Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.102111
Revised: November 22, 2024
Accepted: December 30, 2024
Published online: March 15, 2025
Processing time: 128 Days and 3 Hours
Precancerous lesions of gastric cancer (PLGC) represent a critical pathological stage in the development of intestinal gastric cancer. Early detection and dia
To comprehensively review the current state of research on PLGC, examining development trends and research hotspots.
We conducted a bibliometric analysis of PLGC-related studies published between 2004 and 2023 using the Web of Science Core Collection database. We employed Software, including VOSviewer, CiteSpace, R software, and SCImago Graphica, to map scientific networks and visualize knowledge trends in terms of publication volume, countries/regions, institutions, journals, authors, and keywords.
A total of 4097 articles were included, and overall publication volume showed an increasing trend. Over the past two decades, China published the most articles, followed by the United States, Japan, South Korea, and Italy. Among the top 10 contributors, the United States ranked highest in institutions, authors, and citations and demonstrated the strongest international collaboration. Research keywords in this field were clustered into three main categories: Risk factors, pathogenesis, and diagnosis and treatment. Pathogenesis and molecular biomarkers remain key areas of focus. Future research should explore the mechanisms of gut microbiota, immune microenvironment, metabolic reprogramming, and epigenetics. Ad
PLGC research has rapidly developed in recent years, gaining considerable attention. This bibliometric analysis reveals research state and emerging trends over the past 20 years, providing insights for future studies.
Core Tip: As a “golden turning point” in the development of intestinal-type gastric cancer, the early detection and treatment of precancerous lesions of gastric cancer can reduce the incidence of gastric cancer. This article provides a current review of precancerous lesions of gastric cancer research through bibliometric methods, helping researchers stay informed about the state of research and recent advancements in this field, while offering new ideas and directions for future studies.
- Citation: Jia YP, Liu DC, Cao TL, Jiang HZ, Li T, Li Y, Ding X. Advances and global trends of precancerous lesions of gastric cancer: A bibliometric analysis. World J Gastrointest Oncol 2025; 17(3): 102111
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/102111.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.102111
Gastric cancer is one of the most common malignancies of the digestive system, ranking fifth globally in terms of both incidence and mortality[1]. It has the highest incidence in East Asia and Eastern Europe and is the third leading cause of cancer-related deaths in China[1,2], imposing a substantial public health burden[3]. Recent studies have indicated that although the overall incidence of gastric cancer is expected to continue to decrease in most countries, the number of new cases is projected to increase, particularly among younger individuals[4], highlighting that gastric cancer remains a major global public health challenge.
Precancerous lesions of gastric cancer (PLGC) are pathological changes that predispose tissues to gastric cancer. In 1992, Correa et al[5] proposed a cascade model for the progression of intestinal-type gastric cancer, which has been widely accepted by the academic community. The development of intestinal-type gastric cancer follows the sequence of inflammation, atrophy, intestinal metaplasia, dysplasia/intraepithelial neoplasia, and gastric cancer, known as the Correa cascade[5]. In 2012, the European guidelines on the management of precancerous conditions and lesions in the stomach (MAPS) defined chronic atrophic gastritis and intestinal metaplasia as precancerous conditions, with dysplasia being the most direct precursor of gastric cancer[6]. Dysplasia/intraepithelial neoplasia is characterized by neoplastic epithelial proliferation with cytological and architectural atypia, but without convincing evidence of invasion[6]. In 2019, the World Health Organization (WHO) stated that dysplasia is more appropriately classified for tubular adenocarcinoma, and the 2019 updated European guidelines categorize dysplasia into low-grade and high-grade[7], which has gradually gained recognition and attention from researchers. Studies have shown that approximately 25% of patients with high-grade dysplasia in a cohort of PLGC patients were diagnosed with gastric cancer after one year of follow-up. Therefore, ef
However, preventing the transition from PLGC to gastric cancer remains challenging. The pathogenesis of PLGC is not yet fully elucidated. Diagnosis primarily relies on gastric mucosal biopsies, with the number, depth, and size of biopsy specimens being critical factors for accurate diagnosis and assessment. In terms of treatment, Helicobacter pylori (H. pylori) eradication can delay or block PLGC progression and prevent gastric cancer[8].However, the therapeutic effects of folate supplementation, antioxidants, and vitamins on PLGC remain inconclusive, with varying results across different studies[9,10]. For patients with endoscopically defined PLGC, options such as endoscopic mucosal resection, endoscopic submucosal dissection, or surgical resection may be considered, along with high-quality endoscopic follow-up to monitor patient prognosis. In recent years, increasing attention has been directed toward the immune microenvironment[11,12], metabolic reprogramming[13], and gut microbiota[14,15], although these areas remain under active investigation. There
Bibliometrics refers to the quantitative study of physical or bibliographic units or their substitutes using mathematical and statistical methods[16]. By analyzing various data points in published literature, including authorship, country of origin, affiliated institutions, journal publications, and keywords, bibliometrics can rapidly and accurately reveal research output, key topics, and future trends in a given field[17]. Bibliometrics has been widely applied across various fields of scientific research. Over the past two decades, numerous countries have conducted extensive studies on the pathogenesis, diagnostic criteria, treatment, and prognosis of PLGC. However, no bibliometric analysis has yet been performed specifically on PLGC. Therefore, this study employed bibliometric methods to summarize the current state of research on PLGC, uncover research hotspots, and predict future trends, aiming to provide reference points for in-depth studies and novel drug development in the field of PLGC.
The data for this study were sourced from the Web of Science Core Collection (WoSCC) database. The search formula used was: (TS = (gastric OR stomach) AND TS = (dysplasia OR atypical hyperplasia OR intraepithelial neoplasia OR preinvasive cancer OR preinvasive lesion* OR preinvasive condition* OR precancerous lesion* OR precancerous condi
For the bibliometric analysis and visualization, we used VOSviewer 1.6.20, CiteSpace 6.4. R1, R software, and SCImago Graphica 1.0.45 to analyze publication volume, countries/regions, institutions, journals, authors, and keywords. VOSviewer, a free program used to construct and visualize bibliometric maps[18], was employed to conduct collaborative analyses between institutions and authors, as well as keyword co-occurrence analysis. CiteSpace, which integrates information visualization techniques, bibliometrics, and data mining algorithms in interactive visualizations[19], was used to extract citation data patterns and perform keyword burst analysis. The R software package “bibliometrix”[20](version 4.3.0) (https://www.bibliometrix.org) was used to plot research hotspot maps. SCImago Graphica, a no-code tool for creating of complex visualizations through simple drag-and-drop interactions[21], was used to construct cooperation networks between countries/regions.
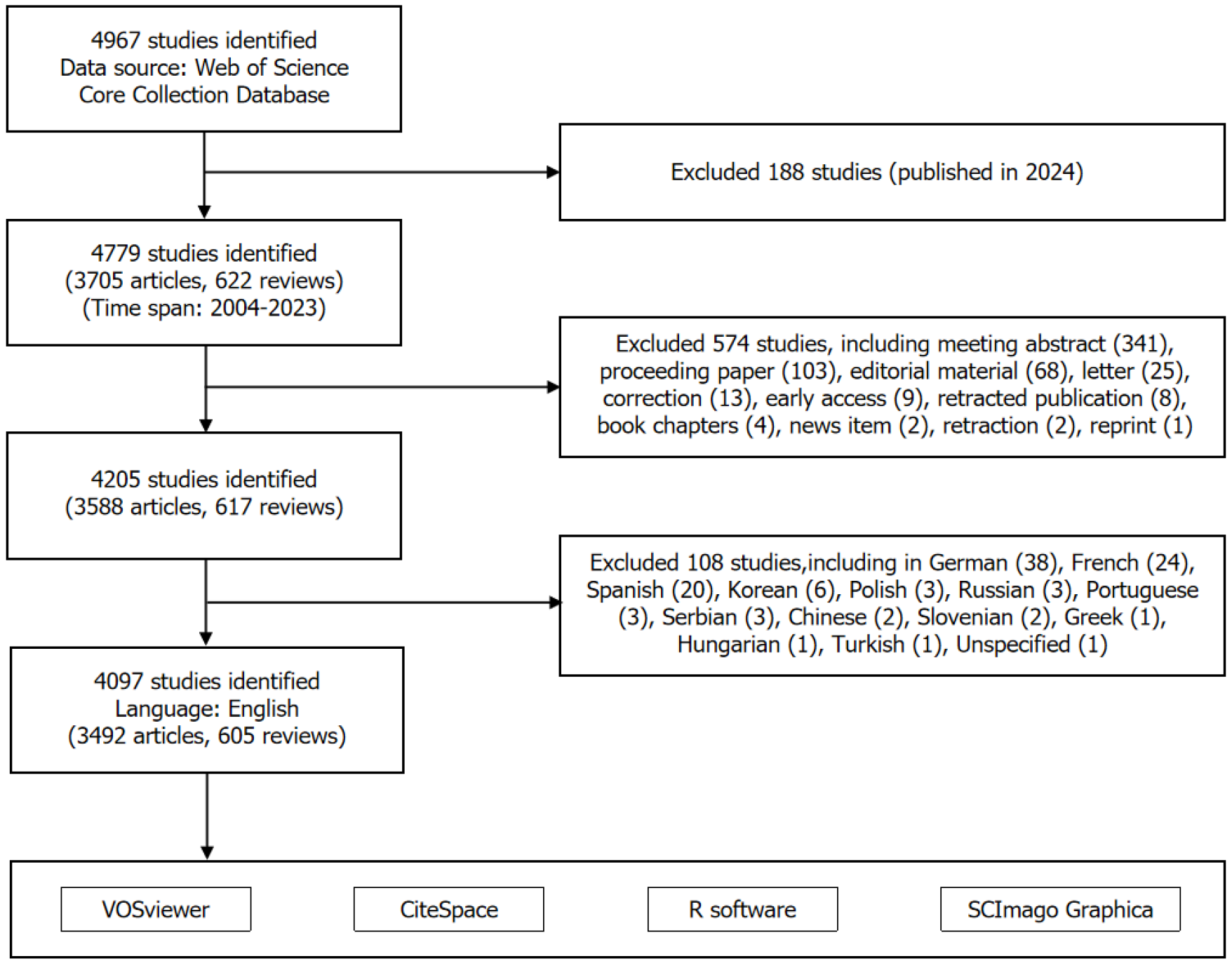
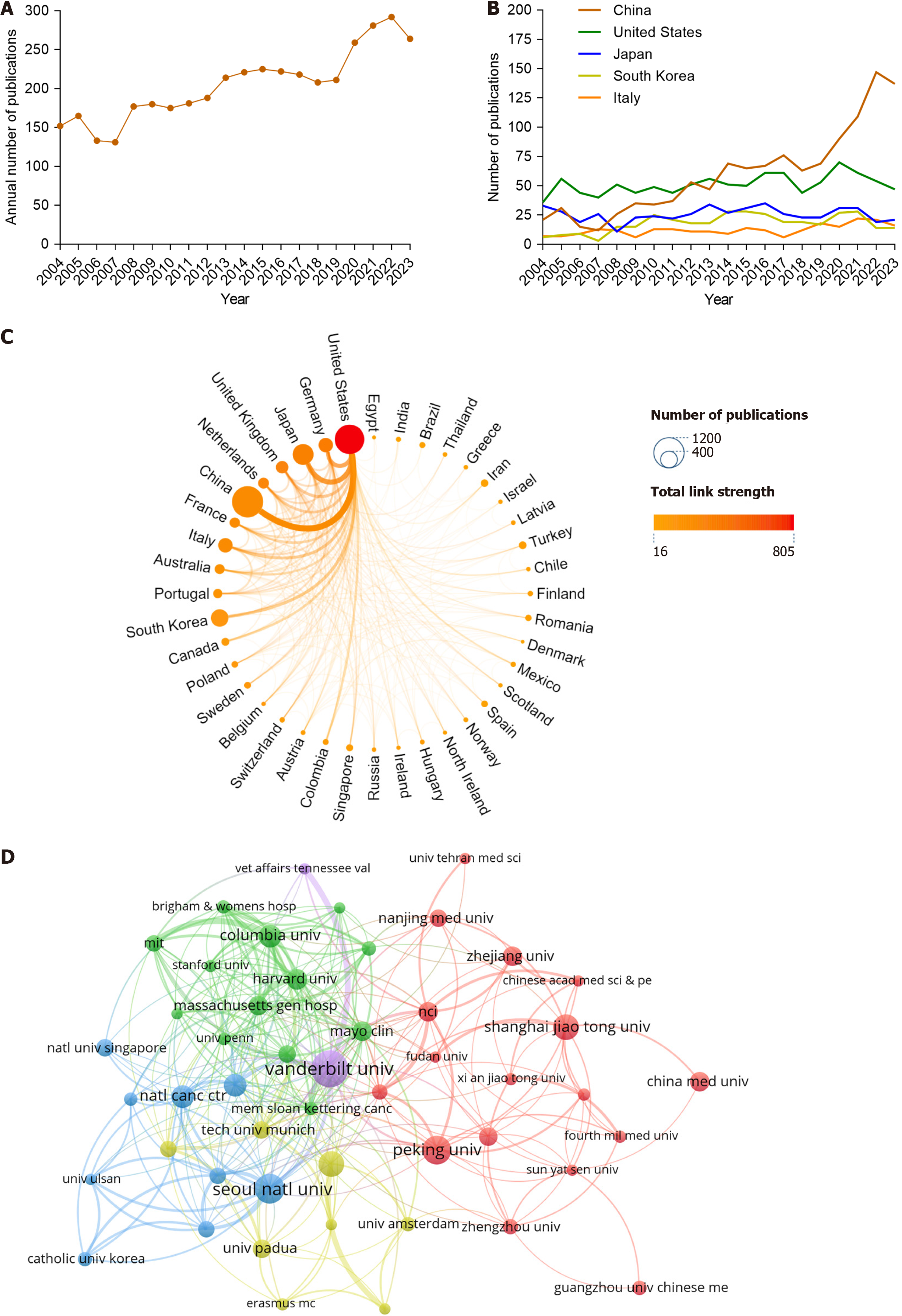
Based on our search strategy, a total of 4967 publications related to PLGC were identified (from 2004 to 2023). After screening, 4097 publications were included in the analysis, comprising 3492 articles and 605 reviews (Figure 1). Collectively, these publications were cited 116044 times, with an h-index of 137. Analysis of the annual publication volume showed that since 2007, the number of publications in the PLGC field has gradually increased. A sharp rise occurred after 2019, with the number of publications increasing from 211 in 2019 to 292 in 2022, representing a 1.38-fold increase (Figure 2A). This suggests that PLGC has gained increasing attention from researchers and contributed to the field’s development.
Research on PLGC has been conducted in 90 countries/regions. The top 10 countries are shown in Table 1, with China and the United States dominating this field, accounting for approximately 53% of the total publications. China led in publication volume with 1147 publications and an h-index of 59. However, the United States had the highest citation count, with 49603 citations and an h-index of 107. Following these countries were Japan (12.52%), South Korea (8.74%), and Italy (6.03%). The annual publication trends of the top five countries are shown in Figure 2B, where China has demonstrated substantial growth in publication volume over the past 5 years, whereas other countries have shown steady trends. A visual analysis of the collaboration between countries/regions is shown in Figure 2C, where the node size represents the number of publications from each country/region, the connections between nodes represent collaborations, and the intensity of the color indicates the strength of the collaborations (total link strength). The United States had the most collaborative publications with other countries, followed by Germany, Japan, the United Kingdom, the Ne
| Country | Count | Citation | H-index | Institutions | Count | Citation | H-index |
| China | 1147 | 21150 | 59 | Harvard University | 130 | 6273 | 41 |
| United States | 1023 | 49603 | 107 | Vanderbilt University | 115 | 8495 | 45 |
| Japan | 513 | 19363 | 66 | United States Department of Veterans Affairs | 112 | 6810 | 44 |
| South Korea | 358 | 9249 | 50 | Veterans Health Administration | 109 | 6678 | 42 |
| Italy | 247 | 8659 | 50 | Peking University | 101 | 3323 | 30 |
| Germany | 245 | 13832 | 61 | Seoul National University | 90 | 3971 | 36 |
| United Kingdom | 182 | 12350 | 50 | Universidade Do Porto | 86 | 4682 | 38 |
| Netherlands | 142 | 10080 | 51 | Harvard Medical School | 81 | 4338 | 35 |
| France | 126 | 7636 | 44 | Shanghai Jiao Tong University | 74 | 1272 | 20 |
| Australia | 117 | 7044 | 40 | University of Texas System | 70 | 3402 | 34 |
Research on PLGC has been conducted in 200 institutions. The top 10 institutions are listed in Table 1. Harvard University (n = 130) and Vanderbilt University (n = 115) had the highest publication volumes. Vanderbilt University had the highest citation count, with 8495 citations and an h-index of 45. The top 10 institutions were primarily located in the United States (n = 6), followed by China (n = 2), South Korea (n = 1), and Portugal (n = 1). An analysis of collaborations between institutions is shown in Figure 2D, where the node size represents the number of publications by each institution, and the thickness of the connecting lines between nodes indicates the strength of the co-authorship relationships. Among the top 10 institutions, Vanderbilt University in the United States had the most collaborations, with strong ties to other institutions. This was followed by Seoul National University in South Korea and Peking University in China.
Research on PLGC has been published across 200 journals, with publication counts ranging from 4 to 168. The top 10 journals are listed in Table 2. The World Journal of Gastroenterology ranked first, with 168 publications, followed by Digestive Diseases and Sciences (n = 77), Gastroenterology (n = 71), and Gastrointestinal Endoscopy (n = 71). Seventy percent of the journals belong to the Q1 quartile of the Journal Citation Reports rankings.
| Journal | Count | JCR (2023) | IF (2023) |
| World Journal of Gastroenterology | 168 | Q1 | 4.3 |
| Digestive Diseases and Sciences | 77 | Q2 | 2.5 |
| Gastroenterology | 71 | Q1 | 25.7 |
| Gastrointestinal Endoscopy | 71 | Q1 | 6.7 |
| Endoscopy | 66 | Q1 | 11.5 |
| PLOS ONE | 59 | Q1 | 2.9 |
| Gut | 58 | Q1 | 23 |
| American Journal of Surgical Pathology | 54 | Q1 | 4.5 |
| Human Pathology | 52 | Q2 | 2.7 |
| Surgical Endoscopy and Other Interventional Techniques | 48 | Q2 | 2.4 |
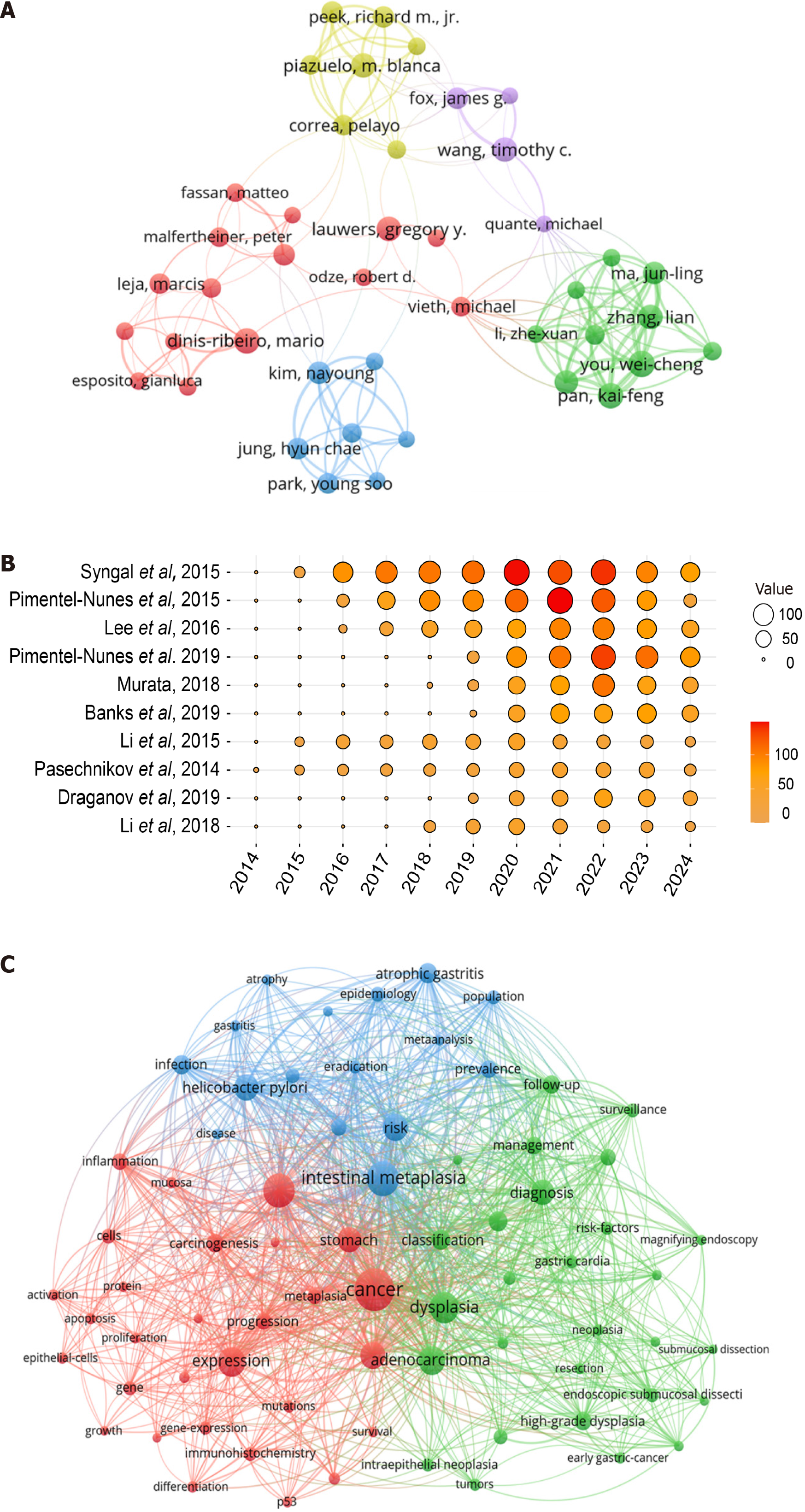
A statistical analysis identified the top 10 authors contributing in PLGC research (Table 3). Mario Dinis-Ribeiro led with 56 publications, followed by Wei-Cheng You (n = 53) and M Blanca Piazuelo (n = 52). The most frequently cited author was Timothy C Wang, with 4780 citations and an h-index of 30. Among the top 10 authors by publication volume, five were from the United States, three from China, and one each from Portugal and the Netherlands. A visualization of the collaboration network between the authors is shown in Figure 3A.
| Author | Country | Count | Citation | Average per item | H-index |
| Mario Dinis-Ribeiro | Portugal | 56 | 4478 | 83.66 | 31 |
| Wei-Cheng You | China | 53 | 2018 | 40.23 | 21 |
| M Blanca Piazuelo | United States | 52 | 3096 | 61.81 | 29 |
| Kai-Feng Pan | China | 45 | 1939 | 45.18 | 21 |
| Timothy C Wang | United States | 41 | 4780 | 119.1 | 30 |
| Gregory Y Lauwers | United States | 40 | 1499 | 38.83 | 22 |
| Richard M Peek | United States | 38 | 3637 | 97.58 | 29 |
| Pelayo Correa | United States | 35 | 3298 | 96.11 | 26 |
| Ernst J Kuipers | Netherlands | 34 | 3521 | 106.29 | 24 |
| Jun-Ling Ma | China | 33 | 1520 | 48.18 | 17 |
A statistical analysis of the top 10 highly cited papers in PLGC research is presented (Table 4), comprising two European guidelines[7,22], two guidelines from the American College of Gastroenterology[23,24], and one guideline from the British Society of Gastroenterology[25]. The remaining papers focused on topics such as H. pylori infection[26], inflammation[27], and biomarkers[28]. A visualization of the citation data for these papers is shown in Figure 3B.
| Title | First author | Journal | Year | Total citations |
| ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes | Sapna Syngal | American Journal of Gastroenterology | 2015 | 1013 |
| Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline | Pedro Pimentel-Nunes | Endoscopy | 2015 | 767 |
| Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis | Yi-Chia Lee | Gastroenterology | 2016 | 561 |
| Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019 | Pedro Pimentel-Nunes | Endoscopy | 2019 | 539 |
| Inflammation and cancer | Mariko Murata | Environmental Health and Preventive Medicine | 2018 | 381 |
| British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma | Matthew Banks | Gut | 2019 | 334 |
| Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer | Qier Li | Tumor Biology | 2015 | 324 |
| Gastric cancer: Prevention, screening and early diagnosis | Victor Pasechnikov | World Journal of Gastroenterology | 2014 | 304 |
| AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States | Peter V Draganov | Clinical Gastroenterology and Hepatology | 2019 | 264 |
| Plasma circular RNA profiling of patients with gastric cancer and their droplet digital RT-PCR detection | Tianwen Li | Journal of Molecular Medicine-Jmm | 2018 | 209 |
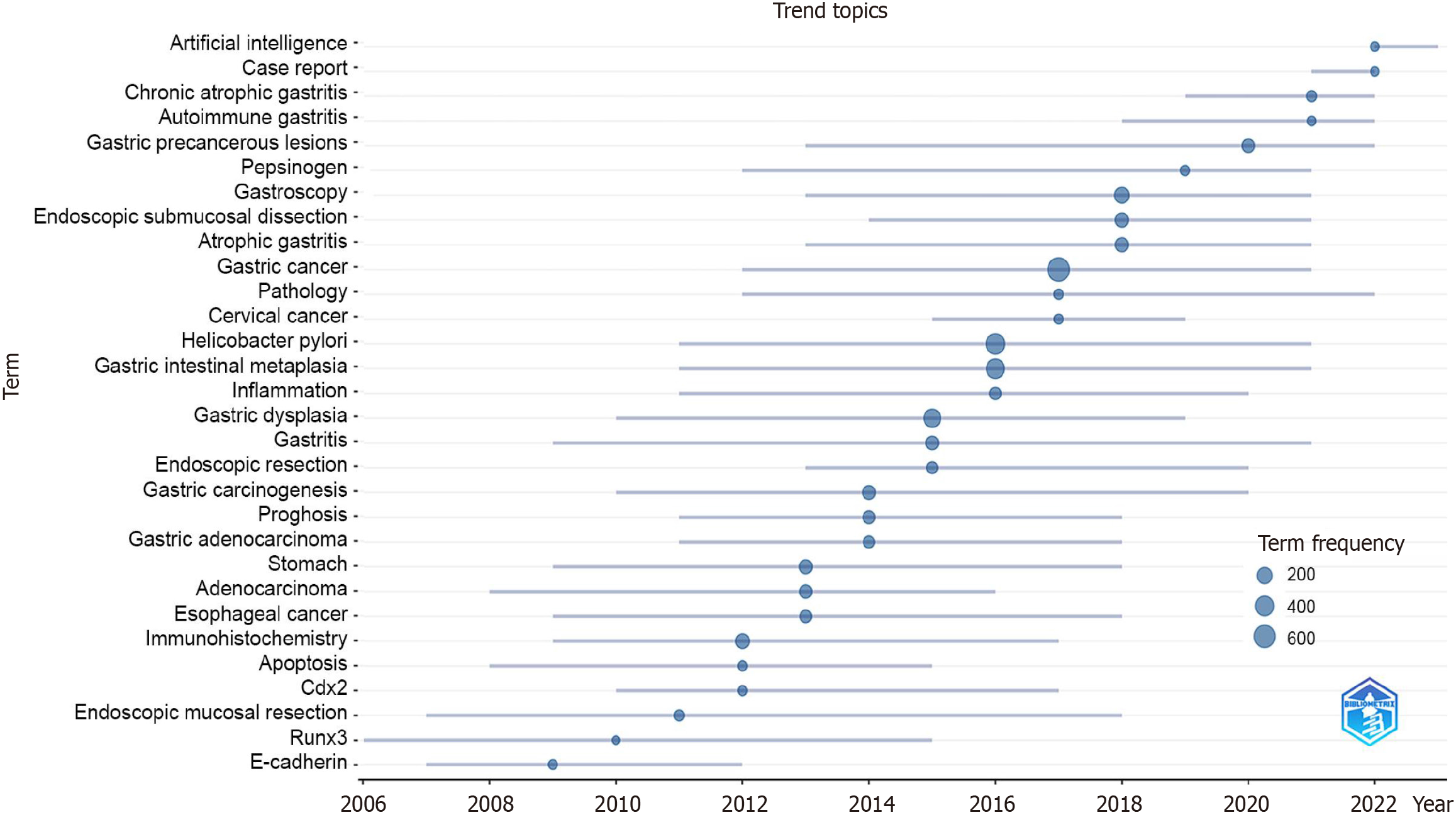
A statistical analysis of keywords in PLGC research was conducted to examine co-occurrence, bursts, and hotspot predictions. Using VOSviewer software, a co-occurrence analysis of keywords was performed (Figure 3C), dividing the network into three clusters that illustrated the foundational knowledge structure of the field. The blue cluster includes terms like “intestinal metaplasia”, “atrophic gastritis”, “helicobacter pylori”, “risk”, and “prevalence”, primarily related to the early pathological stages or risk factors of PLGC development. The red cluster is mainly associated with the me
As the “golden turning point” in the development of intestinal-type gastric cancer, the early detection and treatment of PLGC can reduce the incidence of gastric cancer. This study reviews nearly 20 years of research on PLGC, aiming to summarize the current state of the research using statistical data, track research hotspots, and predict future trends.
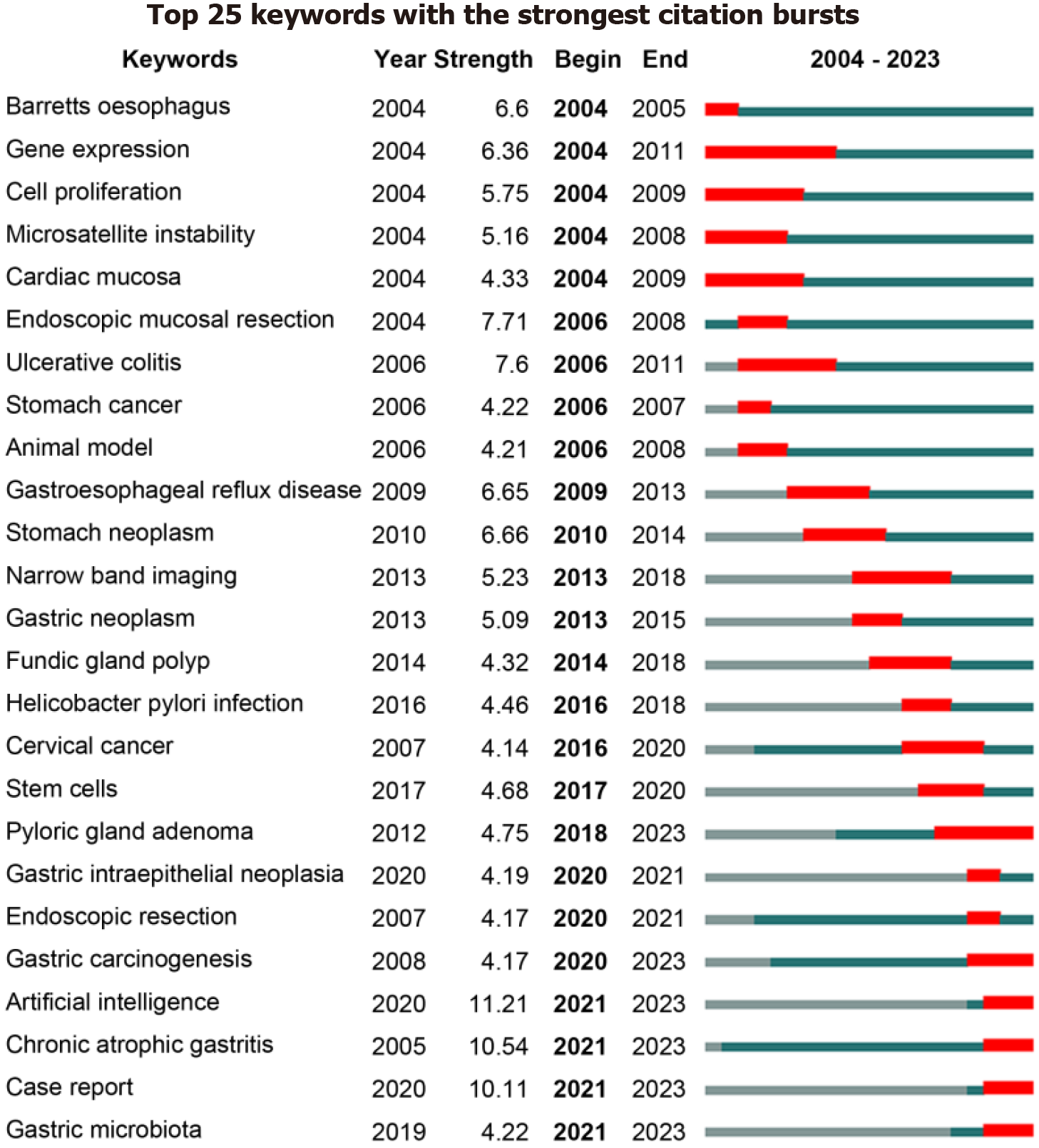
Over the past 20 years, the number of publications on PLGC has shown a general upward trend, with a substantial increase in 2019. This indicates growing interest from researchers in PLGC. A review of the literature reveals that 2019 saw the release of updated European guidelines for the management of epithelial precancerous conditions and lesions in the stomach (MAPS II), providing a reference framework for the diagnosis and treatment of PLGC[7]. In the same year, the United States released an update on clinical practices for endoscopic submucosal dissection, addressing many treat
The establishment of the concept of precancerous lesions has undergone a long evolution. In 1971, the WHO classified gastric cancer precursors into precancerous diseases and precancerous lesions, introducing the concept of precancerous lesions to describe pathological changes prone to transformation into cancerous tissue. In 1992, Correa et al[5] proposed the cascade model for the development of intestinal-type gastric cancer, emphasizing the multi-step and multi-stage process: Inflammation, atrophy, metaplasia, dysplasia, and carcinoma. This model highlight the complexity of the process. After approximately two decades of research, in 2012, Europe formulated guidelines on precancerous conditions and lesions in the stomach, defining chronic atrophic gastritis and intestinal metaplasia as precancerous conditions, and dysplasia as the most direct precancerous lesion[6], which led to a series of extensive clinical and basic research efforts. In 2019, the European guideline was updated, further categorizing dysplasia/intraepithelial neoplasia into low-grade and high-grade forms[7]. That same year, the WHO recommended the use of the term “dysplasia” in the gastrointestinal tract[29], a terminology that has gradually been accepted and applied by researchers. To gain a clearer understanding of the research focuses in the field of PLGC, we employed bibliometric methods to review PLGC-related research over the past 20 years. By integrating the keyword clustering results, we identified the following current research themes in the PLGC field: Risk factors, pathogenesis, as well as diagnosis and treatment.
Risk factors: H. pylori is recognized as a group 1 carcinogen, with a global infection rate of 44.3%[30]. Once H. pylori establishes persistent colonization of the gastric mucosa, it contributes to the development of PLGC through inflammatory responses, free radical production, DNA damage, and molecular events[31]. Pathogenic factors of H. pylori, such as cytotoxin-associated gene A, vacuolating cytotoxin A, and H. pylori-neutrophil-activating protein, exhibit proinflammatory activity, and the accumulation of an inflammatory microenvironment is a key factor in the progression of PLGC[32]. A 16-year follow-up study demonstrated that individuals with a persistent H. pylori infection for 16 years had a substantially higher likelihood of progressing to a more advanced diagnosis (P= 0.001)[33]. H. pylori eradication therapy has been shown to benefit patients with PLGC[34]. In addition to H. pylori, other risk factors for PLGC include age, gender, host genetic variations, and a high-salt diet[7,35].
Pathogenesis: The pathogenesis of PLGC, as identified through keyword clustering in this study, primarily focuses on inflammation, immune microenvironment, metaplasia, cell proliferation, stem cells, gene mutations, and gut microbiota. Chronic inflammation catalyzes cancer development[36] and is associated with genotoxicity, abnormal tissue repair, proliferative responses, invasion, and metastasis[37]. In the inflammatory microenvironment, reactive oxygen species and reactive nitrogen species released by inflammatory cells can damage cells, leading to DNA double-strand breaks, which in turn cause gene mutations, tumorigenic transformation, or cell death[38]. Various immune cells, such as neutrophils, T lymphocytes, macrophages, and fibroblasts, play crucial roles in the progression of PLGC[32]. Factors such as H. pylori infection, bile reflux, and alcohol consumption induce gastric mucosal damage, creating an environment conducive to metaplasia through inflammation and reactive oxygen species production. Long-term metaplasia, caused by sustained exposure, is a risk factor for dysplasia and malignant transformation[39]. Spasmolytic polypeptide-expressing metaplasia represents another form of intestinal metaplasia, with studies showing that interleukin-33 derived from epithelial cells stimulates specialized innate immune cells to produce interleukin-13, which induces chief cells to transdifferentiate into spasmolytic polypeptide-expressing metaplasia, followed by the loss of parietal cells from the gastric corpus[40].
Cancer has been described as a “wounds that will not heal”, where precancerous cells hijack normal regenerative pathways to promote abnormal growth[35]. With advancements in stem cell research, recent studies have identified dysplastic stem cell populations as novel cancer-initiating cells responsible for neoplastic transformation. Xenograft studies showed that CD44v6neg/CD133+/CD166+ (double positive) dysplastic stem cells can clonally evolve into various types of gastric adenocarcinoma, promoting cancer cell heterogeneity through the acquisition of additional gene mu
Diagnosis and treatment: Accurate diagnosis and grading of PLGC are crucial. With the advancements in modern en
In 2018, the National Cancer Institute launched a large-scale research project called the Precancer atlas, aiming to provide spatiotemporal histological and multi-omics mapping strategies for comprehensive characterization of the molecular and cellular features of premalignant lesions and their microenvironment[46]. However, its workflow is complex and requires collaboration across multidisciplinary teams, and the project is still ongoing[47]. Single-cell technology allows for the examination of differences between cell types and states within samples. Using RNA sequencing at single-cell resolution, researchers have characterized heterogeneous cell populations in precancerous lesions and gastric cancer, identifying enriched cyclin D1 mutations in the premalignant disease state, as well as discovering cancer-associated fibroblasts with pro-stemness properties[48]. Spatially resolved analysis techniques can evaluate differences between regions within lesions, changes in tissue structures of different cell types, and alterations in cell neighborhoods and tissue architecture. Researchers have employed bulk, single-cell, and spatial profiling strategies to attempt to map the complex processes of precancerous lesions[49]. With the advancement of aging and epigenetics research, a key molecular marker known as “epigenetic aging” has been associated with precancerous risk and initiation and has been shown to play a crucial role in predicting the timing of cancer onset in patients with precancerous lesions[35,50,51]. The interactions between the immune microenvironment, gut microbiota, and their metabolic products in precancerous lesions remain a hotspot for ongoing research[11].
Based on the above analysis, we predict that the mechanisms of PLGC development and molecular biomarkers of PLGC will remain key areas of ongoing research. Future research should focus on gut microbiota, immune microenvironment, metabolic reprogramming, and epigenetics. Emerging technologies, such as single-cell sequencing, spatially resolved analysis, multi-omics analysis, artificial intelligence, and machine learning, will further enhance research into the mechanisms of PLGC. Traditional formulas or chemical components have been shown to be effective in treating PLGC, demonstrating unique advantages through multiple pathways and multitarget comprehensive interventions[52].
Although we strictly adhered to bibliometric research methods, our study has certain limitations: (1) Selection bias: Our literature search was conducted using the WoSCC database and included only articles and reviews published in English, which may lead to incomplete literature retrieval; (2) Time bias: This study only included literature from 2004 to 2023. Given the rapid pace of research updates, it may not fully reflect the latest scientific trends and findings; and (3) Methodological bias: As the volume of data increases and the demand for more complex analytical methods grows, bibliometric analysis still has limitations in terms of the depth and breadth of data analysis. Future studies could integrate big data and artificial intelligence technologies to provide a more objective, accurate, comprehensive, and multi-faceted representation of the current state of research in the field.
This study systematically analyzed the current state of PLGC research on a global scale, highlighting research status and emerging trends in PLGC over the past 20 years and providing valuable references for future studies. Based on pu
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12252] [Article Influence: 6126.0] [Reference Citation Analysis (6)] |
| 2. | Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 1176] [Article Influence: 588.0] [Reference Citation Analysis (0)] |
| 3. | Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, Zhou Z, Yin P, Zhou M. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. 2023;8:e943-e955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 317] [Article Influence: 105.7] [Reference Citation Analysis (1)] |
| 4. | Arnold M, Park JY, Camargo MC, Lunet N, Forman D, Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020;69:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 5. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 6. | Dinis-Ribeiro M, Areia M, de Vries AC, Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C, Pimentel-Nunes P, Correia R, Ensari A, Dumonceau JM, Machado JC, Macedo G, Malfertheiner P, Matysiak-Budnik T, Megraud F, Miki K, O'Morain C, Peek RM, Ponchon T, Ristimaki A, Rembacken B, Carneiro F, Kuipers EJ; MAPS Participants; European Society of Gastrointestinal Endoscopy; European Helicobacter Study Group; European Society of Pathology; Sociedade Portuguesa de Endoscopia Digestiva. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Virchows Arch. 2012;460:19-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 713] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 8. | Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 450] [Article Influence: 37.5] [Reference Citation Analysis (1)] |
| 9. | Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, Mera R. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 490] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 10. | Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, Cano E, Castro D, Andrade O, Sánchez V, Garcia R, Buiatti E, Aebischer C, Franceschi S, Oliver W, Muñoz N. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst. 2007;99:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Borthwick Bowen M, Helmink BA, Wargo JA, Yates MS. TIME for Bugs: The Immune Microenvironment and Microbes in Precancer. Cancer Prev Res (Phila). 2023;16:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Rhodes JD, Goldenring JR, Lee SH. Regulation of metaplasia and dysplasia in the stomach by the stromal microenvironment. Exp Mol Med. 2024;56:1322-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Won Y, Jang B, Lee SH, Reyzer ML, Presentation KS, Kim H, Caldwell B, Zhang C, Lee HS, Lee C, Trinh VQ, Tan MCB, Kim K, Caprioli RM, Choi E. Oncogenic Fatty Acid Metabolism Rewires Energy Supply Chain in Gastric Carcinogenesis. Gastroenterology. 2024;166:772-786.e14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 14. | Kwon SK, Park JC, Kim KH, Yoon J, Cho Y, Lee B, Lee JJ, Jeong H, Oh Y, Kim SH, Lee SD, Hwang BR, Chung Y, Kim JF, Nam KT, Lee YC. Human gastric microbiota transplantation recapitulates premalignant lesions in germ-free mice. Gut. 2022;71:1266-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Fu K, Cheung AHK, Wong CC, Liu W, Zhou Y, Wang F, Huang P, Yuan K, Coker OO, Pan Y, Chen D, Lam NM, Gao M, Zhang X, Huang H, To KF, Sung JJY, Yu J. Streptococcus anginosus promotes gastric inflammation, atrophy, and tumorigenesis in mice. Cell. 2024;187:882-896.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 148] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 16. | Broadus RN. Toward a definition of “bibliometrics”. Scientometrics. 1987;12:373-379. [RCA] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 287] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Ninkov A, Frank JR, Maggio LA. Bibliometrics: Methods for studying academic publishing. Perspect Med Educ. 2022;11:173-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 468] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 18. | van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4505] [Cited by in RCA: 5632] [Article Influence: 331.3] [Reference Citation Analysis (2)] |
| 19. | Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724-728. [PubMed] |
| 20. | Aria M, Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. J Informetr. 2017;11:959-975. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1736] [Cited by in RCA: 2590] [Article Influence: 287.8] [Reference Citation Analysis (1)] |
| 21. | Hassan-montero Y, De-moya-anegón F, Guerrero-bote VP. SCImago Graphica: a new tool for exploring and visually communicating data. Profesional De La Información. 2022;31. [DOI] [Full Text] |
| 22. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 954] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 23. | Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223-62; quiz 263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1125] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 24. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 348] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 25. | Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 452] [Article Influence: 64.6] [Reference Citation Analysis (1)] |
| 26. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 720] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 27. | Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 431] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 28. | Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L, Wang B, Ye G, Xiao B, Guo J. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36:2007-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 29. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2723] [Article Influence: 453.8] [Reference Citation Analysis (3)] |
| 30. | Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, Derakhshan MH. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 527] [Article Influence: 65.9] [Reference Citation Analysis (1)] |
| 31. | Yang H, Mou Y, Hu B. Discussion on the common controversies of Helicobacter pylori infection. Helicobacter. 2023;28:e12938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Zhang S, Shen Y, Liu H, Zhu D, Fang J, Pan H, Liu W. Inflammatory microenvironment in gastric premalignant lesions: implication and application. Front Immunol. 2023;14:1297101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 33. | Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, Yepez MC, Realpe JL, Schneider BG, Morgan DR, Peek RM Jr, Correa P, Wilson KT, Piazuelo MB. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. 2018;67:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 34. | Zhu F, Zhang X, Li P, Zhu Y. Effect of Helicobacter pylori eradication on gastric precancerous lesions: A systematic review and meta-analysis. Helicobacter. 2023;28:e13013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Stangis MM, Chen Z, Min J, Glass SE, Jackson JO, Radyk MD, Hoi XP, Brennen WN, Yu M, Dinh HQ, Coffey RJ, Shrubsole MJ, Chan KS, Grady WM, Yegnasubramanian S, Lyssiotis CA, Maitra A, Halberg RB, Dey N, Lau KS. The Hallmarks of Precancer. Cancer Discov. 2024;14:683-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Afify SM, Hassan G, Seno A, Seno M. Cancer-inducing niche: the force of chronic inflammation. Br J Cancer. 2022;127:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 37. | Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1198] [Cited by in RCA: 1561] [Article Influence: 120.1] [Reference Citation Analysis (0)] |
| 38. | Mills KD, Ferguson DO, Alt FW. The role of DNA breaks in genomic instability and tumorigenesis. Immunol Rev. 2003;194:77-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 39. | Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 2017;17:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 40. | Meyer AR, Goldenring JR. Injury, repair, inflammation and metaplasia in the stomach. J Physiol. 2018;596:3861-3867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 41. | Min J, Zhang C, Bliton RJ, Caldwell B, Caplan L, Presentation KS, Park DJ, Kong SH, Lee HS, Washington MK, Kim WH, Lau KS, Magness ST, Lee HJ, Yang HK, Goldenring JR, Choi E. Dysplastic Stem Cell Plasticity Functions as a Driving Force for Neoplastic Transformation of Precancerous Gastric Mucosa. Gastroenterology. 2022;163:875-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int J Mol Sci. 2020;21:6402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 43. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 2440] [Article Influence: 271.1] [Reference Citation Analysis (1)] |
| 44. | Zhang M, Zhong J, Song Z, Xu Q, Chen Y, Zhang Z. Regulatory mechanisms and potential therapeutic targets in precancerous lesions of gastric cancer: A comprehensive review. Biomed Pharmacother. 2024;177:117068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Wang P, Li P, Chen Y, Li L, Lu Y, Zhou W, Bian L, Zhang B, Yin X, Li J, Chen J, Zhang S, Shi Y, Tang X. Chinese integrated guideline on the management of gastric precancerous conditions and lesions. Chin Med. 2022;17:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Srivastava S, Ghosh S, Kagan J, Mazurchuk R. The PreCancer Atlas (PCA). Trends Cancer. 2018;4:513-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Srivastava S, Ghosh S, Kagan J, Mazurchuk R; National Cancer Institute’s HTAN Implementation. The Making of a PreCancer Atlas: Promises, Challenges, and Opportunities. Trends Cancer. 2018;4:523-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Kim J, Park C, Kim KH, Kim EH, Kim H, Woo JK, Seong JK, Nam KT, Lee YC, Cho SY. Single-cell analysis of gastric pre-cancerous and cancer lesions reveals cell lineage diversity and intratumoral heterogeneity. NPJ Precis Oncol. 2022;6:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 49. | Chen Z, Lau KS. Advances in Mapping Tumor Progression from Precancer Atlases. Cancer Prev Res (Phila). 2023;16:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, Christensen BC, Gladyshev VN, Heijmans BT, Horvath S, Ideker T, Issa JJ, Kelsey KT, Marioni RE, Reik W, Relton CL, Schalkwyk LC, Teschendorff AE, Wagner W, Zhang K, Rakyan VK. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 639] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 51. | Yu M, Hazelton WD, Luebeck GE, Grady WM. Epigenetic Aging: More Than Just a Clock When It Comes to Cancer. Cancer Res. 2020;80:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 52. | Xu W, Li B, Xu M, Yang T, Hao X. Traditional Chinese medicine for precancerous lesions of gastric cancer: A review. Biomed Pharmacother. 2022;146:112542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |