Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.102187
Revised: November 20, 2024
Accepted: January 2, 2025
Published online: March 15, 2025
Processing time: 126 Days and 2.8 Hours
Liver cancer has a high incidence and mortality worldwide, especially in China. Herein, we investigated the therapeutic effect and mechanism of tetrahydrocurcumin against hepatocellular carcinoma (HCC), with a focus on the of phosphoinositide 3-kinases (PI3K)/AKT signaling pathway.
To investigate the effects and mechanism of tetrahydrocurcumin in HCC cell lines HepG2 and Huh7.
Using Metascape, we analyzed the potential targets of tetrahydrocurcumin in HCC. Molecular docking validation was performed using SYBYL2.0. Cell Counting Kit-8, wound healing, and transwell assays were performed to evaluate the effects of tetrahydrocurcumin on HepG2 and Huh7 cell migration, invasion, and apoptosis. The expression of PI3K/AKT signaling pathway-related proteins was detected by western blotting.
Network pharmacology and molecular docking showed that tetrahydrocurcumin has high binding affinity for phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha. In vitro experiments demonstrated that tetrahydrocurcumin suppressed the migration and invasion of liver cancer cells, promoted their apoptosis, and downregulated the expression of p-PI3K, p-AKT, and B cell leukemia/lymphoma 2, while upregulating caspase-3, p53, and B cell leukemia/lymphoma 2 associated X.
In summary, tetrahydrocurcumin suppresses PI3K/AKT signaling, promotes apoptosis, and prevents the migration and invasion of liver cancer cells.
Core Tip: In this comprehensive study, we delved into the anti-cancer potential of tetrahydrocurcumin, meticulously assessing its impact on the proliferation, migration, and invasion of liver cancer cell lines HepG2 and Huh7. Our findings revealed that tetrahydrocurcumin not only effectively inhibited the proliferation and migration of hepatocellular carcinoma cells but also significantly induced apoptosis through the phosphoinositide 3-kinases/AKT signaling pathway, underscoring its therapeutic potential in combating liver cancer.
- Citation: Bao ZC, Liu ZD, Zhang Y, Dai HJ, Jia H, Ren F, Li N, Zhao L, Wang YW, Lv SY, Zhang Y. To investigate the effect and mechanism of tetrahydrocurcumin on hepatocellular carcinoma based on phosphoinositide 3-kinases/AKT signaling pathway. World J Gastrointest Oncol 2025; 17(3): 102187
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/102187.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.102187
Liver cancer is among the most common malignant tumors, ranking fifth in global incidence and third in mortality[1]. Liver cancer includes primary and secondary liver tumors, with hepatocellular carcinoma (HCC) being the most common. An estimated 500000 new cases of HCC occur worldwide each year, with HCC being very common in developing countries. Further, half of these cases are from China[2,3]. Currently, surgical resection and liver transplantation are the main treatment methods. Despite these, HCC is associated with a high recurrence rate, surgical difficulty, lack of liver donors, and poor prognosis[4]. Therefore, non-surgical treatment of advanced liver cancer represents an important complementary approach. Anti-cancer compounds from traditional Chinese medicine (TCM) have multiple targets, minimal side effects, and moderate cost, thus providing a new direction for anti-tumor drug development. Therefore, research and development of TCM anti-tumor drugs hold significant value.
Since the 1990s, TCM anti-cancer drugs have gradually emerged as a novel direction in cancer research[5]. Studies have shown that the use of TCM alone or in combination with conventional chemotherapy and radiotherapy can enhance the immune response of patients with liver cancer, prolong survival, and improve quality of life[6]. The anticancer effects of curcumin have been widely reported. Research has found that the oral bioavailability of curcumin is low. However, curcumin still is effective, indicating that curcumin metabolites may be responsible for its pharmacological effects[7,8]. Tetrahydrocurcumin (THC), the main metabolite of curcumin in the body, is considered a valuable compound for cancer prevention and treatment because of its superior chemical stability, bioavailability, and high structural similarity to curcumin. Studies have shown that THC exhibits superior anti-angiogenic effects in vivo when compared to those of curcumin. In nude mice implanted with liver cancer cell lines, daily oral administration of THC significantly reduced microvessel density in tumor tissues. Furthermore, compared to higher doses of curcumin, the use of THC resulted in a greater reduction in tumor weight and ascites volume[9]. The superior anti-tumor effects of tetrahydrocurcumin have also been reported in other studies, but its mechanism remains unclear. Therefore, determining the precise molecular mechanisms of action of THC against liver cancer is of major significance for anti-liver cancer drug development.
The composition and mechanisms of action of natural products are complex. Screening for effective ingredients at low cost and high efficiency and elucidating their target genes have become challenges in drug development. Network pharmacology is based on the core concept of systems biology “multi-component-multi-target”[10]. It allows for analyzing the interaction network between drug components and disease-related genes, combined with molecular docking technology to simulate the molecular dynamics of binding[11,12]. In recent years, network pharmacology and molecular docking have been widely applied in the study of TCM. This approach has revealed the regulation of multiple signaling pathways by formulations, informing drug development and reducing costs[13].
In the present study, we analyzed the potential binding targets of THC in HCC using a network pharmacology approach and validating interactions through molecular docking. Subsequently, in vitro experiments were conducted to investigate the effects of THC on the proliferation, invasion, and migration of liver cancer cells. We show that THC suppressed the phosphoinositide 3-kinases (PI3K)/AKT signaling pathway to induce apoptosis and inhibit HCC growth. This study provides a basis for the development of novel and more effective anti-cancer drugs against HCC.
Screening of targets related to THC: We used network pharmacology analysis to determine potential targets of THC. First, we used “Tetrahydrocurcumin” as a search keyword, downloaded the 3-dimensional chemical structure of THC, saved in mol2 format, and uploaded it to SwissTargetPrediction (http://www.swisstargetprediction.ch)[14] for target prediction. After downloading the prediction results, we filtered these and used a “Probability*” value greater than 0 as the filtering criterion. Based on small differences between the selected candidate target proteins and known targets, an appropriate target was selected as the final target. In addition, we conducted a thorough investigation of the literature focusing on “Tetrahydrocurcumin-“ from the PubMed database (https://pubmed.ncbi.nlm.nih.gov) to obtain supplementary information on other potential targets of THC. Based on this, candidate compounds were analyzed and summarized. Finally, all target names were converted to their corresponding gene names using the UniProt database (http://www.uniprot.org). Subsequently, the structural and functional information of the target proteins were organized. Integration and deduplication of these targets were then performed.
Collection and screening of targets: Using the Genecards database (https://www.genecards.org/)[15] and the Online Mendelian Inheritance in Man database (https://omim.org/)[16], with “Hepatocellular carcinoma” as a search keyword, we set the disease targets obtained from the Genecards database with a relevance score ≥ twice the median as the screening criteria. When these targets were merged and deduplicated, the targets related to HCC were identified.
Construction of a network model: Using the Venn diagram analysis tool on the Bioinformatics website(http://www.bioinformatics.com.cn)[17], we imported the targets of curcumin in liver cancer and generated a Venn diagram to identify common targets. These intersecting targets were considered as potential targets of THC. Using Cytoscape 3.9.1 software, we constructed and visualized a comprehensive network of curcumin-related targets and liver cancer-related targets, forming a “Curcumin-Targets-Liver Cancer” network.
Construction of the protein-protein interaction network and screening of core targets: We imported the common targets of curcumin and HCC into the STRING database (https://www.string-db.org/)[18] and set the species to Homo sapiens. We maintained the default settings for other parameters and obtained protein-protein interaction (PPI) network information. Subsequently, we imported this information into the Cytoscape3.9.1 software[19] and conducted network topology analysis using the Centiscape plugin[20]. We used Degree, Betweenness, and Closeness as reference values to identify the core targets of the disease.
Gene ontology function and Kyoto Encyclopedia of genes and genomes pathway enrichment analysis: On the Metascape[21] (https://metascape.org) online enrichment analysis platform, we imported key targets of curcumin and liver cancer, setting the species to “Homo sapiens”. Next, we conducted enrichment analysis of gene ontology (GO) functions and Kyoto Encyclopedia of genes and genomes (KEGG) pathways to gain a deeper understanding of the biological processes, cellular components, molecular functions, and metabolic pathways involved. This batch of data was stored, and visualization operations were performed on Bioinformatics platform (http://www.bioinformatics.com.cn).
Molecular docking: Semiflexible molecular docking of curcumin molecules and identification of key targets were conducted, followed by conformational scoring. The potential biological activity of candidate compounds was predicted by analyzing the relative affinity of compounds to different sites on drug targets and combining the corresponding stereoisomeric information. The 2-dimensional structure sdf files of THC and PDB format files of core target proteins with high degree values were obtained separately from the PubChem (https://pubchem.ncbi.nlm.nih.gov/)[22] and PDB databases (https://www.rcsb.org/)[23], respectively. Using ChemBio3D software, we converted the sdf files of active ingredients into mol2 format files and performed energy minimization processing[24]. To discover more compounds with potential biological functions, it is necessary to search for new information from existing biological experimental data and screen suitable target proteins or active ingredients to achieve better drug design. We imported the target protein and its active ingredients into the Sybyl2.0 software[25], extracted small ligand molecules from the target protein, removed water molecules, performed hydrogenation operations, conducted molecular docking, and evaluated it using a scoring function. The optimal binding site was determined on the basis of the highest score. Molecular docking diagrams were constructed using the high-scoring conformations.
Chemicals and reagents: THC was obtained from Shanghai Yuan Ye Biotechnology Co., Ltd. and dissolved in dimethyl sulfoxide from Sigma to obtain a stock solution. In all cell cultures, the dimethyl sulfoxide concentration was maintained below 0.01%, ensuring no detectable impact on cell growth or cell death. Fetal bovine serum (FBS) was obtained from Hangzhou Siji Green Biological Materials Research Institute. Dulbecco’s modified eagle medium, trypsin, streptomycin-penicillin mixture, and phosphate-buffered saline (PBS) were from EallBio company and obtained from Dalian Saituo Biotechnology Co., Ltd. Cell Counting Kit-8 (CCK8) reagent and PI3K signaling pathway activator 740Y-P were produced by American Glpbio company and were obtained from Shanghai Hongye Biotechnology Co., Ltd. ABW matrix gel reagent was purchased from Shanghai Novartis Pharmaceutical Technology Co., Ltd. Hoechst 33342 staining reagent was purchased from Bi Yun Tian Biotechnology Company.
Cell culture: HepG2cells and Huh7 cells were cultured in Dulbecco’s modified eagle medium high-glucose basal culture medium, supplemented with 10% FBS and 1% pen-strep. Cells were kept at 37 °C in a 5% CO2 incubator.
Cell viability assay: Cells in the logarithmic growth phase were used for viability assays. These were seeded at 3 × 103 cells/well in a96-well culture plate. After reaching 50%-60% confluence, the old culture medium was replaced with 100 μL of fresh complete medium containing different concentrations (0, 20, 40, 60, 80, 100, and 120 μM) of THC, with five replicate wells at each concentration. Cell viability was evaluated at 24, 48, and 72 hours. Subsequently, 10 μL of CCK8 reagent was added to each well, followed by 2-hincubation at 37 °C. The absorbance at 450 nm was then measured using an enzyme-linked immunosorbent assay reader.
Clonogenic assay: Cells were seeded in a six-well plate at 500 cells/well and distributed evenly. Once cells adhered, they were treated with different concentrations of THC (0, 25,50, and 100 μM) in liquid form. Cells were cultured for 1-2 weeks and checked regularly up until clone formation was visible in the 6-well plate. Then, old medium was discarded, and cells were washed gently with PBS three times, fixed with 2 mL 4% paraformaldehyde per well for 15 minutes, and rinsed with PBS three times to remove fixative. Thereafter, 2 mL of 0.1% crystal violet staining solution was added per well, and cells were stained at room temperature for 15 minutes. The cells were washed several times with PBS, air-dried, photographed, and counted.
Wound-healing assay: Cells were seeded at a density of 20 × 104 cells/well in 6-well plates containing 10% FBS. When the cells reached 70% confluence, the tip of a yellow pipette was used to scratch a vertical line at the center of the well, ensuring that the pipette remained vertical without tilting. The original medium was discarded, cells were washed three times with PBS, and low-serum medium containing different concentrations (0, 25, 50, and 100 μM) of curcumin was added. Five fields of view were marked at each scratch, and photographs were taken under a microscope at 0 hour, followed by 48-hour incubation. Photographs were repeatedly taken at the marked points. The results were analyzed using Image J software to calculate the healing percentage using the following formula: Healing percentage = (area at 0 hour - area at 48 hours)/area at 0 hour.
Transwell assay: We used a new two-chamber flat plate to detect cell migration and invasion. For migration experiments, cells were seeded into the upper chamber in serum-free medium. For invasion experiments, the upper chamber was coated with Matrigel. The cell suspension was seeded into the upper chamber at 100 μL per well, with700 μL of medium containing FBS added to the lower chamber. After the cells adhered, serum-free medium was carefully replaced in the upper layer with different drug concentrations. The treatment solution was also prepared in serum-free medium. After 48 hours of drug treatment, the cells that had passed through the membrane on the outside of the upper chamber were fixed with 4%paraformaldehyde and stained with crystal violet solution for 30 minutes, washed with PBS three times, and the chamber was air-dried the chamber. We selected five random fields of view in each upper chamber under an inverted microscope (10 × 10 magnification), observed the cells that had passed through the membrane, photographing them under a microscope, and counted them using ImageJ to obtain the average.
Hoechst 33342 staining experiment: Cells were cultured in six-well plates. After reaching confluence, they were incubated with complete culture medium containing different concentrations of THC. The cells were cultured for 48 hours and an appropriate amount of Hoechst 33342 staining solution was added to ensure full coverage of the sample to be stained. Typically, 1 mL of the staining solution was added to each well of a six-well plate, followed by incubation for 20-30 minutes at a temperature suitable for cell culture. The staining solution was then discarded, cells were washed 2-3 times with PBS or culture medium, and fluorescence was detected. Apoptotic cell nuclei appear as densely stained and fragmented under a fluorescence microscope.
Western blot analysis: A standard immunoblotting procedure was performed to detect protein levels. The following antibodies were used: PI3K p110 beta polyclonal antibody (20584-1-AP, Proteintech, 1:1000), phospho-PI3K p85 rabbit pAb (341468, Zenbio, 1:1000), AKT (T55561F, Abmart, 1:1000), Phospho-Akt (13038T, CST, 1:1000), p53 (10442-1-AP, Proteintech, 1:5000), caspase-3 (A0214, Abclonal, 1:1000), B cell leukemia/lymphoma 2 (Bcl-2) associated X (Bax, T40051F, Abmart, 1:1000), Bcl-2, T40056F, Abmart, 1:1000), beta-actin monoclonal antibody (66009-1-lg, Proteintech, 1:5000). Detection was performed using an ECL kit (Millipore, Billerica, Massachusetts, United States).
Statistical analysis was performed using GraphPad Prism9.0 and IBM SPSS 22.0 software. Each experiment was conducted at least three times, with independent replicates. Data are presented as the mean ± SD. Pairwise comparisons between groups were performed using t-tests, and differences were considered significant at P < 0.05, using one-way analysis of variance (aP < 0.05; bP < 0.01).
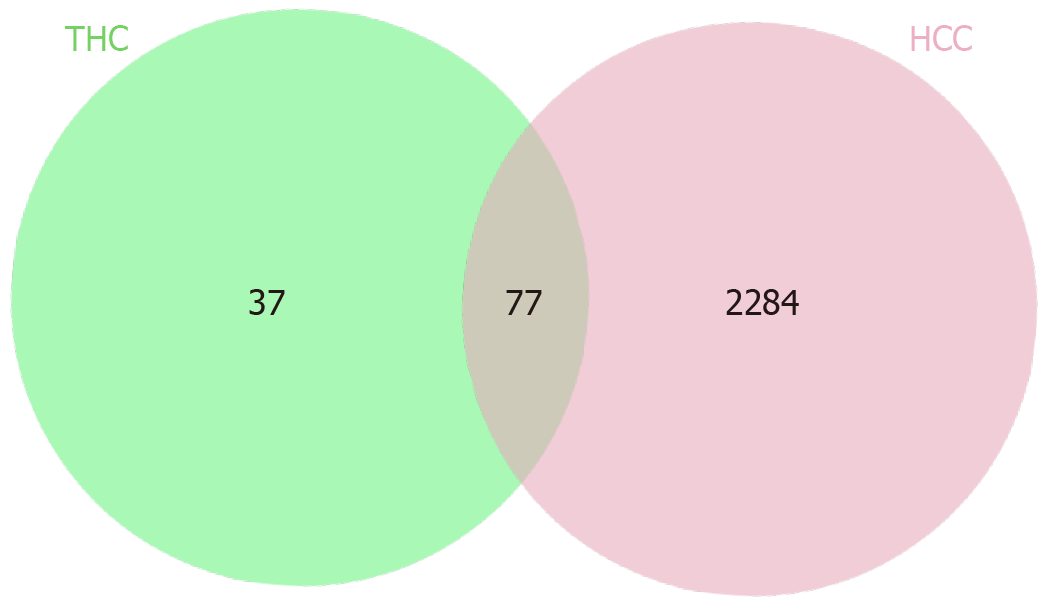
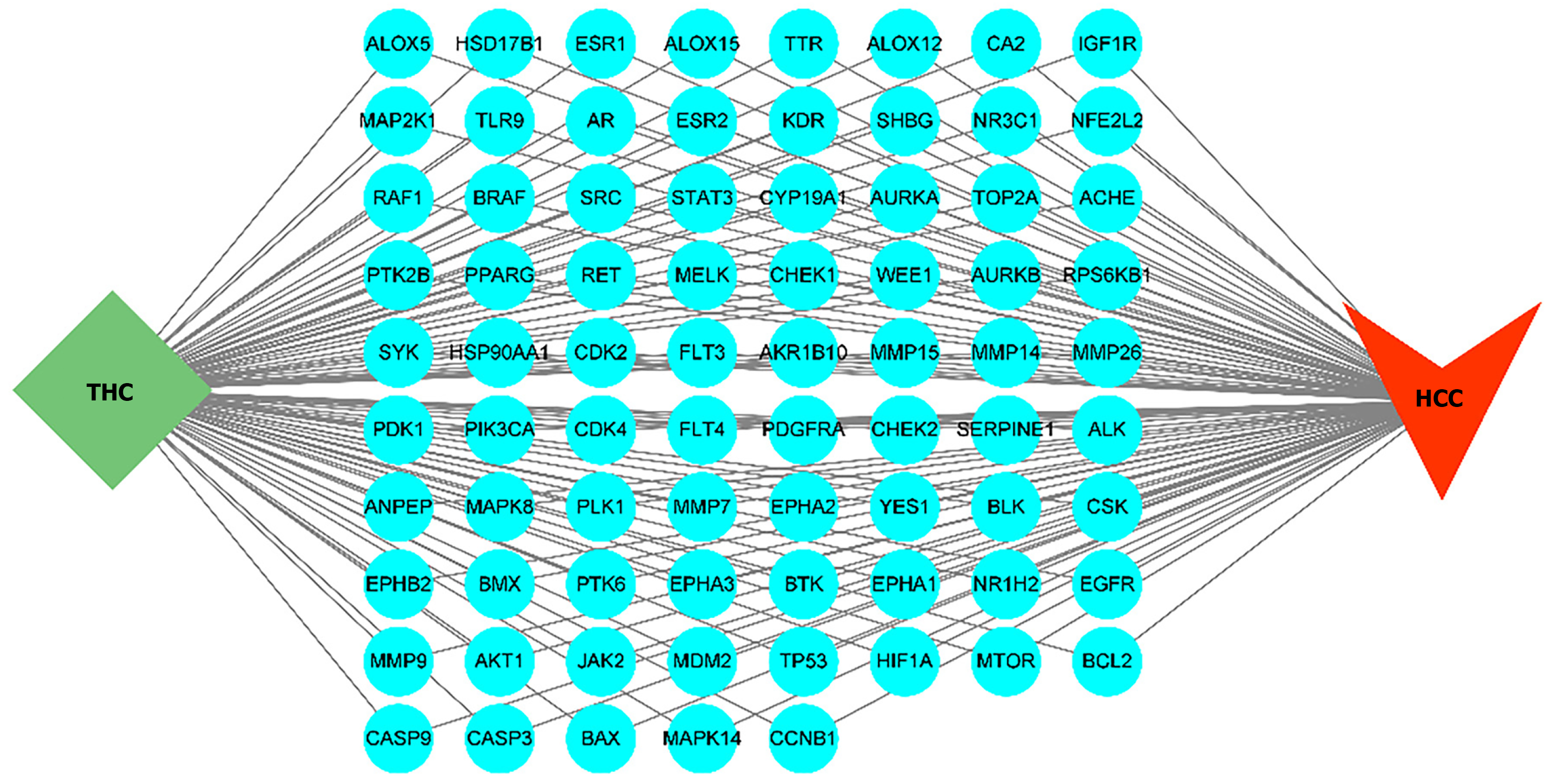
After screening, we identified 114 target proteins related to THC and 2361 target proteins related to liver cancer. Among these, there were 77 overlapping target proteins. A Venn diagram is provided in Figure 1. These 77 common target proteins were considered potential targets of THC for the treatment of liver cancer. We conducted network construction and visualization on these potential targets, and the network structure of “THC-target protein-liver cancer” is shown in Figure 2.
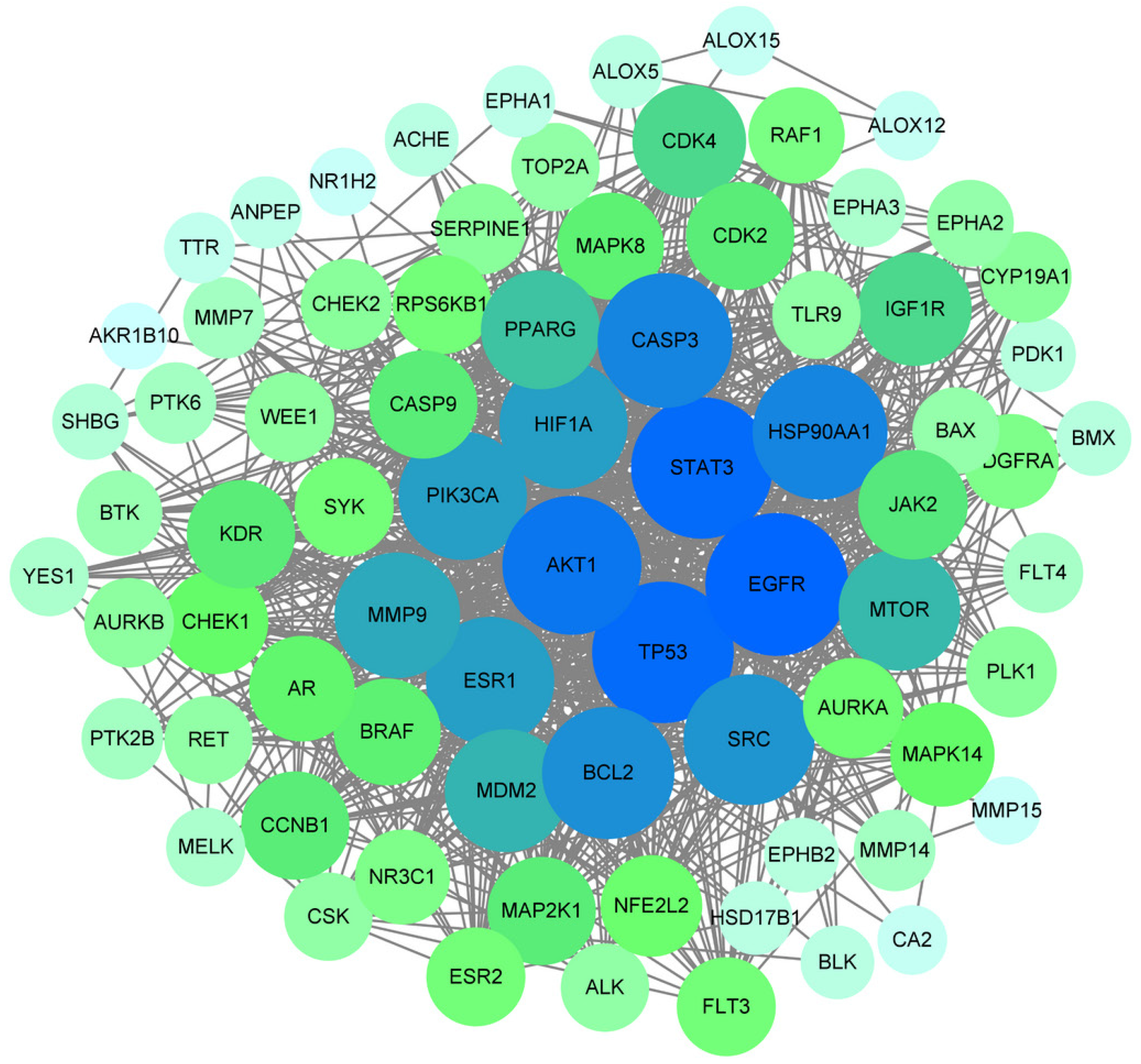
We uploaded the data of the 77 target proteins to the STRING platform and analyzed the PPI network, thereby determining the interactions between target proteins. Subsequently, we imported this information into Cytoscape 3.9.1, where targets are represented by nodes and the interactions between them are represented by edges. After removing the two isolated nodes, the constructed PPI network contained 77 nodes and 767 interaction edges. The PPI network is shown in Figure 3. Using these data, critical target proteins closely related to the occurrence and development of diseases as well as the corresponding drug action sites can be obtained. Using the Centiscape plugin for network topological analysis, we employed degree, betweenness centrality, and closeness centrality as screening criteria, selecting 16 core targets based on these topological parameters, which are displayed in Table 1. The importance of a node in the PPI network increases with its degree, which may indicate greater biological significance[26]. In addition, the correlation coefficient matrix between each target point was obtained through software calculations, based on which the relationships and relative importance between different target points were determined. As shown in Figure 3, the degree was directly proportional to the area.
| Name | Betweenness | Closeness | Degree |
| TP53 | 537.5688911 | 0.011111111 | 58 |
| HSP90AA1 | 411.5735117 | 0.010416667 | 53 |
| STAT3 | 280.5350034 | 0.010309278 | 52 |
| AKT1 | 451.5531513 | 0.010204082 | 50 |
| SRC | 297.0466707 | 0.01 | 49 |
| EGFR | 222.7036191 | 0.00990099 | 48 |
| CASP3 | 233.6425731 | 0.009803922 | 47 |
| PIK3CA | 159.1340985 | 0.00952381 | 46 |
| MTOR | 126.8320074 | 0.009433962 | 43 |
| ESR1 | 202.0774342 | 0.009345794 | 43 |
| HIF1A | 109.3920337 | 0.009259259 | 41 |
| MDM2 | 66.2593338 | 0.008928571 | 37 |
| MMP9 | 230.2669059 | 0.00877193 | 34 |
| PPARG | 269.6527851 | 0.008695652 | 33 |
| KDR | 65.5729245 | 0.008130081 | 26 |
| AR | 63.40344608 | 0.008064516 | 26 |
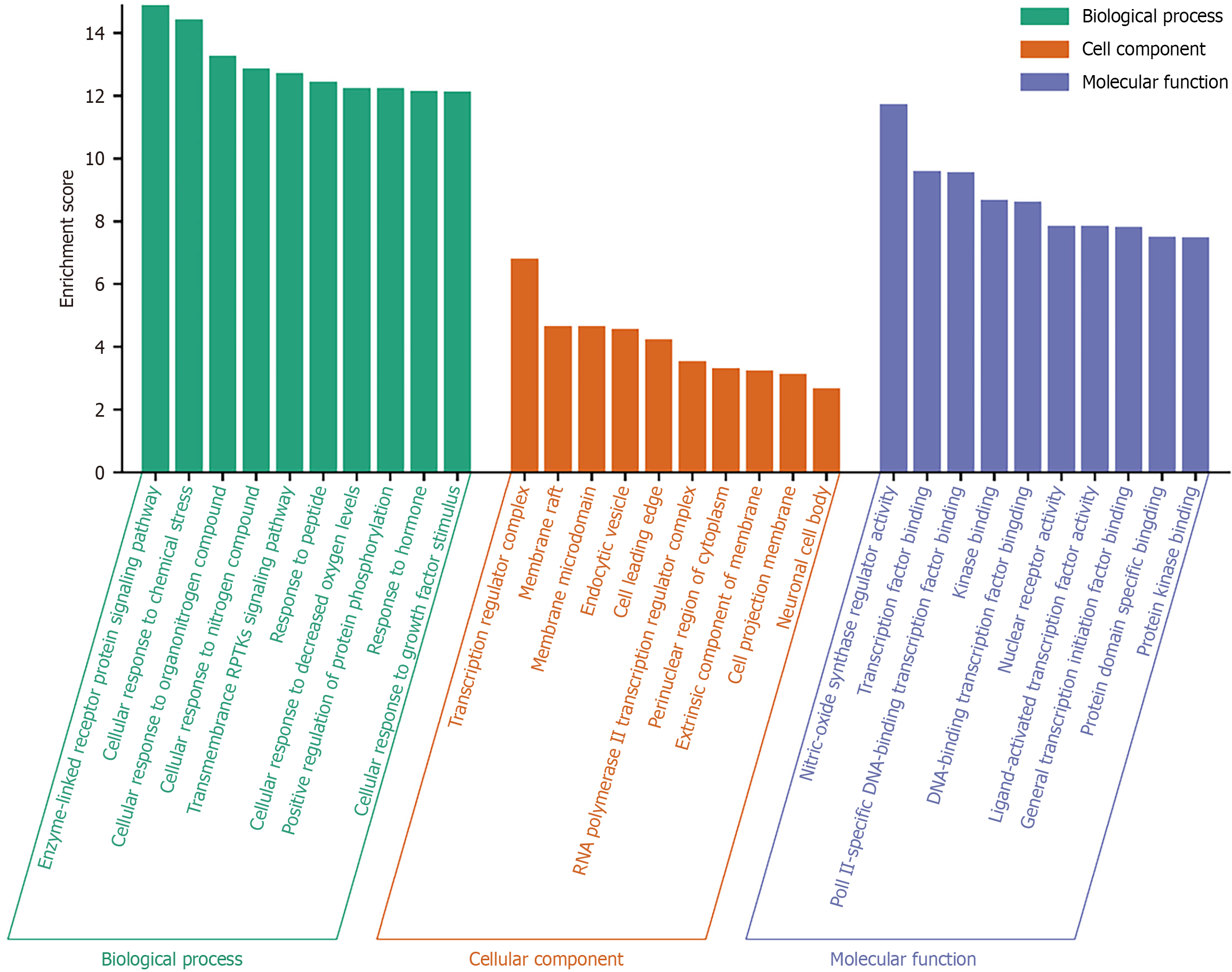
GO functional enrichment analysis results included 495 biological process (BP), 19 cellular component, and 39 molecular function terms. We sorted these based on -log10P values, where a higher value indicated a higher enrichment score and a higher degree of enrichment. We selected the top 10 and plotted a bar graph (Figure 4). Biological process terms included enzyme-linked receptor protein signaling pathway, transmembrane receptor protein tyrosine kinase signaling pathway, and cellular responses to decreased oxygen levels. Cellular component terms mainly include membrane rafts, endocytic vesicles, and RNA polymerase II transcription regulator complexes. Molecular function terms included transcription factor binding, nuclear receptor activity, and protein kinase binding.
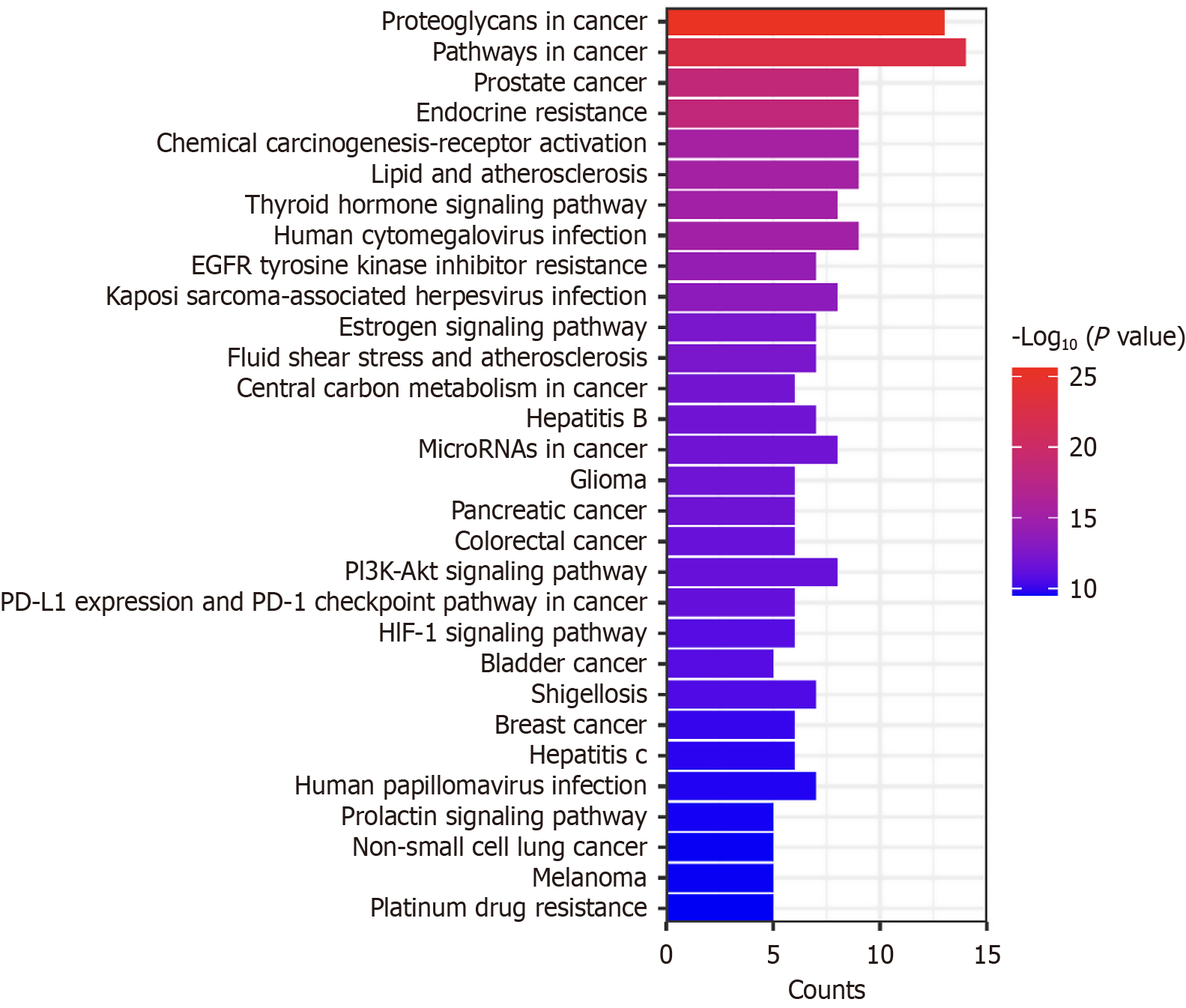
KEGG pathway enrichment analysis revealed 97 pathways related to the core targets. When sorting by P value, a higher value results in a redder color, indicating a higher enrichment level. The top 30 terms were selected, and a bar graph was created (Figure 5). The signaling pathways involved included cancer-related pathways, the activation of chemical carcinogenesis receptors, the PI3K-AKT signaling pathway, and the hypoxia-inducible factor 1 signaling pathway. All of these pathways are implicated in HCC. In addition, curcumin may have therapeutic effects against prostate cancer, hepatitis B, colorectal cancer, and breast cancer. Moreover, based on the enriched terms, curcumin may inhibit tumor cell proliferation and promote apoptosis. This demonstrated that curcumin has unique functions in multiple pathways.
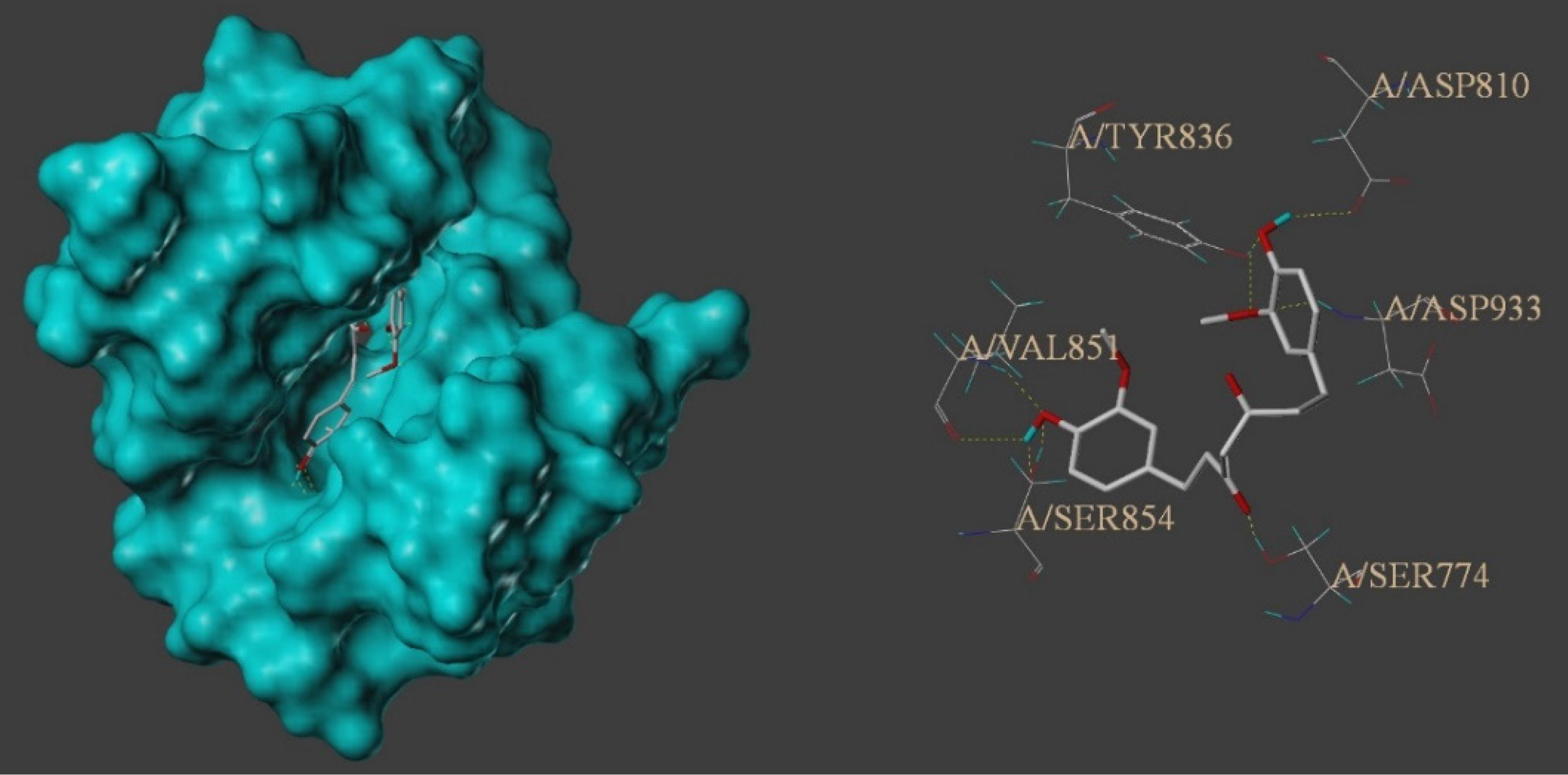
To assess binding between the components of TCM and protein targets, we employed molecular docking. By docking THC with the 16 selected core targets, we set a docking result threshold with a total score ≥ 5. The lower the binding free energy, the more stable the ligand-receptor binding, with a higher total score indicating better binding. A total score ≥ 5 reflected good binding degree between the active ingredient and the target protein, while a total score > 7 indicates a very strong binding activity[27]. Based on the obtained data, corresponding 3-dimensional structural diagrams and other information were generated. For detailed molecular docking data, refer to Table 2. The experimental results showed that THC successfully docked on the 16 key target points. THC exhibited strong binding affinity for phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), kinase insert domain receptor, epidermal growth factor receptor, matrix metallopeptidase 9, heat shock protein 90 alpha family class A member 1, signal transducer and activator of transcription 3, peroxisome proliferator activated receptor gamma, mammalian target of rapamycin (mTOR), and androgen receptor. In addition, it showed excellent binding affinity for AKT1, tumor protein p53, caspase-3, murine double minute 2 (MDM2), estrogen receptor 1, and hypoxia inducible factor 1 subunit alpha, but relatively weak affinity for proto-oncogene tyrosine-protein kinase Src. The highest docking score was noted for the PIK3CA target protein. Therefore, THC compound can be considered a potential lead compound for anti-tumor drugs with good development prospects. Figure 6 shows a schematic of molecular docking. THC formed nine hydrogen bonds in the hydrophobic structure of PIK3CA, composed of amino acid residues TYR836, VAL851, SER854, and ASP810, demonstrating very high binding activity.
| Name | PDB ID | Target protein scoring, total score |
| PIK3CA | 5DXT | 9.2428 |
| KDR | 2XIR | 9.1786 |
| EGFR | 3POZ | 9.0041 |
| MMP9 | 6ESM | 8.7419 |
| HSP90AA1 | 1BYQ | 8.5337 |
| STAT3 | 5AX3 | 7.9753 |
| PPARG | 3B1M | 7.9106 |
| MTOR | 4DRI | 7.8581 |
| AR | 1T65 | 7.6085 |
| AKT1 | 4GV1 | 6.6415 |
| TP53 | 1XQH | 6.5781 |
| CASP3 | 1NMS | 6.2940 |
| MDM2 | 4HG7 | 6.1486 |
| ESR1 | 3CBP | 5.9387 |
| HIF1A | 6GMR | 5.6041 |
| SRC | 1O41 | 4.2536 |
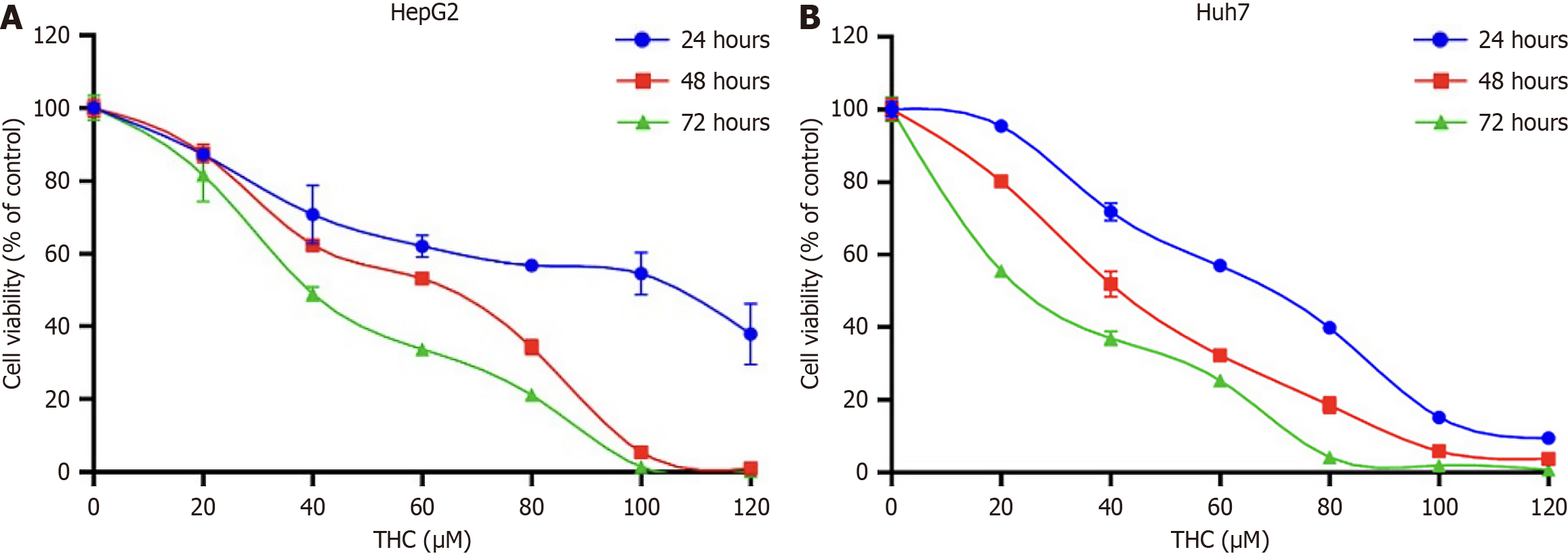
To study the inhibitory effects of THC in different liver cancer cell lines, we used the CCK8 assay to assess cell viability. THC suppressed the proliferation of HepG2 and Huh7 cells to variable extents at 24, 48, and 72 hours after treatment. Cell viability gradually decreased in a time and dose-dependent manner (Figure 7). The half maximal inhibitory concentration (IC50) values of THC in HepG2 cells at 24, 48, and 72 hours were 95.30, 53.84, and 40.21 μM, respectively, while those in Huh7 cells were 62.45, 40.53, and 25.15 μM. Therefore, for subsequent experiments, drug intervention concentrations were chosen as 1/2 of the IC50, the IC50, and two times the IC50, namely 25, 50, and 100 μM, respectively.
To determine the effect of THC on the proliferation of HepG2 and Huh7 cells, we treated these with 25, 50, and 100 μM for 1-2 weeks. The experimental results showed that THC inhibited colony formation by HepG2 and Huh7 cells when compared to that in the blank control group. As the concentration of THC increased, the number of liver cancer cell clones gradually decreased, and a greater effect was noted in Huh7 cells. Furthermore, compared to the control group, the number of cell clones in the group treated with 100 μM THC significantly decreased, showing a significant difference (Figure 8).
Scratch assays of HepG2 and Huh7 cells treated with 25, 50, and 100 μM THC for 48 hours revealed that the migration of the two liver cancer cell lines was suppressed in a concentration-dependent manner. Transwell assays also indicated decreased invasion and migration with increasing drug concentration. Wound-healing assays confirmed these results in both HepG2 and Huh7 liver cancer cells. Cells treated with low concentrations of THC showed faster wound healing rates than those in the high-concentration group. The experimental results are shown in Figure 9.
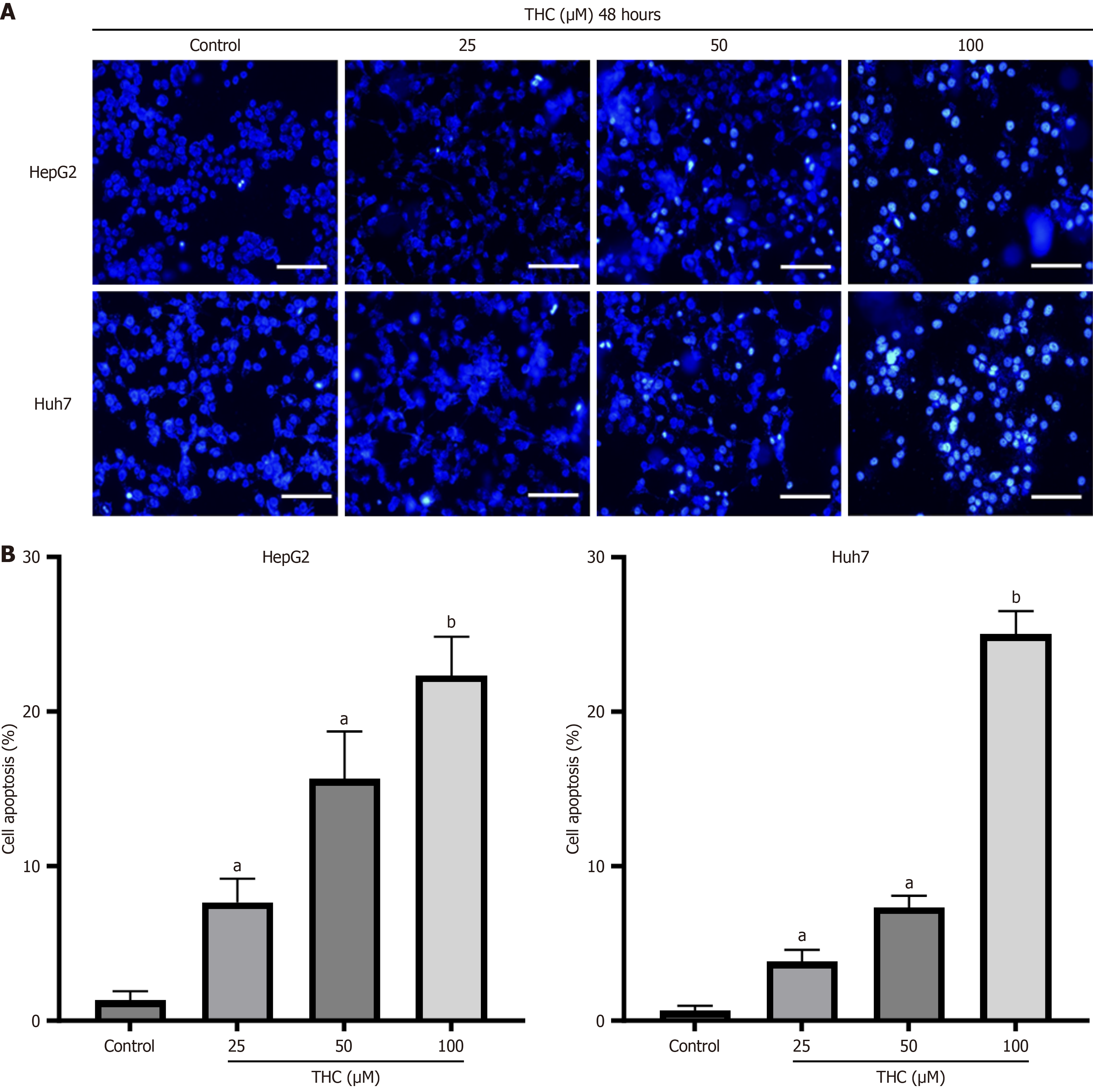
Hoechst 33342 staining is commonly used to detect apoptosis. When cells undergo apoptosis, dense or fragmented staining of the cell nucleus can be observed under a fluorescence microscope[28]. The apoptotic cell death induced by different concentrations of curcumin in HepG2 and Huh7 cells is shown in Figure 10. Under a fluorescence microscope, normal cells with nuclear staining displayed intact round nuclei with relatively light chromatin staining. In contrast, HepG2 and Huh7 cells exposed to different concentrations of curcumin showed altered nuclear morphology and increased chromatin condensation or fragmentation, characteristic of apoptotic cells[29].
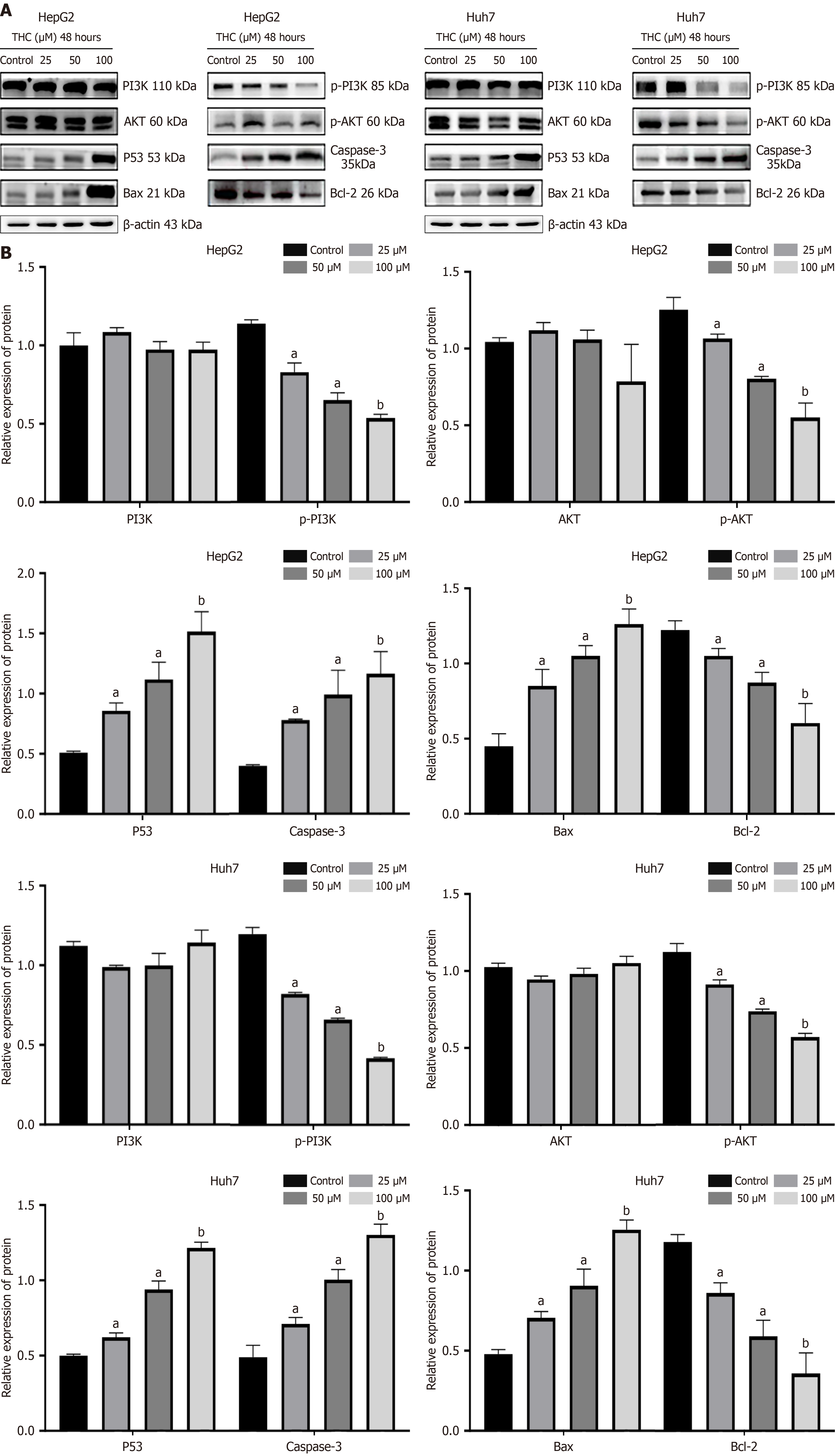
The PI3K/AKT pathway can suppress apoptosis, promoting their survival. Network pharmacology predicted that curcumin may affect liver cancer cell apoptosis through the PI3K/AKT pathway. Thus, we analyzed the expression of PI3K/AKT signaling pathway-related proteins in liver cancer cells using western blot after treatment with curcumin at different concentrations for 48 hours (Figure 11). Curcumin suppressed p-PI3K, p-AKT, and Bcl-2 Levels in a concentration-dependent manner, whereas there was no significant change in the expression of PI3K and AKT. Further, the expression of p53, caspase-3, and Bax was increased. These results indicated that with increasing curcumin concentration, the phosphorylation of the PI3K target proteins was suppressed, as also noted for the downstream AKT protein, reflecting a blockade of PI3K/AKT signaling and enhanced proapoptotic expression.
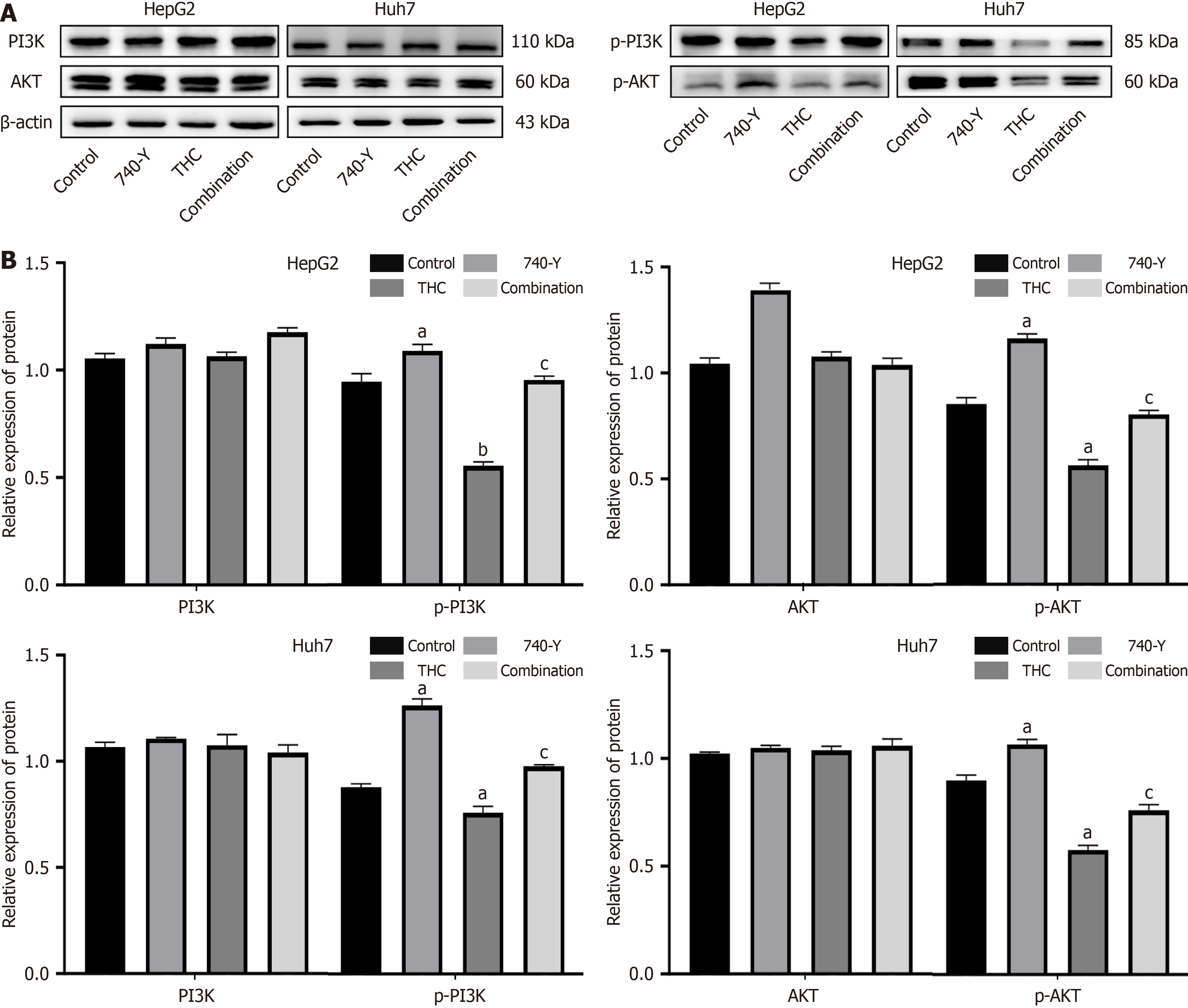
To further investigate the inhibitory effect of THC on PI3K/AKT signaling in HepG2 and Huh7 cells, we performed western blot. Using 0 μM THC as the control group, the experimental groups were treated with 50 μM THC, PI3K activator 740 Y-P[30], or a combination of both. As shown in Figure 12, THC significantly inhibited the expression of p-PI3K and p-AKT in HepG2 and Huh7 cells. However, there was no significant change in the total PI3K and AKT levels. A PI3K activator was used to evaluate the regulatory effects of THC on PI3K/AKT signaling. The inhibition of PI3K/AKT by THC was attenuated by the PI3K activator, resulting in an increase in the expression of p-PI3K and p-AKT, with a more significant effect in Huh7 cells. In summary, THC significantly inhibited the activation of PI3K/AKT signaling pathway, promoting apoptosis to exert an anti-tumor effect in liver cancer cells.
As a Chinese treasure, TCM is attracting increasing attention worldwide and gradually becoming a new direction for the development of anti-tumor drugs. In 2022, Chen et al[31] confirmed that curcumin can be used to HCC, showing that it suppresses HepG2 viability through the adenosine monophosphate-activated protein kinase/unc-51 like autophagy activating kinase 1 autophagy pathway. However, studies have shown that curcumin has low oral bioavailability. THC, the main metabolite of curcumin in the body, is considered a valuable compound for cancer prevention and treatment because of its relatively superior chemical stability, bioavailability, and high structural similarity to curcumin.
Angiogenesis is an essential process for tumor development. Vascular endothelial growth factor is the main mediator of tumor angiogenesis[32]. Studies have shown that in BALB/c nude mice implanted with HepG2 xenograft tumors, daily oral administration of 3000 mg/kg curcumin can significantly reduce microvessel density in tumor tissues[33]. At the molecular level, curcumin triggers a mitochondria-dependent cascade reaction, upregulates p53, and reduces MDM2 to induce cell apoptosis[34]. Molecular docking results also demonstrated that curcumin could stably bind to target proteins kinase insert domain receptor, p53, and MDM2. PIK3CA, one of the core targets, exhibits the strongest binding activity with drug molecules. In this study, relevant drug targets were predicted using network pharmacology and molecular docking, coupled with in vitro experiments to verify that THC induced apoptosis through the proposed mechanism. Apoptosis is a normal physiological event during development, aging, and tissue homeostasis. However, dysregulation of apoptosis during cancer progression render tumor cells resistant to apoptosis[35-37].
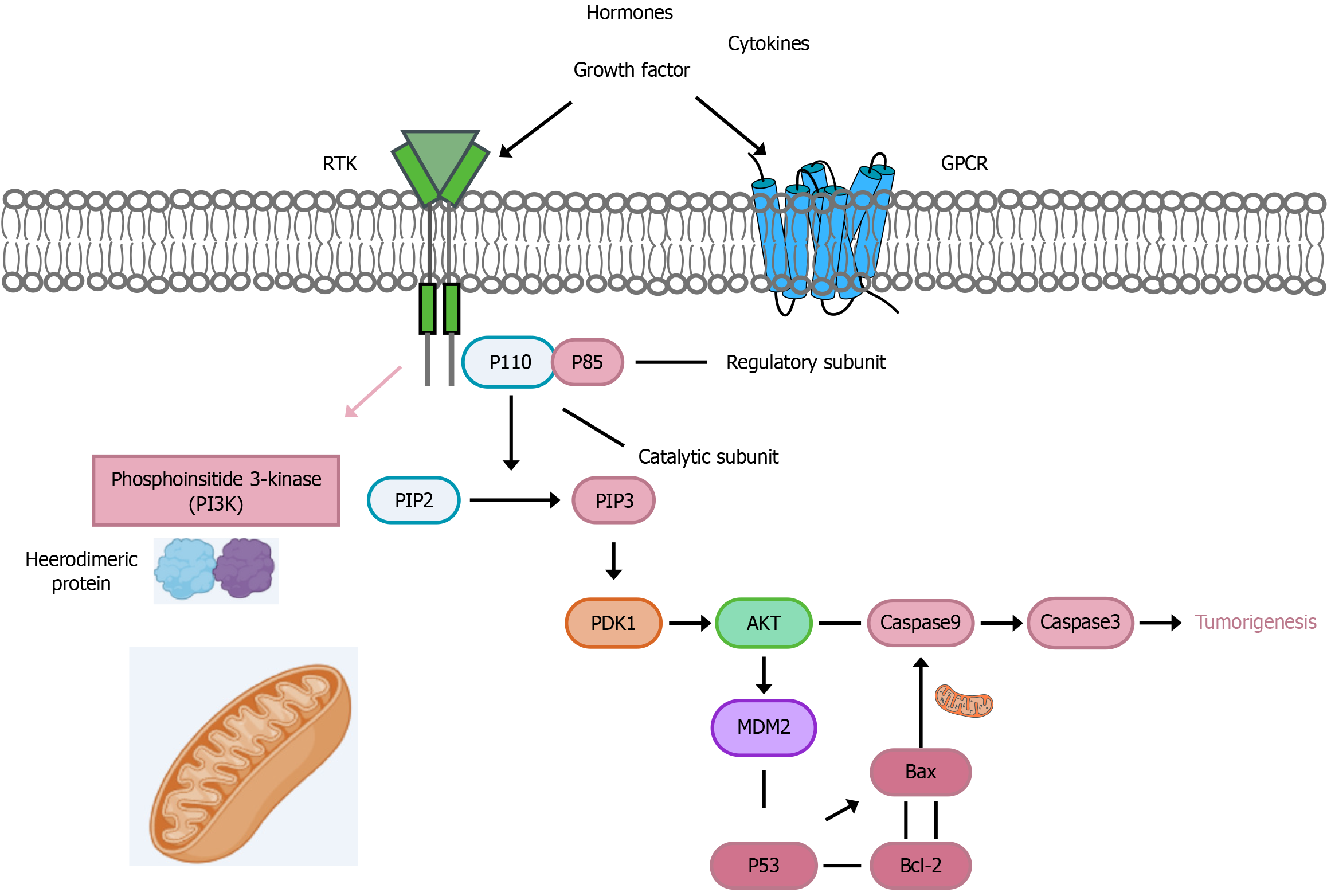
The PI3K/AKT signaling pathway can inhibit cell apoptosis, accelerate the formation of new blood vessels in malignant tumors, and promote invasion and metastasis, thus playing a crucial role in tumor development[38]. Using western blotting, we determined the expression of PI3K/AKT-related proteins. Different concentrations of curcumin downregulated the protein expression of p-PI3K, p-AKT, and Bcl-2 in a dose-dependent manner after 48 hours of treatment, while upregulating the protein expression of p53, caspase-3, and Bax. PI3K, an enzyme belonging to the lipid and serine/threonine kinase family, facilitates lipid synthesis and activates AKT[39,40]. PI3K is a heterodimeric protein consisting of two subunits: A catalytic subunit (p110) and a regulatory subunit (p85)[41]. Hormones, cytokines, and growth factors activate the PI3K/AKT signaling pathway and its downstream targets. To trigger the dimerization and phosphorylation of PI3K, growth factors bind to G protein coupled receptor and receptor tyrosine kinases receptors on the cell surface[42,43]. The conversion of phosphatidylinositol bisphosphate to phosphatidylinositol trisphosphate is mediated by the p110 catalytic subunit. Once phosphatidylinositol trisphosphate is generated, it triggers the AKT signaling cascade by upregulating pyruvate dehydrogenase kinase 1. Both PI3K and AKT signaling proteins require the activation of their respective functions via phosphorylation[44]. AKT can affect the activity of p53 by phosphorylating MDM2, causing phosphorylated MDM2 to translocate to the nucleus and bind to p53, leading to p53 degradation. Degradation of the p53 protein causes an increase in the expression of the anti-apoptotic factor Bcl-2, while expression of the pro-apoptotic protein Bax decreases, affecting the activation of the endogenous apoptotic pathway[45]. Similarly, AKT can inhibit the activity of the protease caspase-3, preventing the activation of the apoptosis cascade, ultimately leading to tumor formation (Figure 13). Caspase-3, also known as cysteine aspartate-specific protease 3, specifically cleaves poly adenosine diphosphate-ribose polymerase 1 (PARP) and acetyl-DEVD-7-amino-4-methylcoumarin, leading to DNA fragmentation and promoting apoptosis[46]. To further validate the ability of THC to inhibit tumor growth by affecting the expression of proteins in the PI3K/AKT signaling pathway, liver cancer cells were treated with a combination of signaling pathway agonists and THC. The experimental results showed that the inhibition of PI3K/AKT was attenuated by a PI3K agonist. Overall, these findings indicate that THC significantly suppresses PI3K/AKT signaling, resulting in anti-tumor effects.
This study characterized the mechanism of action of curcumin in treating HCC through network pharmacology analysis, molecular docking, and other bioinformatics, coupled with molecular biology methods. Although we verified based data mining and cell phenotype that curcumin can affect liver cancer cells, the specific mechanism through which curcumin affects liver cancer cells requires further elucidation. In 2011, Wu et al[46] reported that the main metabolites of curcumin induce autophagic cell death by coordinating the PI3K/AKT/mTOR and mitogen-activated protein kinase (MAPK) signaling pathways in human leukemia HL-60 cells. At the molecular level, Western blot analysis indicated that curcumin inhibited PI3K/AKT/mTOR/p70S6K and activated extracellular signal-regulated kinase 1/2 and JNK1/2 early, leading to autophagy in HL-60 cells[47]. Kang et al[47] studied the effect of dihydrocurcumin on the cell cycle of human breast cancer cells and found that dihydrocurcumin can cause mitochondrial concentration-dependent functional impairment. After 48 hours of dihydrocurcumin treatment, the percentage of mitochondrial membrane potential decreased compared to that of untreated cells. Dihydrocurcumin treatment dose-dependently increased Bax levels and significantly decreased Bcl-2 levels, indicating that the various pathways involved in the effects of dihydrocurcumin on human breast cancer cells may involve the apoptotic pathway. Additionally, after treatment with different concentrations of dihydrocurcumin for 48 hours, the expression of cytochrome c and activated caspase-9 and caspase-3 increased significantly, whereas that of ICAD and PARP[48]. Numerous studies have shown that caspase-3 plays a crucial role in apoptosis. Once activated, caspase-3 carries out various functions, including cytoplasmic endonuclease, PARP cleavage, and ICAD activation, ultimately leading to apoptosis[49].
Current cancer treatments include surgery, chemotherapy, and radiotherapy. In addition to limited efficacy, there are several major issues with these treatments, especially radiotherapy and chemotherapy, which may damage and weaken the patient’s immune system and have various other side effects, such as gastrointestinal reactions, bone marrow toxicity, cardiac toxicity, neurotoxicity, and the emergence of secondary tumors, which altogether greatly decrease patient quality of life. Curcumin, a natural substance with anti-tumor effects, is effective when used in conjunction with traditional treatment methods to increase treatment efficacy, reduce toxic side effects, and improve patient quality of life and survival. It is worth noting that excessive activation of the PI3K/AKT signaling pathway has been found in many cancer types[50-52] Excessive activation of the PI3K/AKT signaling pathway affects downstream pathways, leading to uncontrolled induction of angiogenesis, cell proliferation, and inhibition of apoptosis, which are key factors in cancer initiation and progression. Therefore, targeting the PI3K/AKT pathway has great therapeutic potential. However, further preclinical research is needed to validate the specific molecular mechanisms of targeting the PI3K/AKT pathway through direct and indirect inhibitors as monotherapy or in combination with other drugs. Furthermore, more clinical trials are needed to determine the safety of these interventions as well as larger-scale efficacy studies to establish clinical outcomes. Although curcumin acts as a nonspecific inhibitor of the PI3K/AKT signaling pathway, it also affects other pathways involved in cancer development[52]. In the future, we will investigate the specific molecular mechanisms of action of THC in HCC. Additionally, we will conduct further in vivo drug experiments and clinical validation studies to determine the role of curcumin in HCC, providing scientific evidence for the development of novel and more effective anti-cancer drugs, and offering new insights for the prevention and treatment of HCC.
In this study, the effects of THC on HCC and its mechanism of action were investigated using a combination of network pharmacology, molecular docking, and in vitro experiments. It was found that THC has a high binding affinity to target proteins, such as PIK3. In vitro experiments confirmed that it can inhibit the proliferation of HCC cells, reducing migration and invasion, while promoting apoptosis. THC exerted its anti-tumor effects by downregulating the expression of p-PI3K, p-AKT within the PI3K/AKT signaling pathway while upregulating the expression of caspase-3, p53 and Bax. These results suggest that THC is a potential candidate for the development of new and more effective anti-cancer drugs, providing a basis for novel approaches to prevention and treatment of HCC.
| 1. | Lan T, Li H, Zhang D, Xu L, Liu H, Hao X, Yan X, Liao H, Chen X, Xie K, Li J, Liao M, Huang J, Yuan K, Zeng Y, Wu H. KIAA1429 contributes to liver cancer progression through N6-methyladenosine-dependent post-transcriptional modification of GATA3. Mol Cancer. 2019;18:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 344] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 2. | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1066] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56601] [Article Influence: 7075.1] [Reference Citation Analysis (134)] |
| 4. | Möller K, Safai Zadeh E, Görg C, Dong Y, Cui X, Lim A, de Molo C, Serra C, Martín Algíbez A, Berzigotti A, Piscaglia F, Faiss S, Dietrich CF. Focal Liver Lesions other than Hepatocellular Carcinoma in Cirrhosis: Diagnostic Challenges. J Transl Int Med. 2022;10:308-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Bochuan W, Yong Z, Qiuyun Z, Zhiqiang Z, Changyong L, Zhendong W, Chen B, Yuhan W, Xueyi GE, Ying Q, He YU, Xiaohong GU. Reveal the mechanisms of prescriptions for liver cancer' treatment based on two illustrious senior TCM physicians. J Tradit Chin Med. 2023;43:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Yang G, Yan H, Tang Y, Yuan F, Cao M, Ren Y, Li Y, He Z, Su X, Yao Z, Deng M. Advancements in understanding mechanisms of hepatocellular carcinoma radiosensitivity: A comprehensive review. Chin J Cancer Res. 2023;35:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 7. | Novaes JT, Lillico R, Sayre CL, Nagabushnam K, Majeed M, Chen Y, Ho EA, Oliveira ALP, Martinez SE, Alrushaid S, Davies NM, Lakowski TM. Disposition, Metabolism and Histone Deacetylase and Acetyltransferase Inhibition Activity of Tetrahydrocurcumin and Other Curcuminoids. Pharmaceutics. 2017;9:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Okada K, Wangpoengtrakul C, Tanaka T, Toyokuni S, Uchida K, Osawa T. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J Nutr. 2001;131:2090-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Liu W, Zhang Z, Lin G, Luo D, Chen H, Yang H, Liang J, Liu Y, Xie J, Su Z, Cao H. Tetrahydrocurcumin is more effective than curcumin in inducing the apoptosis of H22 cells via regulation of a mitochondrial apoptosis pathway in ascites tumor-bearing mice. Food Funct. 2017;8:3120-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt HHHW. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43:136-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 698] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 11. | Stanzione F, Giangreco I, Cole JC. Use of molecular docking computational tools in drug discovery. Prog Med Chem. 2021;60:273-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 12. | Crampon K, Giorkallos A, Deldossi M, Baud S, Steffenel LA. Machine-learning methods for ligand-protein molecular docking. Drug Discov Today. 2022;27:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 13. | Yuan H, Ma Q, Cui H, Liu G, Zhao X, Li W, Piao G. How Can Synergism of Traditional Medicines Benefit from Network Pharmacology? Molecules. 2017;22:1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 321] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 14. | Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357-W364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 2663] [Article Influence: 443.8] [Reference Citation Analysis (0)] |
| 15. | Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1-1.30.33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 3351] [Article Influence: 335.1] [Reference Citation Analysis (0)] |
| 16. | Amberger JS, Hamosh A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr Protoc Bioinformatics. 2017;58:1.2.1-1.2.12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 465] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 17. | Jia A, Xu L, Wang Y. Venn diagrams in bioinformatics. Brief Bioinform. 2021;22:bbab108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 18. | Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT, Pyysalo S, Bork P, Jensen LJ, von Mering C. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51:D638-D646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1815] [Cited by in RCA: 5015] [Article Influence: 1671.7] [Reference Citation Analysis (0)] |
| 19. | Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24663] [Cited by in RCA: 35635] [Article Influence: 1619.8] [Reference Citation Analysis (7)] |
| 20. | Scardoni G, Tosadori G, Faizan M, Spoto F, Fabbri F, Laudanna C. Biological network analysis with CentiScaPe: centralities and experimental dataset integration. F1000Res. 2014;3:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, Benner C, Chanda SK. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3766] [Cited by in RCA: 9742] [Article Influence: 1391.7] [Reference Citation Analysis (0)] |
| 22. | Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49:D1388-D1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2091] [Cited by in RCA: 2347] [Article Influence: 469.4] [Reference Citation Analysis (0)] |
| 23. | Shin JM, Cho DH. PDB-Ligand: a ligand database based on PDB for the automated and customized classification of ligand-binding structures. Nucleic Acids Res. 2005;33:D238-D241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Habeeb MM, Al-Attas AS, Al-Raimi DS. Spectroscopic studies and molecular orbital calculations of charge transfer complexation between 3,5-dimethylpyrazole with DDQ in acetonitrile. Spectrochim Acta A Mol Biomol Spectrosc. 2015;142:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Liu Y, Sun W, Shen N, Hao W, Xin H, Che F, Cui Y. Network pharmacology and molecular docking combined with widely targeted metabolomics to elucidate the potential compounds and targets of Euphorbia helioscopia seeds for the treatment of pulmonary fibrosis. Comput Biol Med. 2023;160:107007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Wei X, Hou W, Liang J, Fang P, Dou B, Wang Z, Sai J, Xu T, Ma C, Zhang Q, Cheng F, Wang X, Wang Q. Network Pharmacology-Based Analysis on the Potential Biological Mechanisms of Sinisan Against Non-Alcoholic Fatty Liver Disease. Front Pharmacol. 2021;12:693701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Hsin KY, Ghosh S, Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One. 2013;8:e83922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 28. | Fuchs H, Jahn K, Hu X, Meister R, Binter M, Framme C. Breaking a Dogma: High-Throughput Live-Cell Imaging in Real-Time with Hoechst 33342. Adv Healthc Mater. 2023;12:e2300230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Jiang XL, Tai H, Xiao XS, Zhang SY, Cui SC, Qi SB, Hu DD, Zhang LN, Kuang JS, Meng XS, Li SM. Cangfudaotan decoction inhibits mitochondria-dependent apoptosis of granulosa cells in rats with polycystic ovarian syndrome. Front Endocrinol (Lausanne). 2022;13:962154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 30. | Chen L, Liu Y, Wang Z, Zhang L, Xu Y, Li Y, Zhang L, Wang G, Yang S, Xue G. Mesenchymal stem cell-derived extracellular vesicles protect against abdominal aortic aneurysm formation by inhibiting NET-induced ferroptosis. Exp Mol Med. 2023;55:939-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 69] [Reference Citation Analysis (0)] |
| 31. | Chen Y, Li Q, Ren S, Chen T, Zhai B, Cheng J, Shi X, Song L, Fan Y, Guo D. Investigation and experimental validation of curcumin-related mechanisms against hepatocellular carcinoma based on network pharmacology. J Zhejiang Univ Sci B. 2022;23:682-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Johnson BM, Johnson AM, Heim M, Buckley M, Mortimer B, Berry JL, Sewell-Loftin MK. Biomechanical stimulation promotes blood vessel growth despite VEGFR-2 inhibition. BMC Biol. 2023;21:290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 33. | Yoysungnoen P, Wirachwong P, Changtam C, Suksamrarn A, Patumraj S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J Gastroenterol. 2008;14:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 34. | Gao Y, Li J, Ma M, Fu W, Ma L, Sui Y, Wang Y. Prognostic prediction of m6A and ferroptosis-associated lncRNAs in liver hepatocellular carcinoma. J Transl Int Med. 2024;12:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 35. | Jung YY, Shanmugam MK, Chinnathambi A, Alharbi SA, Shair OHM, Um JY, Sethi G, Ahn KS. Fangchinoline, a Bisbenzylisoquinoline Alkaloid can Modulate Cytokine-Impelled Apoptosis via the Dual Regulation of NF-κB and AP-1 Pathways. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Bordoloi D, Banik K, Shabnam B, Padmavathi G, Monisha J, Arfuso F, Dharmarajan A, Mao X, Lim LHK, Wang L, Fan L, Hui KM, Kumar AP, Sethi G, Kunnumakkara AB. TIPE Family of Proteins and Its Implications in Different Chronic Diseases. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Ye Y, Liu M, Wu F, Ou S, Wang W, Fei J, Xie F, Bai L. TRMT6 promotes hepatocellular carcinoma progression through the PI3K/AKT signaling pathway. Eur J Med Res. 2023;28:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Iksen, Pothongsrisit S, Pongrakhananon V. Targeting the PI3K/AKT/mTOR Signaling Pathway in Lung Cancer: An Update Regarding Potential Drugs and Natural Products. Molecules. 2021;26:4100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 39. | Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122:4-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 1138] [Article Influence: 189.7] [Reference Citation Analysis (1)] |
| 40. | Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2247] [Cited by in RCA: 2610] [Article Influence: 130.5] [Reference Citation Analysis (0)] |
| 41. | Kirtonia A, Pandey AK, Ramachandran B, Mishra DP, Dawson DW, Sethi G, Ganesan TS, Koeffler HP, Garg M. Overexpression of laminin-5 gamma-2 promotes tumorigenesis of pancreatic ductal adenocarcinoma through EGFR/ERK1/2/AKT/mTOR cascade. Cell Mol Life Sci. 2022;79:362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 42. | Lee JH, Chinnathambi A, Alharbi SA, Shair OHM, Sethi G, Ahn KS. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol Res. 2019;150:104504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 43. | Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 972] [Cited by in RCA: 1130] [Article Influence: 161.4] [Reference Citation Analysis (0)] |
| 44. | Kwon JW, Oh JS, Seok SH, An HW, Lee YJ, Lee NY, Ha T, Kim HA, Yoon GM, Kim SE, Oh PR, Lee SH, Voon DC, Kim DY, Park JW. Combined inhibition of Bcl-2 family members and YAP induces synthetic lethality in metastatic gastric cancer with RASA1 and NF2 deficiency. Mol Cancer. 2023;22:156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 45. | Kim C, Wang XD, Jang S, Yu Y. PARP1 inhibitors induce pyroptosis via caspase 3-mediated gasdermin E cleavage. Biochem Biophys Res Commun. 2023;646:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Wu JC, Lai CS, Badmaev V, Nagabhushanam K, Ho CT, Pan MH. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol Nutr Food Res. 2011;55:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Kang N, Wang MM, Wang YH, Zhang ZN, Cao HR, Lv YH, Yang Y, Fan PH, Qiu F, Gao XM. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem Toxicol. 2014;67:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 48. | Unnisa A, Greig NH, Kamal MA. Inhibition of Caspase 3 and Caspase 9 Mediated Apoptosis: A Multimodal Therapeutic Target in Traumatic Brain Injury. Curr Neuropharmacol. 2023;21:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 49. | Mangiapane LR, Nicotra A, Turdo A, Gaggianesi M, Bianca P, Di Franco S, Sardina DS, Veschi V, Signore M, Beyes S, Fagnocchi L, Fiori ME, Bongiorno MR, Lo Iacono M, Pillitteri I, Ganduscio G, Gulotta G, Medema JP, Zippo A, Todaro M, De Maria R, Stassi G. PI3K-driven HER2 expression is a potential therapeutic target in colorectal cancer stem cells. Gut. 2022;71:119-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 50. | Han SH, Lee JH, Woo JS, Jung GH, Jung SH, Han EJ, Kim B, Cho SD, Nam JS, Che JH, Jung JY. Myricetin induces apoptosis and autophagy in human gastric cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Heliyon. 2022;8:e09309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 51. | Yang MH, Lee JH, Ko JH, Jung SH, Sethi G, Ahn KS. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules. 2019;24:1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 52. | Zhang X, Zhao L, Zhai G, Ji J, Liu A. Multifunctional Polyethylene Glycol (PEG)-Poly (Lactic-Co-Glycolic Acid) (PLGA)-Based Nanoparticles Loading Doxorubicin and Tetrahydrocurcumin for Combined Chemoradiotherapy of Glioma. Med Sci Monit. 2019;25:9737-9751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |