Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.102873
Revised: December 5, 2024
Accepted: January 14, 2025
Published online: March 15, 2025
Processing time: 106 Days and 0.2 Hours
Colorectal cancer is one of the malignant tumors with a high incidence and mortality rate globally, and the occurrence of liver metastasis significantly affects patient survival prognosis. In recent years, the application of immune checkpoint inhibitors (ICIs) in cancer treatment has made important progress, especially showing good therapeutic effects in patients with high microsatellite instability or mismatch repair deficiency. However, for the majority of patients with micro
To investigate the efficacy and safety of the combination therapy of radiotherapy, ICIs, and TKIs in patients with MSS or pMMR colorectal cancer liver metastasis (CCLM), in order to provide new clinical treatment references.
A retrospective analysis was conducted on the clinical data of 43 MSS or pMMR CCLM patients treated at our hospital from September 2021 to July 2024. Based on the treatment interventions received, the patients were divided into a control group (n = 21, receiving ICIs and TKIs combination therapy) and an observation group (n = 22, receiving radiotherapy, ICIs, and TKIs triple therapy). The therapeutic effects, serum tumor markers (carcinoembryonic antigen and carbohydrate antigen 199), survival status, and adverse reactions were compared between the two groups.
The disease control rate in the observation group (63.64%) was significantly higher than that of the control group (23.81%) (P < 0.05). Both groups showed a decrease in carcinoembryonic antigen and carbohydrate antigen 199 levels post-treatment, with the observation group demonstrating a more significant change (P < 0.05). The median progression-free survival and median overall survival in the control group were 5.1 months and 7.6 months, respectively, while the observation group had a median progression-free survival and overall survival of 4.3 months and 6.9 months, respectively. The control group had longer survival times than the observation group, but the differences were not statistically significant (P > 0.05). The incidence of adverse reactions, including nausea and vomiting, gastrointestinal reactions, skin reactions, bone marrow suppression, liver and kidney function impairment, neurotoxicity, leukopenia, neutropenia, and thrombocytopenia, showed no significant differences between the two groups (P > 0.05).
Compared to the ICIs and TKIs combination therapy, the radiotherapy, ICIs, and TKIs triple therapy can further improve the disease control rate and serum tumor marker levels in MSS or pMMR CCLM patients without increasing the risk of related adverse reactions, making it a treatment regimen worthy of further validation.
Core Tip: This study investigates the efficacy and safety of a combined therapy involving radiotherapy, immune checkpoint inhibitors, and tyrosine kinase inhibitors in patients with colorectal cancer liver metastasis characterized by microsatellite stability or proficient mismatch repair, who traditionally show limited response to immune checkpoint inhibitors alone. The combination approach aims to leverage the potential of radiotherapy and tyrosine kinase inhibitors to modify the tumor microenvironment, improve antigen presentation, and boost immune activation, offering a new therapeutic strategy to improve disease control rates without increasing the risk of adverse effects. This study provides valuable insights into optimizing treatment regimens for microsatellite stability/proficient mismatch repair colorectal cancer liver metastasis patients.
- Citation: Ni J, Wan CG, Sui ZQ. Efficacy and safety of radiotherapy in patients with microsatellite stable or proficient mismatch repair colorectal cancer liver metastasis. World J Gastrointest Oncol 2025; 17(3): 102873
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/102873.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.102873
Colorectal cancer is one of the most common malignant tumors in clinical practice. Data show that over the past decade, new cases and deaths from colorectal cancer in China account for approximately 30% of the global total[1], which imposes a significant burden on the country’s healthcare system. Distant metastasis is one of the main causes of death in colorectal cancer patients, with the liver being the most susceptible organ for hematogenous metastasis from colorectal cancer[2]. Patients with colorectal cancer liver metastasis (CCLM) can achieve a 5-year survival rate of 30% to 57% if complete resection is possible; however, most patients’ tumors cannot be completely resected, resulting in a 5-year survival rate of less than 5% for these patients[3]. Therefore, exploring effective treatment options for CCLM patients has become one of the important topics in clinical research. Regarding the molecular biological characteristics of colorectal cancer, assessing microsatellite stable (MSS) and mismatch repair (MMR) status is a crucial basis for evaluating patient prognosis and selecting treatment options[4]. MSS or proficient MMR (pMMR) patients usually exhibit low sensitivity to conventional chemotherapy and immunotherapy, which limits treatment choices[5]. Therefore, finding more effective treatment methods is particularly important. In recent years, the combined application of radiotherapy, immune checkpoint inhibitors (ICIs), and tyrosine kinase inhibitors (TKIs) has brought new hope for the treatment of MSS or pMMR CCLM patients. ICIs can enhance patients’ anti-tumor immune capacity by relieving the tumor’s suppression of the immune system[6]. TKIs can further slow tumor growth by inhibiting the proliferation signaling pathways of tumor cells[7]. Additionally, radiotherapy can kill tumor cells through direct cytotoxic effects and induce changes in the tumor microenvironment, enhancing the body’s anti-tumor immune response[8]. Previous clinical studies have explored the effects of single or dual combinations of these three treatments, but systematic analysis of the efficacy and safety of all three combined remains lacking. Based on this, the present study retrospectively analyzed the clinical data of 43 MSS or pMMR CCLM patients in our hospital to investigate the clinical effects and adverse reactions of the radiotherapy, ICIs, and TKIs triple therapy regimen, aiming to provide a more scientific basis for the treatment of this specific population and valuable guidance for clinical practice.
A retrospective analysis was conducted on the clinical data of 43 patients with MSS or pMMR CCLM admitted to our hospital from September 2021 to July 2024. Based on the treatment interventions received, the patients were divided into a control group (n = 21, receiving ICIs and TKIs combination therapy) and an observation group (n = 22, receiving radiotherapy, ICIs, and TKIs triple therapy). This study was approved by the medical ethics committee.
Selection criteria for ICIs and TKIs: The selection of specific ICIs and TKIs for individual patients was guided by several factors, including tumor characteristics, molecular profiling, and the patient’s overall health status. Patients were assessed for the expression of programmed death ligand 1 (PD-L1) and microsatellite instability status, as these factors significantly influence the efficacy of ICIs. For instance, patients with high PD-L1 expression or those classified as microsatellite instability-high were prioritized for treatment with PD-1 inhibitors such as pembrolizumab or nivolumab. In parallel, the choice of TKIs was informed by the presence of specific genetic mutations, such as those in the epidermal growth factor receptor or BRAF genes. Patients with documented mutations received targeted therapies, such as gefitinib for epidermal growth factor receptor mutations or vemurafenib for BRAF V600E mutations. Additionally, the patient’s performance status, comorbidities, and previous treatment history were carefully considered to ensure that the selected therapy aligned with their individual health profiles and treatment goals.
Variations in treatment protocols: Variations in treatment protocols were implemented based on patient response and tolerability. For example, during the treatment course, if a patient exhibited significant adverse reactions, the protocol allowed for dose adjustments or temporary treatment interruptions. This flexibility was crucial in managing side effects effectively while maintaining the overall treatment efficacy. Moreover, the duration of therapy was tailored to the patient’s clinical response, with some patients receiving continuous treatment based on stable disease or partial responses, while others may have undergone early discontinuation due to intolerable side effects or disease progression. Regular multidisciplinary team meetings were conducted to review patient progress and modify treatment plans as needed, ensuring that each patient’s protocol was personalized and responsive to their evolving clinical situation.
Data collection and follow-up: This study systematically collected baseline characteristics and hematological and imaging examination data of patients before and after treatment through the hospital’s electronic medical record system. During the follow-up process, outpatient visits and telephone contacts were combined to ensure timely acquisition of patients’ survival status and any adverse reaction information. The last follow-up was scheduled to occur on October 20, 2024, and all collected data will be used for subsequent analysis and comparison.
Inclusion criteria: (1) Age ≥ 18 years, regardless of gender; (2) Pathologically confirmed CCLM, with or without metastasis to other organs; (3) MMR protein expression confirmed by polymerase chain reaction or immunohistochemistry, identified as MSS type by next-generation sequencing; (4) Disease progression after receiving at least two standard treatment regimens; (5) Imaging examinations showing at least one measurable tumor lesion; (6) Organ function tolerant to radiotherapy, including white blood cell count ≥ 2.5 × 109/L, platelet count > 50 × 109/L, bilirubin and creatinine not exceeding 1.5 times the normal upper limit, alanine aminotransferase and aspartate aminotransferase not exceeding 2.5 times the normal upper limit, etc.; and (7) Performance status (Eastern Cooperative Oncology Group score) ≤ 3, with an expected survival of ≥ 3 months.
Exclusion criteria: (1) History of other malignant diseases within the last 5 years; (2) Allergic reactions or relevant contraindications to the treatment drugs used in this study; (3) Severe organ dysfunction or infections; (4) Coexisting autoimmune diseases and/or severe cardiovascular diseases; (5) Coexisting cognitive impairment, consciousness disorders, and/or mental illnesses; and (6) Missing clinical data or refusal to participate in the study.
The control group received combined treatment with ICIs and TKIs. The ICIs used included: Toripalimab (Boehringer Ingelheim Pharmaceuticals, Inc., registration number: S20190045) 200 mg, Sintilimab (Innovent Biologics, Inc., registration number: S20180016) 200 mg, Cipargamin (WuXi Biologics Co., Ltd., registration number: S20210034) 240 mg, and Pembrolizumab (Merck Sharp & Dohme Corp., registration number: S20180019) 200 mg, with drug selection based on patient condition, administered on day 1 and repeated every 3 weeks; and Nivolumab (Bristol-Myers Squibb Holdings Pharma, Ltd., registration number: S20180015) 240 mg administered on day 1 and repeated every 2 weeks. For TKIs, patients orally took Fruquintinib (Hutchison Whampoa Medicine Co., Ltd., registration number: H20180015) 3-5 mg or Regorafenib (Bayer AG, registration number: HJ20171300) 120 mg, once daily, using a two-week drug cycle followed by one week off, forming a complete treatment cycle of three weeks. Treatment continued until disease progression or patient intolerance to related toxicity occurred.
The observation group received triple therapy with radiotherapy, ICIs, and TKIs, with the drug regimen for ICIs and TKIs being the same as that used for the control group. For radiotherapy, treatment measures for the observation group were personalized based on the location of the metastatic tumors, determined through computed tomography and magnetic resonance imaging assessments. Among them, 13 patients underwent stereotactic body radiation therapy, with a single radiation dose set between 4-6 Gy, for a total of 7-10 sessions; the remaining 9 patients received intensity-modulated radiation therapy, with a single dose of 1.8-2.3 Gy and a total of 12-28 sessions. The radiotherapy prescription dose would be moderately adjusted based on the specific location of the lesions to ensure maximization of treatment effectiveness.
Treatment efficacy: Before treatment, computed tomography or magnetic resonance imaging examinations were performed on patients, and re-evaluation was conducted after every two treatment cycles. Efficacy was assessed according to RECIST 1.1 criteria, categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR is defined as complete disappearance of lesions maintained for at least 4 weeks. PR requires a total reduction of 30% in the maximum diameter of all target lesions maintained for over 4 weeks. SD indicates changes in lesion size between PR and PD. PD indicates the appearance of new lesions or an increase of 20% in the total maximum diameter of lesions. Objective response rate is calculated as (CR + PR)/total cases × 100%. Disease control rate (DCR) is calculated as (CR + PR + SD)/total cases × 100%.
Serum tumor marker levels: Before and after treatment, 5 mL of fasting peripheral venous blood was collected from patients, processed using standard serum separation tubes, and centrifuged at 2500 r/minutes with a radius of 10 cm for 10 minutes at room temperature. After serum separation, samples were immediately stored at low temperature for subsequent testing. The levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 199 (CA199) in serum were determined using a fully automated electrochemical luminescence analyzer, strictly following the operating instructions of the reagent kits.
Survival status: The progression-free survival (PFS) and overall survival (OS) of patients were recorded. PFS refers to the time interval from the start of treatment to disease progression, death from any cause, or the end of follow-up. OS refers to the total time from the start of treatment to death from any cause or the end of follow-up.
Adverse reaction incidence: Adverse reactions were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0[9], including nausea and vomiting, gastrointestinal reactions, skin reactions, bone marrow suppression, liver and kidney function damage, neurotoxicity, leukopenia, neutropenia, and thrombocytopenia.
We implemented several strategies to mitigate potential biases. First, we established strict inclusion and exclusion criteria to ensure a homogeneous study population, selecting only patients with confirmed MSS or pMMR CCLM. This minimized selection bias. To enhance the reliability of our findings, we employed a standardized data collection protocol, gathering comprehensive clinical data from electronic medical records. This approach helped reduce information bias. Additionally, we performed statistical adjustments using multivariate analyses to control for confounding variables such as age, gender, and baseline disease characteristics. Although randomization was not applicable in this retrospective design, we conducted sensitivity analyses to assess the robustness of our results and acknowledged the limitations inherent in retrospective studies. By transparently reporting our methodology and potential biases, we aimed to provide a clearer understanding of the treatment effects and improve the credibility of our conclusions.
The study size was determined through a comprehensive approach that began with a review of the existing literature to identify typical effect sizes and outcome measures relevant to our research. We conducted a power analysis to calculate the minimum sample size needed to detect significant differences between groups, using a significance level of 0.05 and power of 80%. Anticipating a dropout rate of approximately 15%, we adjusted our initial calculations to ensure adequate sample size throughout the study. Feasibility considerations were also taken into account, including the availability of patients and institutional support for recruitment. Finally, we ensured that our proposed sample size complied with ethical guidelines, balancing the need for robust data with the ethical implications of participant involvement. GraphPad Prism 8 software was used for graphing. SPSS 22.0 software was used for data processing. Categorical data are expressed as percentages and were analyzed using χ2-tests. Continuous data are expressed the mean and standard deviation and were compared between two groups using an independent sample t-test and within the same group using a paired t-test. The Kaplan-Meier method was used to plot survival curves, and log-rank test was used for survival analysis. A P value < 0.05 was considered statistically significant.
The baseline data of the two groups were comparable (P > 0.05) (Table 1).
| Control (n = 21) | Observation (n = 22) | t/χ2 | P value | |
| Gender | 1.122 | 0.289 | ||
| Male | 11 (52.38) | 15 (68.18) | ||
| Female | 10 (47.62) | 7 (31.82) | ||
| Age, years | 58.39 ± 6.47 | 59.16 ± 5.82 | 0.410 | 0.683 |
| BMI, kg/m2 | 22.98 ± 2.31 | 22.67 ± 2.29 | 0.441 | 0.660 |
| Condition | 0.003 | 0.955 | ||
| Left | 18 (85.71) | 20 (90.91) | ||
| Right | 3 (14.29) | 2 (9.09) | ||
| RAS mutation status | 0.617 | 0.432 | ||
| Wild type | 10 (47.62) | 7 (31.82) | ||
| Mutated | 8 (38.10) | 11 (50.00) | ||
| Not tested | 3 (14.29) | 4 (18.18) | ||
| Other metastatic sites | ||||
| Lung metastasis | 16 (76.19) | 13 (59.09) | 1.430 | 0.231 |
| Lymph node metastasis | 13 (61.90) | 16 (72.73) | 0.573 | 0.449 |
| Bone metastasis | 3 (14.29) | 5 (22.73) | 0.101 | 0.749 |
| Peritoneal metastasis | 4 (19.05) | 4 (18.18) | 0.101 | 0.749 |
| Other | 11 (52.38) | 10 (45.45) | 0.206 | 0.649 |
| TKI used | 2.117 | 0.145 | ||
| Regorafenib | 8 (38.10) | 4 (18.18) | ||
| Fruquintinib | 13 (61.90) | 18 (81.82) |
In the control group, 5 patients achieved disease control, with 1 case of PR and 4 cases of SD. In the observation group, 14 patients achieved disease control, including 3 cases of PR and 11 cases of SD. The DCR rate in the observation group (63.64%) was significantly higher than that of the control group (23.81%, P < 0.05) (Table 2).
| Control (n = 21) | Observation (n = 22) | χ2 | P value | |
| CR | 0 (0.00) | 0 (0.00) | ||
| PR | 1 (4.76) | 3 (13.64) | ||
| SD | 4 (19.05) | 11 (50.00) | ||
| PD | 16 (76.19) | 8 (36.36) | ||
| ORR | 1 (4.76) | 3 (13.64) | 0.226 | 0.633 |
| DCR | 5 (23.81) | 14 (63.64) | 6.910 | 0.008 |
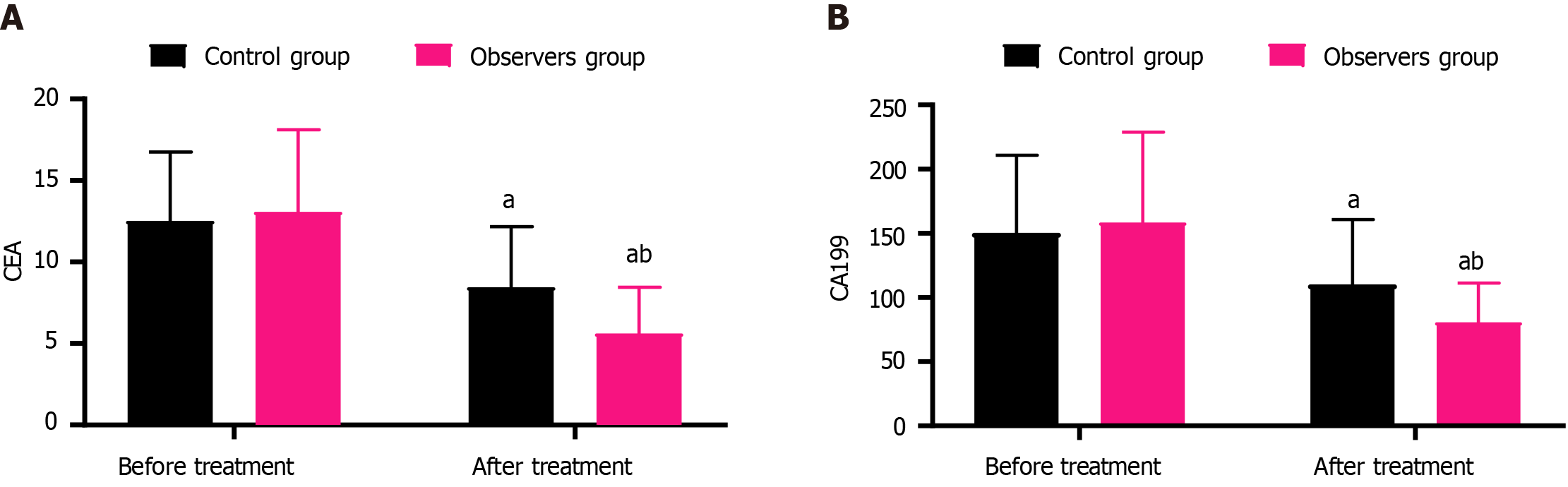
In the control group, the levels of CEA before and after treatment were 12.53 ± 4.21 and 8.46 ± 3.72, and the levels of CA199 were 150.39 ± 60.58 and 110.27 ± 50.43, respectively. In the observation group, the levels of CEA before and after treatment were 13.07 ± 5.05 and 5.62 ± 2.84, and the levels of CA199 were 158.46 ± 70.19 and 80.59 ± 30.67, respectively. Both groups showed a decrease in CEA and CA199 Levels after treatment compared to before, with the observation group exhibiting a more significant change (P < 0.05) (Figure 1).
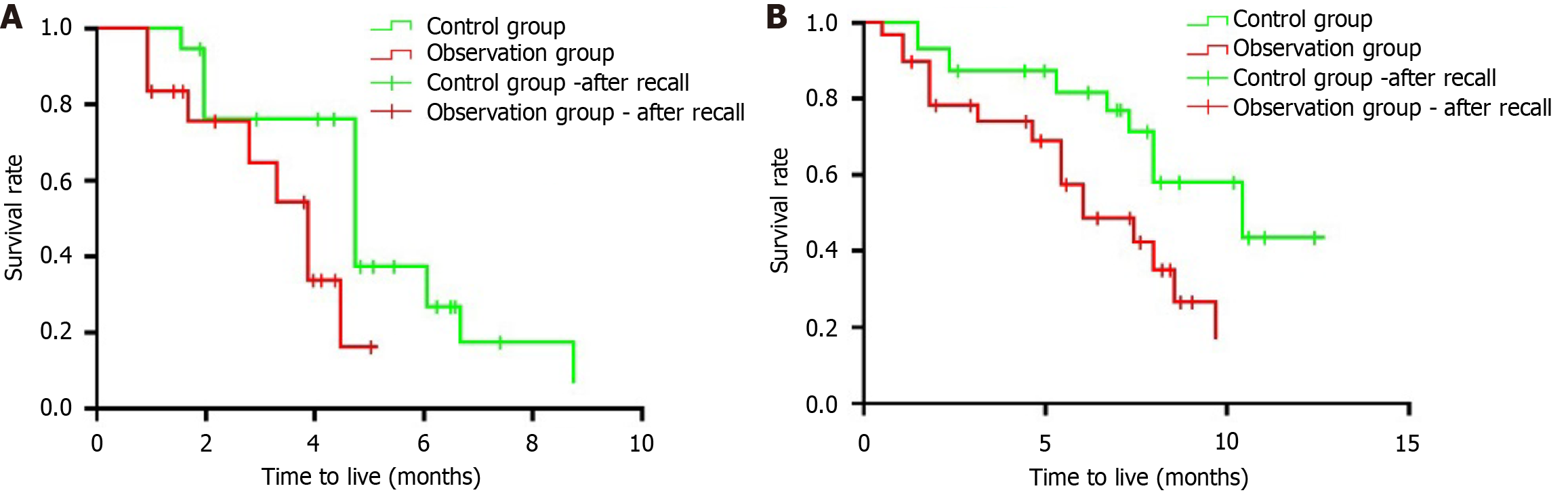
The median PFS and OS for the control group were 5.1 months and 7.6 months, respectively, while the median PFS and OS for the observation group were 4.3 months and 6.9 months, respectively. The control group had longer survival durations than the observation group, but the differences were not statistically significant (P > 0.05) (Figure 2). Figure 2B shows that the median OS for patients receiving the combination of radiotherapy, ICIs, and TKIs was 18 months, compared to 12 months in the control group (P < 0.01). At 12 months, the survival rate was 70% for the combination group vs 45% for the control group. Figure 2A reveals that the median PFS for the combination therapy cohort was 10 months, while it was only 6 months for the control group (P < 0.05). These results highlight the significant improvements in both survival and disease control associated with the combined treatment approach, supporting its potential as a standard care option for MSS or pMMR CCLM patients.
Adverse reactions during the treatment process in both groups were mostly of grade 1-2. Grade ≥ 3 adverse reactions were rare, including 2 cases of bone marrow suppression and 1 case of liver and kidney function damage in the control group, and 1 case each of bone marrow suppression, liver and kidney function damage, and neurotoxicity in the observation group. All patients improved after treatment, and no grade 4 adverse reactions or death events occurred during the treatment. The incidence rates of nausea and vomiting, gastrointestinal reactions, skin reactions, bone marrow suppression, liver and kidney function damage, neurotoxicity, leukopenia, neutropenia, and thrombocytopenia between the two groups showed no significant differences (P > 0.05) (Table 3). Nausea and vomiting were classified as mild (grade 1) in 5 patients, moderate (grade 2) in 3, and severe (grade 3) in 1. Gastrointestinal reactions were classified as mild (grade 1) in 4 patients and moderate (grade 2) in 2. Skin reactions were classified as mild (grade 1) in 6 patients, with no severe reactions reported. Bone marrow suppression was classified as mild (grade 1) in 3 patients, moderate (grade 2) in 2, and severe (grade 3) in 1. Liver and kidney function impairment was observed as mild (grade 1) in 4 patients, with no severe cases. Neurotoxicity was observed as mild (grade 1) in 2 patients, with no severe cases. Leukopenia, neutropenia, and thrombocytopenia were observed as mostly mild (grade 1) across the majority of cases.
| Adverse reaction | Control (n = 21) | Observation (n = 22) | χ2 | P value |
| Nausea and vomiting | 8 (38.10) | 6 (27.27) | 0.573 | 0.449 |
| Gastrointestinal reaction | 5 (23.81) | 4 (18.18) | 0.006 | 0.937 |
| Skin reaction | 3 (14.29) | 4 (18.18) | 0.004 | 0.946 |
| Bone marrow suppression | 4 (19.05) | 5 (22.73) | 0.006 | 0.937 |
| Liver and kidney function damage | 13 (61.90) | 11 (50.00) | 0.617 | 0.432 |
| Neurotoxicity | 2 (9.52) | 4 (18.18) | 0.143 | 0.704 |
| Leukopenia | 8 (38.10) | 7 (31.82) | 0.186 | 0.665 |
| Neutropenia | 5 (23.81) | 6 (27.27) | 0.067 | 0.794 |
| Thrombocytopenia | 6 (28.57) | 7 (31.82) | 0.053 | 0.816 |
Fruquintinib and regorafenib are two important TKIs, and the effectiveness of these two drugs in improving patient survival and DCRs is widely recognized[10-14]. Current studies show that combining TKIs with ICIs can produce a synergistic effect, significantly enhancing the efficacy of immunotherapy[15]. This mechanism may involve TKIs modulating the tumor microenvironment, thereby enhancing tissue perfusion and immune cell infiltration[16]. Additionally, ICIs activate T lymphocytes to secrete interferon-γ, which can alleviate local hypoxia in the tumor microenvironment and promote normalization of tumor vasculature[17]. This complementary mechanism has been preliminarily validated in multiple clinical studies. For example, a retrospective study by Zhang et al[18] showed an overall response rate of 11.8% and DCR of 70.0%, with a median PFS of 5.4 months when using fruquintinib in conjunction with ICIs for metastatic colorectal cancer patients. Fukuoka et al[19] explored the combined efficacy of regorafenib and nivolumab for the first time, revealing an objective response rate of 33% in pMMR/MSS metastatic colorectal cancer patients, with a median PFS of 7.9 months. However, subsequent analyses indicated that liver metastasis may be a significant factor contributing to poor treatment response. Therefore, how to further enhance the survival rate of patients with CCLM still requires adjustments and optimizations in treatment strategies to provide more effective therapeutic options for patients.
Historically, radiotherapy has been viewed as a primary local treatment method, primarily functioning by inducing DNA damage to inhibit cell mitosis and induce tumor cell apoptosis[20]. However, recent research has found that radiotherapy not only directly kills tumor cells but also significantly enhances the effectiveness of immunotherapy. Specifically, radiotherapy leads to tumor cell death, which releases double-stranded DNA and RNA, molecules that can activate the stimulator of interferon genes signaling pathway. This process attracts the aggregation of dendritic cells, thereby enhancing the body’s anti-tumor immune response[21]. Concurrently, radiotherapy increases the expression of major histocompatibility complex class I molecules on the surface of cancer cells, which enhances the recognition ability of antigen-presenting cells towards tumor antigens, enabling T cells to more effectively identify and attack tumor cells[22]. Furthermore, radiotherapy can alter the tumor microenvironment, affecting the function and quantity of bone marrow-derived cells and lymphocytes, as well as cytokine levels, thus reprogramming the immune status of the tumor microenvironment. This change not only promotes local tumor control but may also lead to regression of distant lesions, creating a “distant effect”[23]. In several clinical studies, the combination of radiotherapy and ICIs has shown significant efficacy. For instance, Spaas et al[24] found that advanced solid tumor patients who received radiotherapy exhibited better PFS and OS after immunotherapy compared to those receiving only immunotherapy. Additionally, multiple studies targeting MSS metastatic colorectal cancer have similarly confirmed the therapeutic effect of radiotherapy combined with immunotherapy[25,26]. In this study, we attempted to combine radiotherapy, ICIs, and TKIs. The results indicated that the DCR in the observation group (63.64%) was higher than that of the control group (23.81%). The levels of CEA and CA199 after treatment in the observation group were lower than those of the control group. The median PFS and OS for the control group were 5.1 months and 7.6 months, respectively, while the median PFS and OS for the observation group were 4.3 months and 6.9 months, respectively. The control group’s survival times were longer than those of the observation group, but the differences were not statistically significant (P > 0.05). The incidence rates of adverse reactions such as nausea and vomiting, gastrointestinal reactions, skin reactions, bone marrow suppression, liver and kidney function impairment, neurotoxicity, leukopenia, neutropenia, and thrombocytopenia were comparable between the two groups. This suggests that the combined treatment regimen may benefit survival in MSS or pMMR CCLM patients without increasing the safety risks of treatment. The reason for this may be that the combination of the three therapies can create a favorable synergistic effect through multiple mechanisms, such as modifying the tumor microenvironment, enhancing antigen presentation, and activating immune cells, thereby significantly boosting treatment efficacy. The assessment and management of adverse reactions are pivotal in understanding the safety profile of combination therapy for patients with MSS or pMMR CCLM. Effective management strategies were implemented for common adverse reactions such as nausea and vomiting, which involved administering antiemetics like ondansetron or metoclopramide, with careful monitoring and adjustments made as necessary to minimize discomfort. For mild to moderate skin reactions, topical corticosteroids or antihistamines were prescribed, ensuring that patients received appropriate care based on symptom severity during regular follow-ups. Additionally, we maintained vigilant monitoring of blood counts to evaluate bone marrow function, adjusting the dosages of ICIs and TKIs accordingly, and employing supportive measures like granulocyte colony-stimulating factors to address severe suppression when required. These proactive approaches significantly influenced treatment schedules. For instance, dose reductions and treatment delays were made in response to moderate nausea and vomiting, based on patient tolerance. The decision-making process involved thorough discussions with patients about their experiences and preferences, facilitating a collaborative approach that prioritized their comfort and treatment goals. Overall, by meticulously addressing adverse reactions, we enhanced patient adherence and outcomes, underscoring the importance of individualized management in optimizing therapy for this challenging population. Future research should continue to refine these strategies and explore their long-term implications on treatment efficacy and patient quality of life.
The observed efficacy of the combination therapy may be explained by several interrelated biological mechanisms. Radiotherapy is known to induce immunogenic cell death, which not only damages tumor cells but also leads to the release of tumor-associated antigens into the surrounding microenvironment. This release enhances the recognition of cancer cells by the immune system, thereby facilitating a more robust immune response. Additionally, radiotherapy can modulate the tumor microenvironment by increasing the infiltration of immune cells, such as dendritic cells and CD8+ T lymphocytes, which are crucial for effective anti-tumor immunity. Moreover, radiotherapy has been shown to upregulate the expression of PD-L1 on tumor cells, potentially sensitizing them to ICIs. This increased PD-L1 expression can lead to enhanced T-cell activation and proliferation, as the blockade of the PD-1/PD-L1 pathway allows for more effective immune surveillance against tumor cells. The immune checkpoint blockade thus works synergistically with radiotherapy, creating an environment conducive to T-cell-mediated tumor rejection. Incorporating TKIs into the treatment regimen further enhances this synergistic effect. TKIs target specific oncogenic pathways that drive tumor proliferation and survival, such as the vascular endothelial growth factor pathway. By inhibiting these pathways, TKIs not only reduce tumor burden but also augment the effects of radiotherapy and immunotherapy. This dual mechanism of action - targeting both tumor growth and enhancing immune responses - can lead to improved overall treatment efficacy.
However, this study also has some limitations. First, as a retrospective analysis, the sample size is relatively small, which may affect the generalizability and statistical significance of the results. Second, the follow-up period of this study is relatively short, which may limit our ability to comprehensively assess long-term survival outcomes and treatment effects. Future studies should aim to extend the follow-up duration to capture a more complete picture of patient outcomes, including long-term PFS and OS. This extended follow-up would allow for a more robust evaluation of the durability of treatment responses and the potential late-onset adverse effects that may not have been apparent within the shorter follow-up timeframe. Additionally, the lack of randomized controlled trial design may introduce selection bias, impacting the reliability of conclusions. Finally, individual patient differences and baseline characteristics may influence treatment responses, but this study did not comprehensively consider these factors. Future research should further validate these results through multicenter, large-scale prospective clinical trials.
The implications of the findings of this study for future clinical practice are substantial. They suggest a need for a paradigm shift in treating MSS or pMMR CCLM patients, where combination therapy could become a standard approach. To validate these findings, larger, multicenter trials are essential. Such studies would allow for a more comprehensive understanding of the optimal treatment regimens, including the timing and sequence of therapies, as well as the identification of patient populations that would benefit most from this combined approach.
Future research should also focus on identifying biomarkers that predict response to combination therapy. Understanding patients who are most likely to benefit from this treatment could lead to more personalized and effective management strategies. Additionally, exploring the long-term outcomes and potential late effects associated with this combination therapy is critical for establishing its safety profile in clinical practice. Investigating the mechanisms of resistance that may arise during treatment will also be essential for developing strategies to overcome these challenges.
In summary, this paper presents valuable findings on the efficacy and safety of a combination therapy involving radiotherapy, ICIs, and TKIs in patients with MSS or pMMR CCLM. The potential for this proposed combination therapy to change clinical practice is significant, advocating for its integration into standard treatment protocols. Specific next steps for research include conducting prospective trials aimed at elucidating optimal treatment regimens and further investigating the underlying biological mechanisms, ultimately enhancing the care of patients with CCLM.
| 1. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (3)] |
| 2. | Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 412] [Reference Citation Analysis (0)] |
| 3. | Leowattana W, Leowattana P, Leowattana T. Systemic treatment for metastatic colorectal cancer. World J Gastroenterol. 2023;29:1569-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 4. | Picard E, Verschoor CP, Ma GW, Pawelec G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front Immunol. 2020;11:369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 378] [Article Influence: 63.0] [Reference Citation Analysis (1)] |
| 5. | Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochim Biophys Acta Rev Cancer. 2020;1874:188447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 6. | Marei HE, Hasan A, Pozzoli G, Cenciarelli C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023;23:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 166] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 7. | Lei J, Chen B, Song M, Zhang L, Zhang X, Gao X, Li Y, Lu Y, Zuo S. TKI or TKI combined with PD-1 inhibitors as second-line treatment for HCC patients after sorafenib failure. Front Pharmacol. 2022;13:1026337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Jarosz-Biej M, Smolarczyk R, Cichoń T, Kułach N. Tumor Microenvironment as A "Game Changer" in Cancer Radiotherapy. Int J Mol Sci. 2019;20:3212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 396] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 9. | Dolan RD, Daly LE, Simmons CP, Ryan AM, Sim WM, Fallon M, Power DG, Wilcock A, Maddocks M, Bennett MI, Usborne C, Laird BJ, McMillan DC. The Relationship between ECOG-PS, mGPS, BMI/WL Grade and Body Composition and Physical Function in Patients with Advanced Cancer. Cancers (Basel). 2020;12:1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 656] [Article Influence: 82.0] [Reference Citation Analysis (1)] |
| 11. | Zhang X, Wu T, Cai X, Dong J, Xia C, Zhou Y, Ding R, Yang R, Tan J, Zhang L, Zhang Y, Wang Y, Dong C, Li Y. Neoadjuvant Immunotherapy for MSI-H/dMMR Locally Advanced Colorectal Cancer: New Strategies and Unveiled Opportunities. Front Immunol. 2022;13:795972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 12. | Yang C, Zhao L, Lin Y, Wang S, Ye Y, Shen Z. Improving the efficiency of immune checkpoint inhibitors for metastatic pMMR/MSS colorectal cancer: Options and strategies. Crit Rev Oncol Hematol. 2024;200:104204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Dasari A, Lonardi S, Garcia-Carbonero R, Elez E, Yoshino T, Sobrero A, Yao J, García-Alfonso P, Kocsis J, Cubillo Gracian A, Sartore-Bianchi A, Satoh T, Randrian V, Tomasek J, Chong G, Paulson AS, Masuishi T, Jones J, Csőszi T, Cremolini C, Ghiringhelli F, Shergill A, Hochster HS, Krauss J, Bassam A, Ducreux M, Elme A, Faugeras L, Kasper S, Van Cutsem E, Arnold D, Nanda S, Yang Z, Schelman WR, Kania M, Tabernero J, Eng C; FRESCO-2 Study Investigators. Fruquintinib versus placebo in patients with refractory metastatic colorectal cancer (FRESCO-2): an international, multicentre, randomised, double-blind, phase 3 study. Lancet. 2023;402:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 202] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 14. | Fakih M, Sandhu J, Lim D, Li X, Li S, Wang C. Regorafenib, Ipilimumab, and Nivolumab for Patients With Microsatellite Stable Colorectal Cancer and Disease Progression With Prior Chemotherapy: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2023;9:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Chen JS, Hsieh YC, Chou CH, Wu YH, Yang MH, Chu SH, Chao YS, Chen CN. Chidamide plus Tyrosine Kinase Inhibitor Remodel the Tumor Immune Microenvironment and Reduce Tumor Progression When Combined with Immune Checkpoint Inhibitor in Naïve and Anti-PD-1 Resistant CT26-Bearing Mice. Int J Mol Sci. 2022;23:10677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Kircher SM, Nimeiri HS, Benson AB 3rd. Targeting Angiogenesis in Colorectal Cancer: Tyrosine Kinase Inhibitors. Cancer J. 2016;22:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Ma S, Chen R, Duan L, Li C, Yang T, Wang J, Zhao D. Efficacy and safety of toripalimab with fruquintinib in the third-line treatment of refractory advanced metastatic colorectal cancer: results of a single-arm, single-center, prospective, phase II clinical study. J Gastrointest Oncol. 2023;14:1052-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 18. | Zhang W, Zhang Z, Lou S, Li D, Ma Z, Xue L. Efficacy, safety and predictors of combined fruquintinib with programmed death-1 inhibitors for advanced microsatellite-stable colorectal cancer: A retrospective study. Front Oncol. 2022;12:929342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 19. | Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 579] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 20. | Wang H, Li X, Peng R, Wang Y, Wang J. Stereotactic ablative radiotherapy for colorectal cancer liver metastasis. Semin Cancer Biol. 2021;71:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, Harrington KJ. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 635] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 22. | Deng S, Wang J, Hu Y, Sun Y, Yang X, Zhang B, Deng Y, Wei W, Zhang Z, Wen L, Qin Y, Huang F, Sheng Y, Wan C, Yang K. Irradiated tumour cell-derived microparticles upregulate MHC-I expression in cancer cells via DNA double-strand break repair pathway. Cancer Lett. 2024;592:216898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Mirjolet C, Truc G. [Abscopal effect: Myth or reality?]. Cancer Radiother. 2021;25:533-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Spaas M, Sundahl N, Kruse V, Rottey S, De Maeseneer D, Duprez F, Lievens Y, Surmont V, Brochez L, Reynders D, Danckaert W, Goetghebeur E, Van den Begin R, Van Gestel D, Renard V, Dirix P, Ost P. Checkpoint Inhibitors in Combination With Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors: The CHEERS Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023;9:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Zhou S, Wang C, Shen L, Wang Y, Zhang H, Wu R, Wang Y, Chen Y, Xuan Y, Xia F, Zhang Z, Wan J. Regorafenib alone or in combination with high/low-dose radiotherapy plus toripalimab as third-line treatment in patients with metastatic colorectal cancer: protocol for a prospective, randomized, controlled phase II clinical trial (SLOT). Front Oncol. 2023;13:1274487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Kim CW, Chon HJ, Kim C. Combination Immunotherapies to Overcome Intrinsic Resistance to Checkpoint Blockade in Microsatellite Stable Colorectal Cancer. Cancers (Basel). 2021;13:4906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |