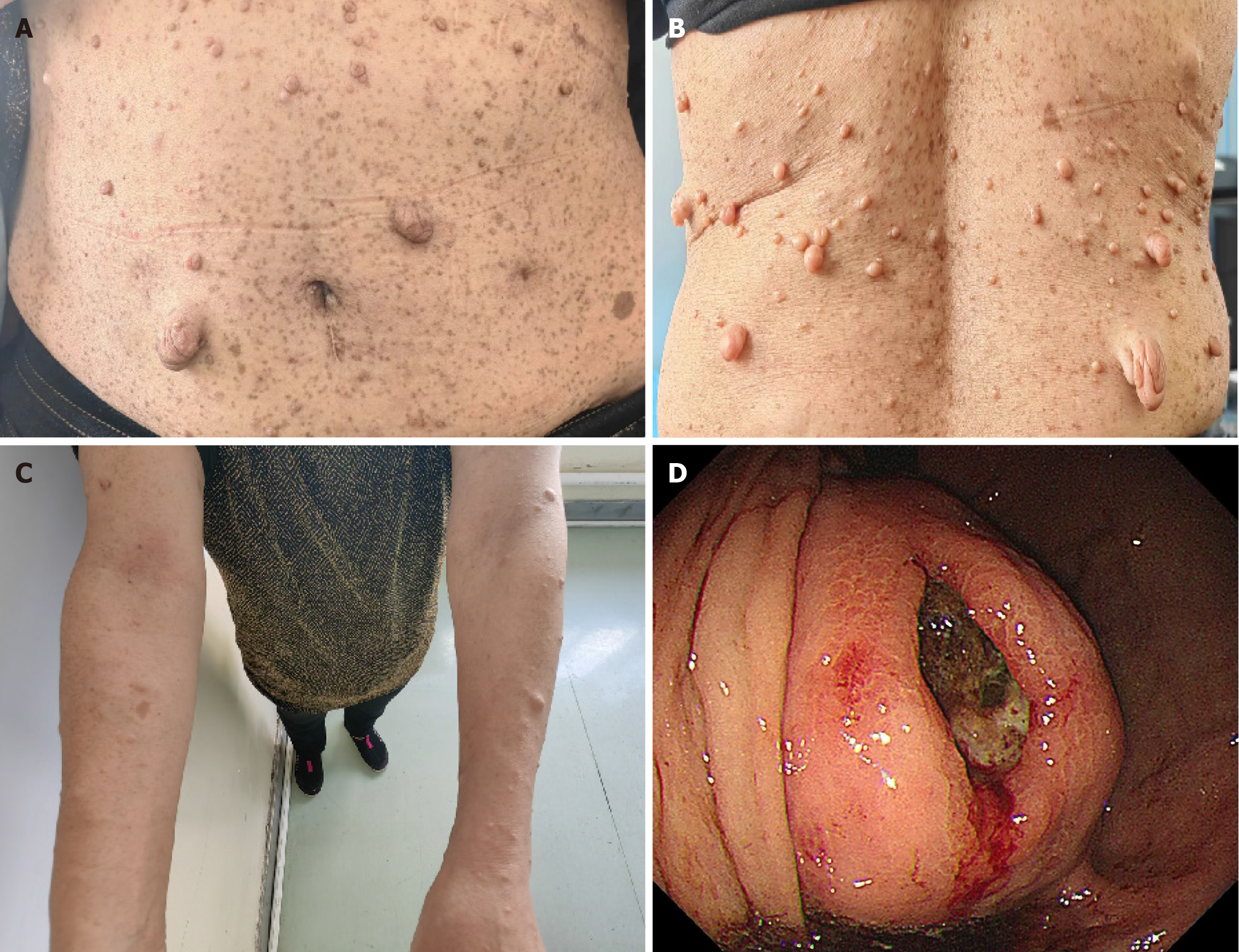
The patient was alert but exhibited poor mental status and a pale appearance, without cyanosis or jaundice of the skin or mucous membrane. Scattered, coffee-colored spots of varying sizes were observed, which were not raised above the skin surface, with lentil-sized raised points that matched the skin color and had a soft texture present on the face, trunk, and limbs (Figure 2A-C). The abdomen was distended, with a large palpable mass approximately 18 cm × 14 cm in the left upper abdomen. There was slight tenderness in the left abdomen, but no rebound tenderness, visible dilated veins on the abdominal wall, or intestinal peristalsis waves. The liver and spleen were not palpable, and Murphy’s sign was negative.
An urgent complete blood count revealed the following results: Red blood cell count 1.99 × 1012/L and hemoglobin 43 g/L. A blood transfusion was administered as symptomatic treatment to stabilize the patient’s condition.
Gastroscopy revealed a substantial volume of coffee-colored liquid in the gastric cavity. After rinsing, a large submucosal elevation was observed at the stomach fundus, covered with dirt-like deposits and exhibiting ulcer formation (Figure 2D). Chest and abdominal computed tomography (CT) scans (both plain and enhanced) revealed a large dense soft tissue lesion in the splenic gastric recess, measuring approximately 18 cm × 14 cm. The lesion had indistinct boundaries with the gastric wall, compressing the adjacent stomach and spleen, and communicated locally with the gastric cavity, exhibiting ulcer changes in the gastric recess occupying lesion. The enhanced scan shows heterogeneous enhancement, suggesting an interstitial tumor (Figure 3A). Additionally, multiple soft tissue nodules were present in the abdominal wall (Figure 3B and C).
On reviewing the patient’s medical history, she had a past diagnosis of neurofibromatosis and multiple CALMs on her body. The patient was admitted for weakness and dizziness due to anemia. During hospitalization, she underwent surgery for GIST. Postoperatively, pathology indicated a GIST (spindle cell type), and genetic testing confirmed the diagnosis of NF1.
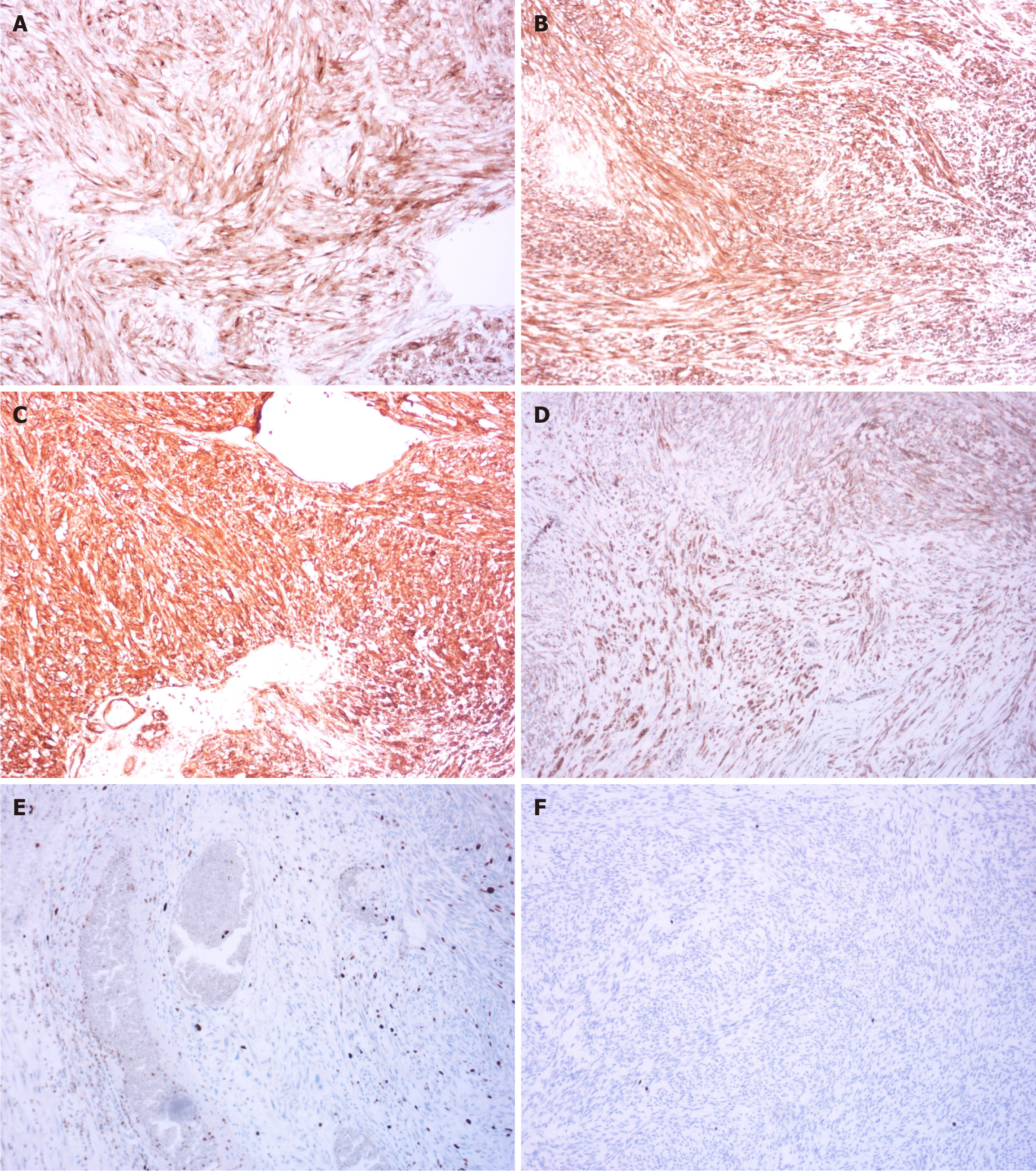
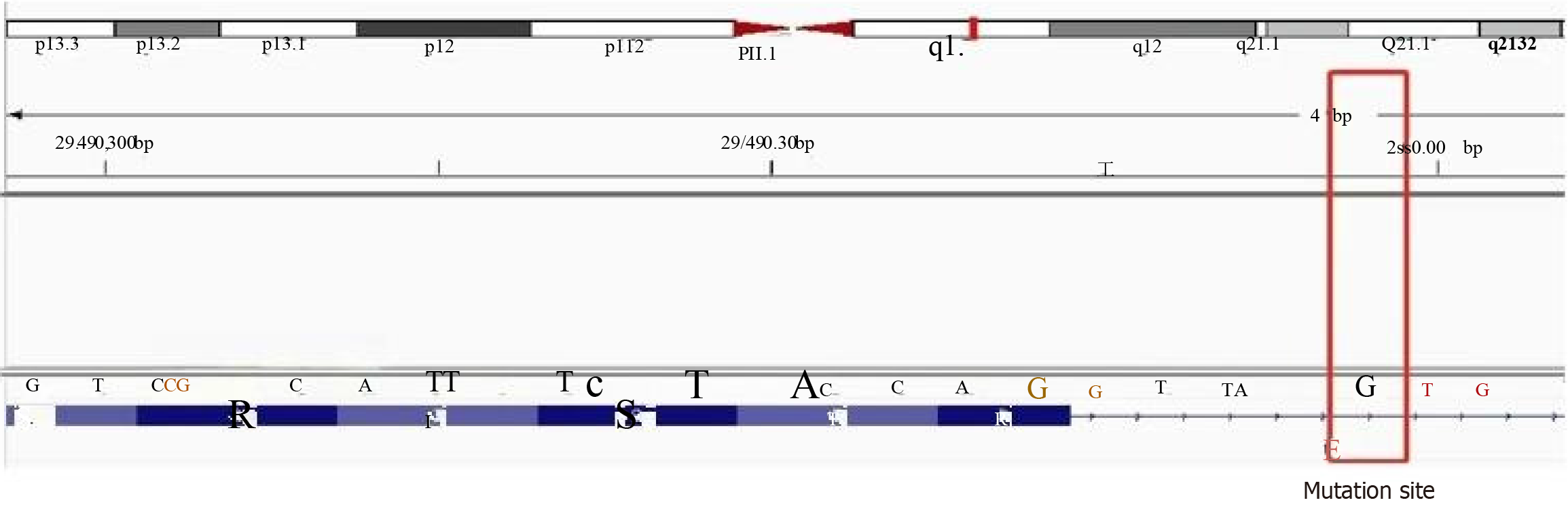
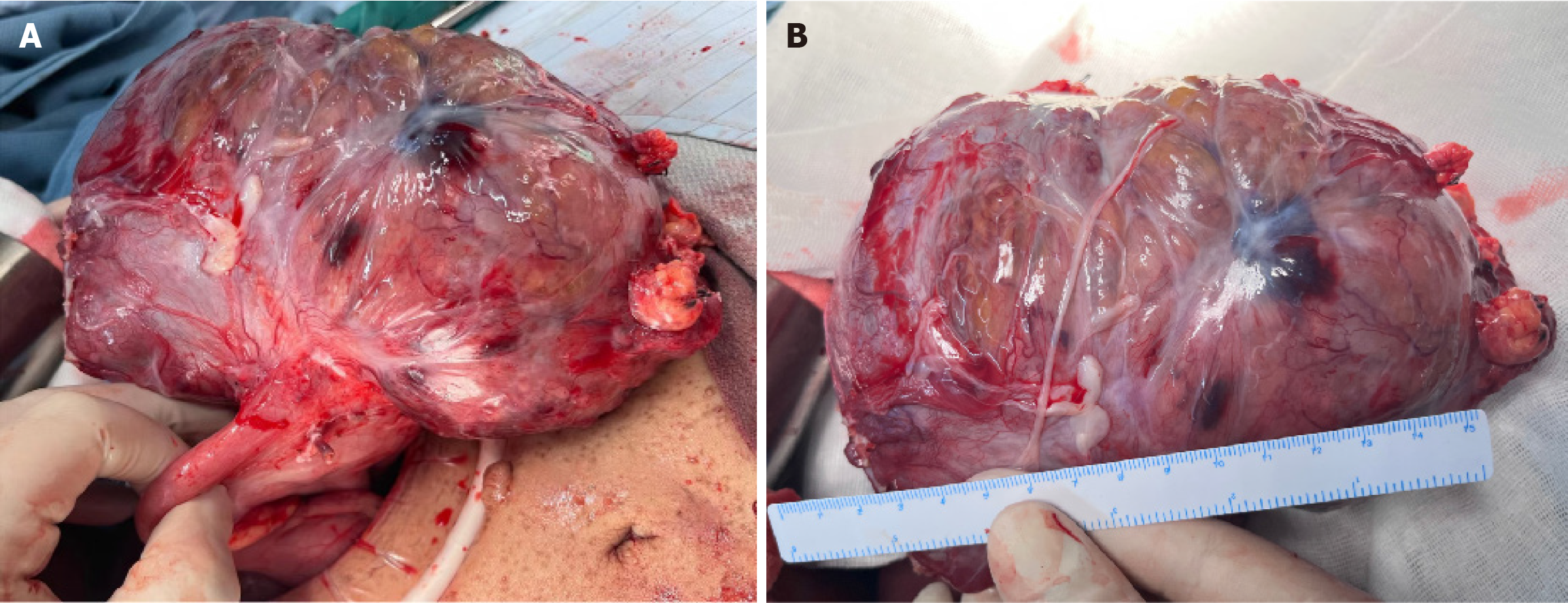
After multidisciplinary consultation, the patient underwent laparoscopic exploratory surgery, which confirmed that the tumor originated from the stomach fundus, leading to resection of a large GIST. Pathological results showed a GIST with necrosis, measuring 18 cm × 14 cm × 11 cm, categorized as high risk, with approximately 5 mitoses per 10 high-power fields. No tumor was found at the surgical margins. Immunohistochemistry results revealed positivity for CD117, delay of germination 1, CD34, succinate dehydrogenase complex iron sulfur subunit B, Ki-67 (5%), and phosphorylated histone H3 (< 1%) (Figure 4). Subsequently, next-generation sequencing genetic testing detected an NM_001042492.3 NF1: c.479+5G>A: p.? variant, not found in the 1000G, ExAC, gnomAD, and iJGVD databases, but documented as pathogenic in the ClinVar database (ID = 565507, with a rating of two stars) (Figure 5). Blood RNA analysis indicated that this variant caused a frameshift in exon 4, resulting in a premature stop codon (p.Gln97Valfs*13), supporting its pathogenic nature.
The patient was discharged on the 7th day after surgery without any complications. Following a definitive diagnosis, the patient received oral regorafenib as adjuvant therapy, which was discontinued after six months. Twelve months later, follow-up examinations showed no signs of tumor recurrence or metastasis (Figure 3D). The patient is scheduled to undergo annual follow-up evaluations with abdominal CT, magnetic resonance imaging, and gastrointestinal endoscopy.
NF1 is an autosomal dominant genetic disorder caused by pathogenic variants in the NF1 gene, which encodes the neurofibromin protein, located on chromosome 17q11.2. The term “neurofibromas” was first introduced by von Recklinghausen in 1882, leading to the alternative name for the disease, von Recklinghausen’s disease. CALMs and neurofibromas are hallmark symptoms of NF1, while GISTs represent a rare complication. Individuals with NF1 typically exhibit CALMs on their skin, appearing as brownish circular, oval, or irregular patches with clear boundaries, ranging in diameter from a few millimeters to several centimeters. The color intensifies during childhood and gradually lightens in adulthood. CALMs can appear on any skin surface except the palms and soles, and are commonly found on the trunk, buttocks, and lower limbs, with less frequent facial involvement. In NF1 patients, CALMs are generally concentrated on the trunk and vary in size from a few millimeters to several centimeters (Figure 2A and B). The more typical the appea
Patients with NF1 often exhibit benign tumors originating from peripheral nerve sheaths. These tumors appear as multiple rubbery, flesh-colored, soft nodules, or subcutaneous lumps. They are slightly prominent, dome-shaped, easily compressible, and have palpable borders, ranging from 1 to 2 cm in diameter, and are randomly distributed throughout the body. Neurofibromas are more common on the torso and limbs (Figure 2A-C). On examination, subcutaneous lumps with a rubbery texture, well-defined borders, consistent elasticity, and tenderness upon compression can be felt. Deeper subcutaneous lesions are more challenging to palpate and require imaging for identification. CT scans revealed multiple soft tissue nodules on the abdominal wall, some on the skin surface and others beneath the skin (Figure 3B and C). Nodules in this area can be mistaken for abdominal metastases, necessitating careful differentiation. Additionally, the patient experienced skin itching, possibly related to significant infiltration of mast cells[3].
GISTs are caused by mutations in KIT and platelet derived growth factor receptor alpha (PDGFRA) in approximately 90% of cases, with a small number of wild-type GISTs being associated with NF1. NF1-related GISTs are linked to inactivation of the NF1 allele gene, with KIT and PDGFRA mutations typically absent[5]. The incidence of GISTs in NF1 patients is 7%, which is 45 times higher than that in the general population[6]. In NF1-related GISTs, tumors are more commonly found in the small intestine (90%) and are rarely located in the stomach[7]. In this patient, there was a large intra-abdominal tumor located between the stomach and spleen with unclear boundaries, causing compression of the stomach and spleen. The tumor communicated locally with the stomach cavity, showing ulcerative changes and heterogeneous enhancement on imaging (Figure 3A). During surgery, the tumor was observed to originate from the stomach wall, with anemia accompanying the ulceration caused by the GIST (Figure 6). Pathological examination post-surgery revealed positivity for CD117, delay of germination 1, and CD34 (Figure 4A-C), aiding in diagnosis of the disease[8]. The location, size, and mitotic activity of the tumor are prognostic factors for GISTs. The Ki-67 index is associated with recurrence and survival[9]. NF1-related GISTs have a better prognosis compared to sporadic ones[7]. Genetic testing in this patient revealed a mutation at intron 4 c.479+5G>A NM_001042492.3, leading to exon 4 skipping and the introduction of a stop codon (p.Gln97Valfs*13), resulting in NF1 protein inactivation (Figure 5). NF1 protein stabilizes the guanosine-triphosphate hydrolase activity of the RAS protein, leading to activation of the downstream RAS/RAF/MEK pathway and tumor formation[10,11]. The mutation rate of the NF1 gene is high, and there is no mutation hotspot[12]. Mutations can be located in the entire coding and non-coding regions, including different types of variations[13]. Even small changes in gene copy number can involve deletions, duplications, frameshifts, splicing, nonsense, missense, chro
Due to the absence of KIT and PDGFRA mutations in NF1-related GIST, imatinib is ineffective for these patients[17]. The European Society for Medical Oncology guidelines advise against the use of imatinib for treating NF1-GIST[18]. Currently, no standard treatment strategy exists for this disease, and only case reports have noted the effectiveness of regorafenib in NF1-associated GIST[19]. The molecular mechanism of regorafenib’s antitumor effect on NF1-GIST remains unclear, but it may be linked to its role as a multi-kinase inhibitor that can inhibit RAF-1, BRAF, and members of the vascular endothelial growth factor receptor family. The patient received oral regorafenib for six months post-discharge, and no recurrence was observed after one year of follow-up. However, its long-term efficacy requires further study. Given the central role of the RAS signaling pathway in NF1, small molecule inhibitors have become a focus of clinical research[16]. Selumetinib, a small molecule MEK1/2 inhibitor, has shown promising results in treating NF1-related neurofibromas, and its efficacy in NF1-associated GIST warrants further validation[20].
Due to the diversity of NF1 clinical symptoms and the complexity of its association with GISTs, clinicians continue to face significant challenges.
| 1. | Kresak JL, Walsh M. Neurofibromatosis: A Review of NF1, NF2, and Schwannomatosis. J Pediatr Genet. 2016;5:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 2. | Uusitalo E, Leppävirta J, Koffert A, Suominen S, Vahtera J, Vahlberg T, Pöyhönen M, Peltonen J, Peltonen S. Incidence and mortality of neurofibromatosis: a total population study in Finland. J Invest Dermatol. 2015;135:904-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 3. | Hernández-Martín A, Duat-Rodríguez A. An Update on Neurofibromatosis Type 1: Not Just Café-au-Lait Spots, Freckling, and Neurofibromas. An Update. Part I. Dermatological Clinical Criteria Diagnostic of the Disease. Actas Dermosifiliogr. 2016;107:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Shah KN. The diagnostic and clinical significance of café-au-lait macules. Pediatr Clin North Am. 2010;57:1131-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Takazawa Y, Sakurai S, Sakuma Y, Ikeda T, Yamaguchi J, Hashizume Y, Yokoyama S, Motegi A, Fukayama M. Gastrointestinal stromal tumors of neurofibromatosis type I (von Recklinghausen's disease). Am J Surg Pathol. 2005;29:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 6. | Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 326] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Nishida T, Tsujimoto M, Takahashi T, Hirota S, Blay JY, Wataya-Kaneda M. Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J Gastroenterol. 2016;51:571-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Pan D, Liang P, Xiao H. Neurofibromatosis type 1 associated with pheochromocytoma and gastrointestinal stromal tumors: A case report and literature review. Oncol Lett. 2016;12:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Mandalà S, Lupo M, Guccione M, La Barbera C, Iadicola D, Mirabella A. Small bowel gastrointestinal stromal tumor presenting with gastrointestinal bleeding in patient with type 1 Neurofibromatosis: Management and laparoscopic treatment. Case report and review of the literature. Int J Surg Case Rep. 2021;79:84-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 11. | Tamura R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int J Mol Sci. 2021;22:5850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 12. | Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1137] [Cited by in RCA: 1063] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 13. | Kehrer-Sawatzki H, Mautner VF, Cooper DN. Emerging genotype-phenotype relationships in patients with large NF1 deletions. Hum Genet. 2017;136:349-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 14. | Hsiao MC, Piotrowski A, Callens T, Fu C, Wimmer K, Claes KB, Messiaen L. Decoding NF1 Intragenic Copy-Number Variations. Am J Hum Genet. 2015;97:238-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | van Minkelen R, van Bever Y, Kromosoeto JN, Withagen-Hermans CJ, Nieuwlaat A, Halley DJ, van den Ouweland AM. A clinical and genetic overview of 18 years neurofibromatosis type 1 molecular diagnostics in the Netherlands. Clin Genet. 2014;85:318-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Primers. 2017;3:17004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 547] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 17. | von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol. 2018;36:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 228] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 18. | ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii21-iii26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 19. | Fujimi A, Nagamachi Y, Yamauchi N, Tamura F, Kimura T, Miyajima N, Inomata H, Nishisato T, Yoshida M, Takada K, Kobayashi K, Kato J. Gastrointestinal Stromal Tumor in a Patient with Neurofibromatosis Type 1 That Was Successfully Treated with Regorafenib. Intern Med. 2019;58:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Armstrong AE, Belzberg AJ, Crawford JR, Hirbe AC, Wang ZJ. Treatment decisions and the use of MEK inhibitors for children with neurofibromatosis type 1-related plexiform neurofibromas. BMC Cancer. 2023;23:553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |