Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.101174
Revised: December 4, 2024
Accepted: January 16, 2025
Published online: March 15, 2025
Processing time: 161 Days and 2.7 Hours
Hepatocellular carcinoma (HCC) is a malignant disease with high incidence and mortality worldwide. This study focuses on the TP53 target protein to investigate the potential therapeutic effect of tetrahydrocurcumin (THC) on HCC and its mechanism of action. The research hypothesis is that THC can inhibit the proliferation, migration, and invasion of HCC cells, and promote their apoptosis by regulating the TP53 target protein.
To explore the mechanism by which THC inhibits HCC cell proliferation via the TP53 signaling pathway.
Potential targets of THC and HCC were identified from multiple databases. The core targets were subjected to analyses using Gene Ontology and Kyoto Encyclopedia of Genes and Genomes databases, and visualization processing, using the online platform Metascape to identify the key molecules and signaling pathways involved in the action of THC against HCC. The molecular mechanisms of action of THC against TP53 in the inhibition of HCC cells were verified using cell counting kit-8, Transwell, apoptosis, and western blotting assays.
Molecular docking results showed that THC had a high score for the TP53 target protein. In vitro experiments indicated that THC effectively inhibited the proliferation and migration of HCC cells, and affected the expression levels of TP53, MDM2, cyclin B, Bax, Bcl-2, caspase-9, and caspase-3.
THC induces the apoptosis of HCC cells through the TP53 signaling pathway, thereby inhibiting their proliferation and migration.
Core Tip: This study investigated the molecular mechanisms by which tetrahydrocurcumin (THC), a derivative of curcumin, inhibits hepatocellular carcinoma cells. Utilizing a comprehensive approach that integrated network pharmacology, molecular docking, and in vitro experiments, the TP53 signaling pathway was identified as a critical mediator of the antitumor activity of THC.
- Citation: Bao ZC, Zhang Y, Liu ZD, Dai HJ, Ren F, Li N, Lv SY, Zhang Y. Tetrahydrocurcumin-induced apoptosis of hepatocellular carcinoma cells involves the TP53 signaling pathway, as determined by network pharmacology. World J Gastrointest Oncol 2025; 17(3): 101174
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/101174.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.101174
Hepatocellular carcinoma (HCC) is the leading cause of cancer-related deaths globally, and liver cirrhosis is known to cause 90% of HCC cases, based on epidemiological results[1]. Treatment options for HCC are limited[2], with tumor resection and liver transplantation being the most commonly used treatment methods. However, there are some problems with these methods, such as a high recurrence probability, difficulty in performing the operations, lack of a liver source, and a poor prognosis. Therefore, it is of great significance to identify effective drugs and active ingredients for the treatment of liver failure to inhibit the further development of liver disease and prevent death.
The combination of traditional Chinese medicine (TCM) and Western medicine has had a positive impact on the field of tumor treatment[3]. The anti-inflammatory properties, anti-cancer activity, and preventive effects of curcumin (CUR) against Alzheimer’s disease[4] have been widely reported. Tetrahydrocurcumin (THC), a CUR metabolite, is superior to CUR in terms of solubility, chemical stability, bioavailability, and antioxidant activity[5]. In addition, THC is more easily absorbed by the intestine than CUR, and studies have shown that THC reduces the occurrence of rectal cancer, increases the death of leukemia cells, and inhibits lymphosarcoma metastasis[6-8].
Network pharmacology is a new discipline based on systems biology theory, biological system network analysis, and the selection of specific signal nodes for multitarget drug design[9]. It is an effective tool for combined analysis of databases of TCM targets for various diseases and validating the identified targets at the molecular level, thus providing evidence for TCM molecular targets and possible mechanisms of action[10]. Network pharmacological methods have been used to predict the basis and mechanism of action of medicinal substances used in TCM, and it is a promising method to reveal the pharmacological mechanism of action of these substances[9].
In this study, the target mechanism of THC was predicted using network pharmacology, and the interaction between the drug and target was verified using molecular docking. In vitro cell counting kit-8 (CCK-8), cell cloning, scratch, and transwell assays were used to study the effects of different concentrations of THC on the proliferation, invasion, migration, and apoptosis of HCC cells. Western blotting confirmed the molecular mechanism by which THC promoted the apoptosis of HCC cells by acting on the target protein, TP53. This study provides a new approach for the research and development of novel anticancer drugs.
Collection and screening of THC-related targets: When predicting drug molecule targets, the potential pharmacological activity of new compounds is usually determined using three-dimensional (3D) structural information and the relationship between the distances between compounds and their targets in the 3D space. The UniProt database[10] (https://www.uniprot.org/) was used to convert target names into gene symbols and to summarize them. Preliminary experiments were performed to predict drug targets based on the similarity of small-molecule structures. The 3D chemical structure of THC was uploaded to SwissTargetPrediction[11](http://swisstargetprediction.ch/) for target prediction, followed by a literature search using the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) with “tetrahydrocurcumin” as the key word to identify other potential THC targets.
Collection and screening of HCC-related targets: Preliminary experiments were conducted to obtain HCC-related targets using the key word “hepatocellular carcinoma” in the GeneCards[12] (https://www.genecards.org/) and OMIM[13] (https://www.omim.org/) databases, with a median relevance score ≥ 2 times as the screening criterion.
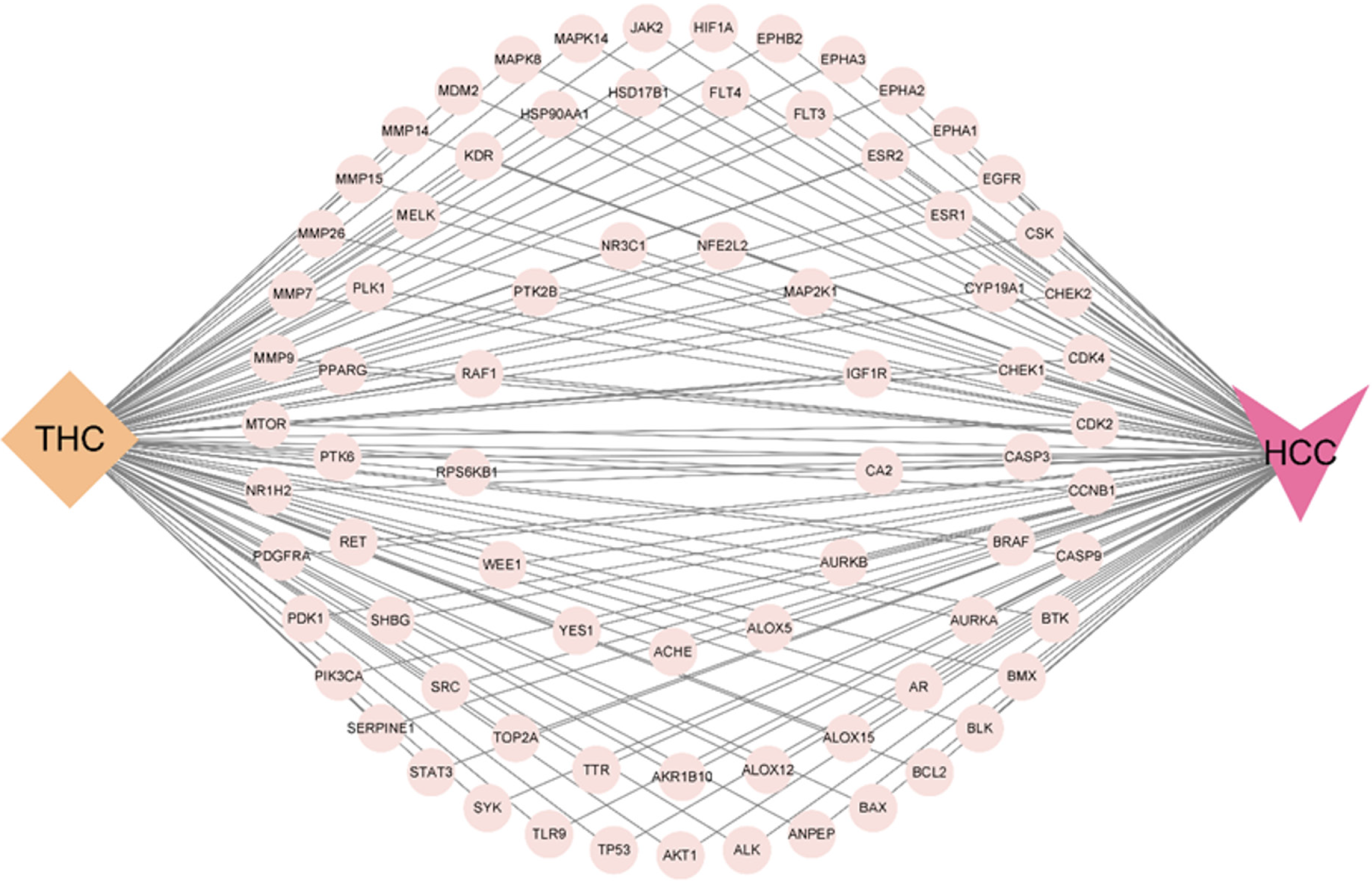
Screening of common targets between THC and hepatocytes and establishment of a network model: The drug targets of THC and the disease targets of HCC were imported into Venny2.1 (https://bioinfogp.cnb.csic.es/tools/venny/)[14], and a Venn diagram was constructed using an online Venn diagram analysis tool to obtain common therapeutic targets; that is, potential targets for the treatment of HCC with THC. Cytoscape3.9.1 software was used to establish a network and visualize the target information, resulting in a “THC-Target-HCC” network.
Establishment of a protein-protein interaction network and screening of core targets: The common targets of THC and HCC were imported into the STRING database[15] (https://cn.string-db.org/) to obtain their protein-protein interaction (PPI) network information, which was then imported into Cytoscape3.9.1 software[16]. The Centiscape plugin[17] was used for network analysis to select core disease targets. The degree (connectivity), betweenness (intermediary centrality), and closeness (proximity centrality) values were used as reference values to identify the core disease targets.
Gene Ontology functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis: The core targets obtained for THC and HCC were imported into Metascape (https://metascape.org/gp/index.html), with the species set to “Homo sapiens” for Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The resulting data were saved and visualized using an online visualization tool (https://www.bioinformatics.com.cn/).
Molecular docking: Molecular docking was performed on the THC molecules and key targets selected as described above, and the molecular interactions were scored. Drug targets were predicted for their potential biological activity based on their relative affinities for different sites of the compound, combined with the corresponding 3D structural information. Two-dimensional structural SDF files of THC and PDB format files of the core target proteins with a high degree were obtained from the PubChem[18] (https://pubchem.ncbi.nlm.nih.gov/) and PDB[19] (https://www.rcsb.org/) databases, respectively. Using ChemBio3D software, the SDF files of the active ingredients were converted to MOL2 files and subjected to energy minimization processing[20]. The target proteins and their active ingredients were then imported into Sybyl2.0 software, where the ligand molecules were extracted from the target proteins, water molecules were removed, and molecular docking was performed. A scoring function was used to evaluate them, with the highest score considered the optimal binding site, and a schematic diagram of molecular docking was constructed.
Cell culture and treatment: HepG2 cells and Huh7 cells were cultured in complete medium comprising 500 mL of high-glucose Dulbecco’s modified Eagle medium with 10% fetal bovine serum (FBS; 50 mL) and 1% antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin), and cultured at 37 °C in an incubator containing 5% CO2. The following experiments were performed when cells reached the logarithmic phase of growth.
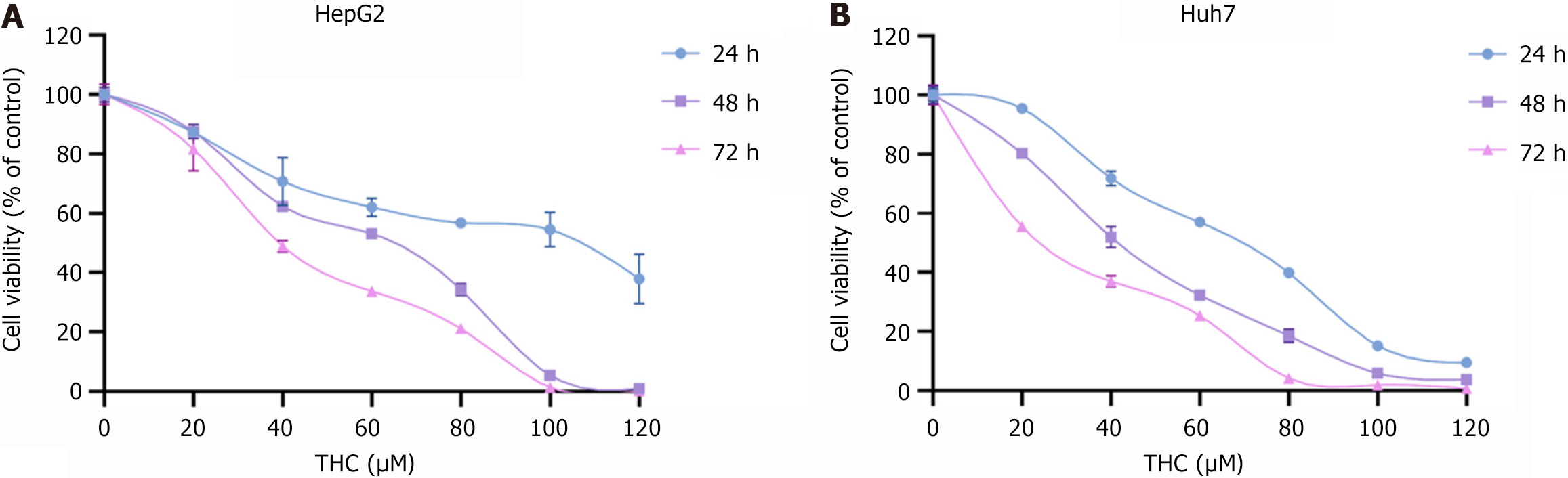
Cell viability was measured using a CCK-8 assay kit. HepG2 cells and Huh7 cells were inoculated into 96-well culture plates, and then exposed to different concentrations of THC (0, 20, 40, 60, 80, 100, and 120 μM), with five wells for each concentration, for 24, 48, and 72 hours. Subsequently, 10 μL of CCK-8 reagent was added to each well and the cells were incubated in a cell incubator at 37 °C for 2 hours. The absorbance was measured at 450 nm using an enzyme meter.
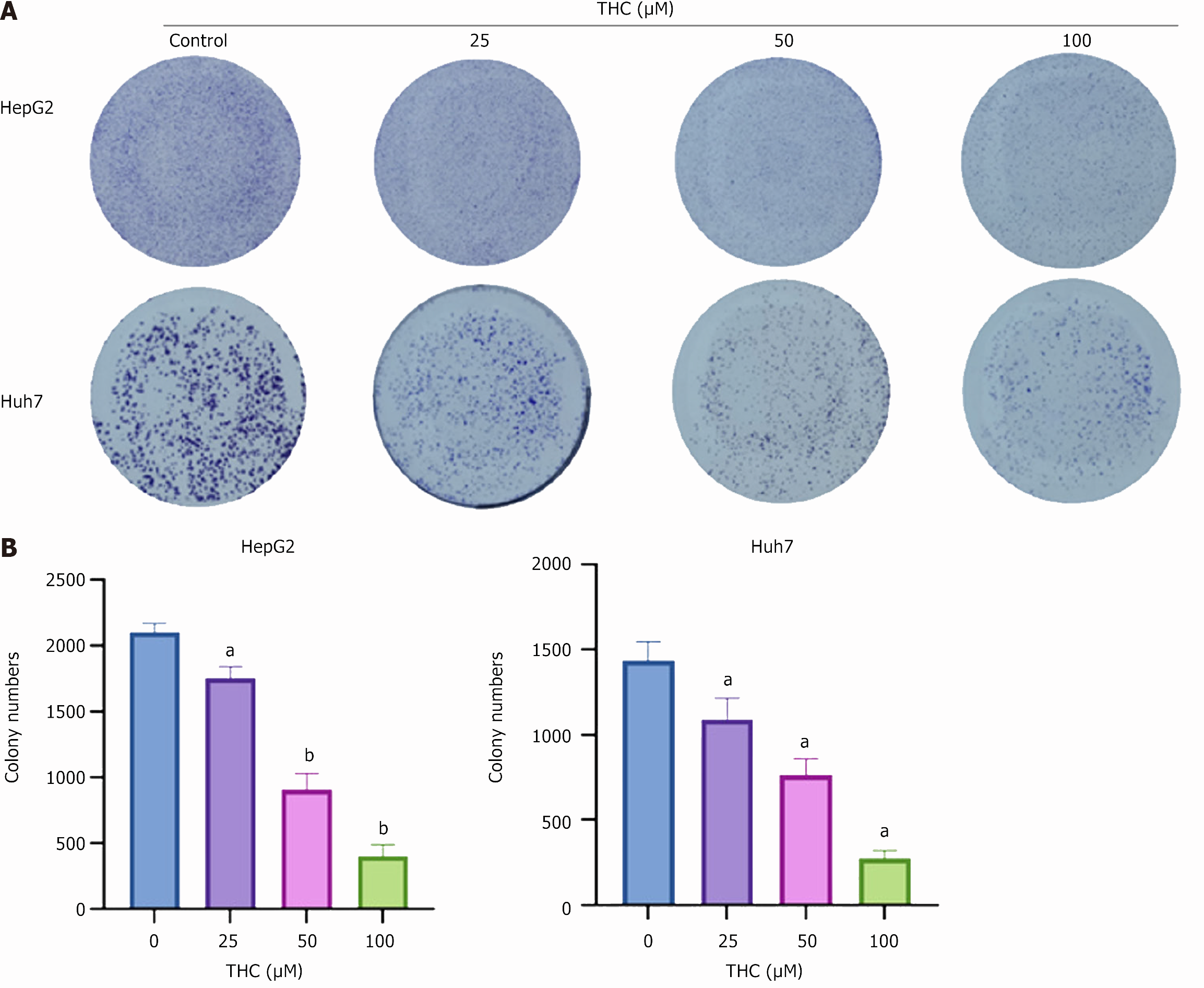
Colony-formation experiment: HepG2 and Huh7 cells in the logarithmic growth stage were inoculated in six-well plates at 500 cells per well. After cell adhesion, the medium was replaced with different concentrations of THC (0, 25, 50, 100 μM) and cultured in a cell incubator for 1-2 weeks, and the cell status was observed at any time. The culture was stopped when clonal clusters formed, as observed under a microscope. The cells were then gently washed three times with PBS, fixed with 4% paraformaldehyde for 15 minutes, washed three times with PBS, stained with crystal violet for 30 minutes, washed several times with PBS, and dried. The cell monolayers were counted and photographed.
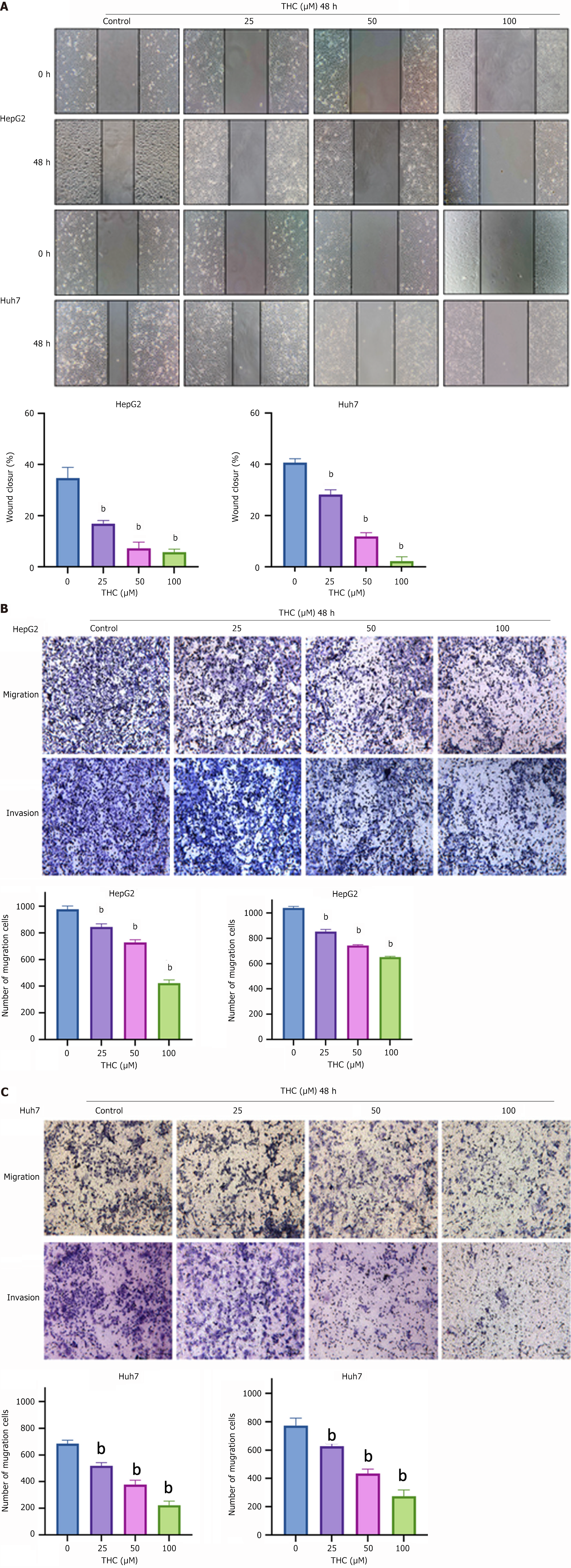
Wound-scratch experiment: After trypsin digestion and low-speed centrifugation, the cells were counted. They were then inoculated into a six-well Petri dish at 20 × 104 cells per well. When the cells were 70% adherent to the wall, a vertical scratch was made in the middle of the well using a 200 μL pipette tip. Low-serum medium containing different concentrations of THC (0, 25, 50, or 100 μM) was added to the wells. Five fields were selected for each scratch and photographs were taken under a microscope. This was recorded as 0 hours. After culturing in a cell incubator for 48 hours, photographs were taken again. ImageJ software (National Institutes of Health, Bethesda, MD, United States) was used for analysis, and the wound-healing percentage was calculated using the following formula: (area at 0 hours - area at 48 hours)/area at 0 hours.
Transwell experiment: HepG2 and Huh7 cells of logarithmic growth were resuspended in serum-free medium, and the migration and invasion abilities of the cells were examined using a two-compartment plate. The upper chamber was coated with matrix glue, cells in serum-free medium (100 μL per well) were inoculated in the upper chamber, and 700 μL of media containing FBS was added to the lower chamber. After the cells attached to the wall, the serum-free medium in the upper chamber was replaced with different concentrations of serum-free THC. After 48 hours, the outer surface of the upper chamber was fixed with 4% paraformaldehyde, and the cells passing through the pores were stained with crystal violet solution for 30 minutes, washed three times with PBS, and dried with an inverted microscope (10 × 10). Five fields of view of each upper chamber were randomly selected to observe and photograph the cells passing through the membrane. The average value count obtained using ImageJ was used for statistical analysis.
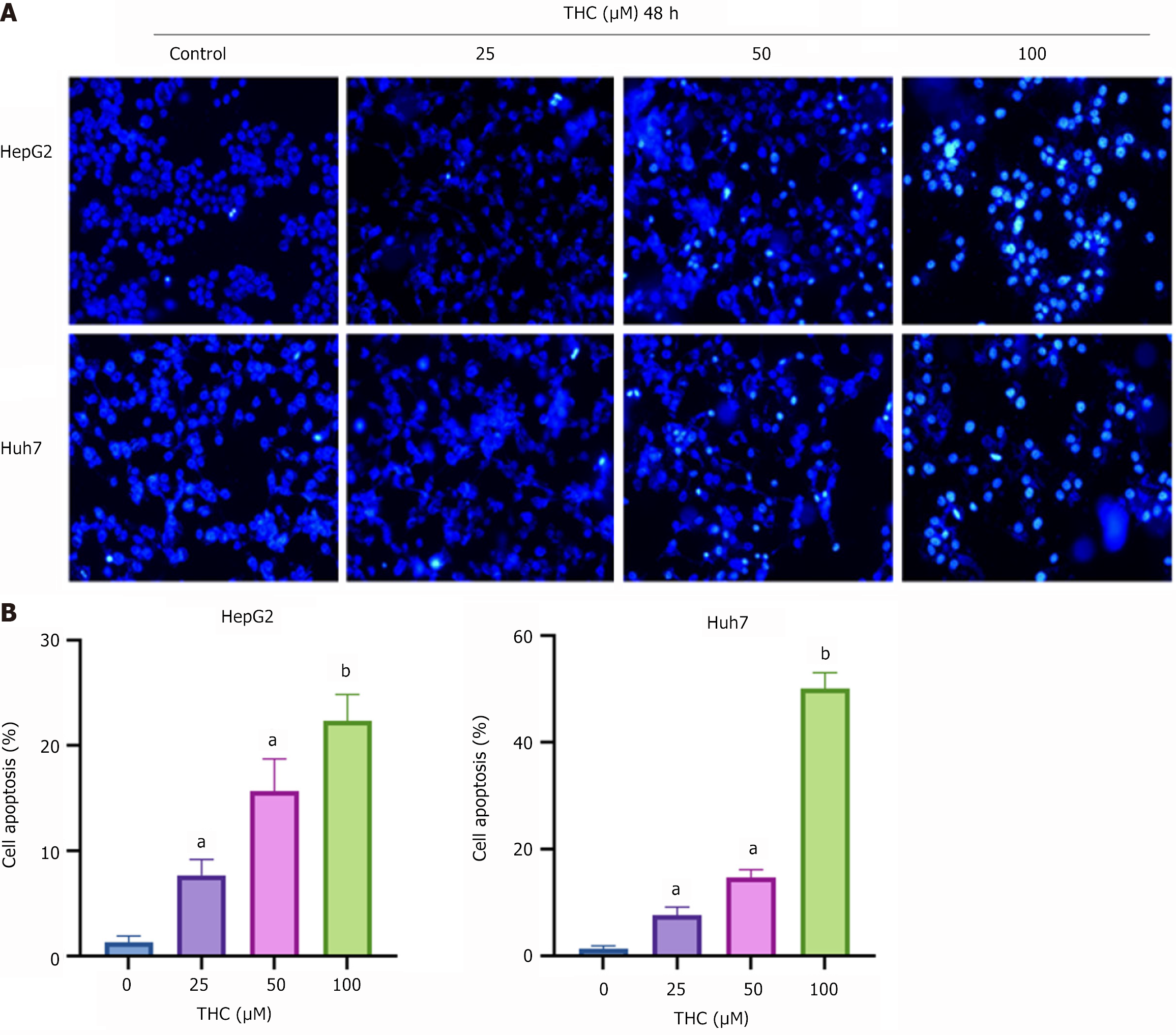
Hoechst 33342 staining: HepG2 and Huh7 cells in the logarithmic growth stage were thinly diluted and inoculated into a six-well plate at 20 × 104 cells per well. After the cells were affixed to the wall, they were washed with PBS and THC prepared in complete medium was added. After 48 hours, the THC was discarded, 1 mL of Hoechst 33342 dye solution was added to each well, and the cells were placed in an incubator at 37 °C for 20-30 minutes. The cells were washed 2-3 times with PBS and fluorescence was detected using a fluorescence microscope.
Western blotting analysis: HepG2 and Huh7 cells in six-well plates were treated with THC were washed three times with PBS. Radioimmunoprecipitation assay lysis buffer was added at 100 mL per well and the samples were shaken well on ice, scraped to with a cell scraper, and transferred to an Eppendorf tube, which was centrifuged in a pre-cooled centrifuge at 12000 rpm at 4 °C for 30 minutes. The resulting supernatant was collected and transferred to a fresh Eppendorf tube. A protein concentration detection kit was used to determine the protein concentration according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, United States). After the standard curve was obtained, the protein concentration was standardized in each Eppendorf tube using 1 × loading buffer, and the protein was denatured by boiling at 100 °C for 5-10 minutes in a metal bath, and stored at -20 °C for subsequent experiments. The obtained protein sample was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane, which was incubated in a blocking solution prepared with skim milk for 2 h. The PVDF membrane was then incubated with the corresponding primary antibody overnight. The membranes were washed with Tris-buffered saline with Tween 20 (TBST) and incubated with the corresponding secondary antibodies for 2 hours at room temperature. The membranes were washed again with TBST. Enhanced chemiluminescent reagent was added to the PVDF membrane according to the manufacturer’s instructions and images were captured. ImageJ software was used to analyze the grey values of the bands.
Statistical analysis was performed using GraphPad Prism 9.0 (GraphPad, San Diego, CA, United States) and IBM SPSS 22.0 (IBM, Armonk, NY, United States). Each experiment was performed on at least three independent occasions. Data are expressed as the mean ± SD. Student’s t-tests were used for pairwise comparisons between groups, with P < 0.05 as the cut-off value.
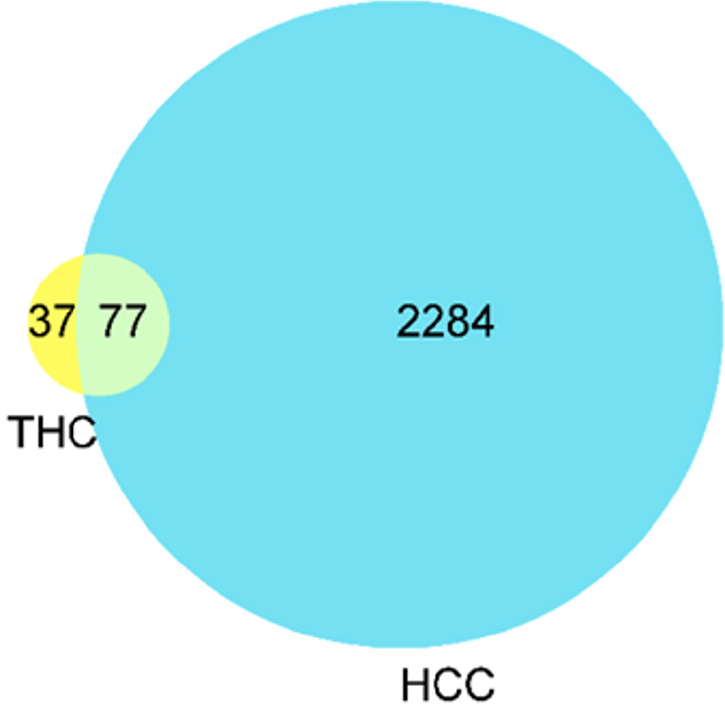
Seventy-seven targets overlapped with those related to the drug mechanism of action and the disease. A Wayne diagram of overlapping targets, namely the “THC-target-HCC” network diagram (Figure 1), was established to visualize potential target information and imported into Cytoscape 3.9.1 for visualization (Figure 2).
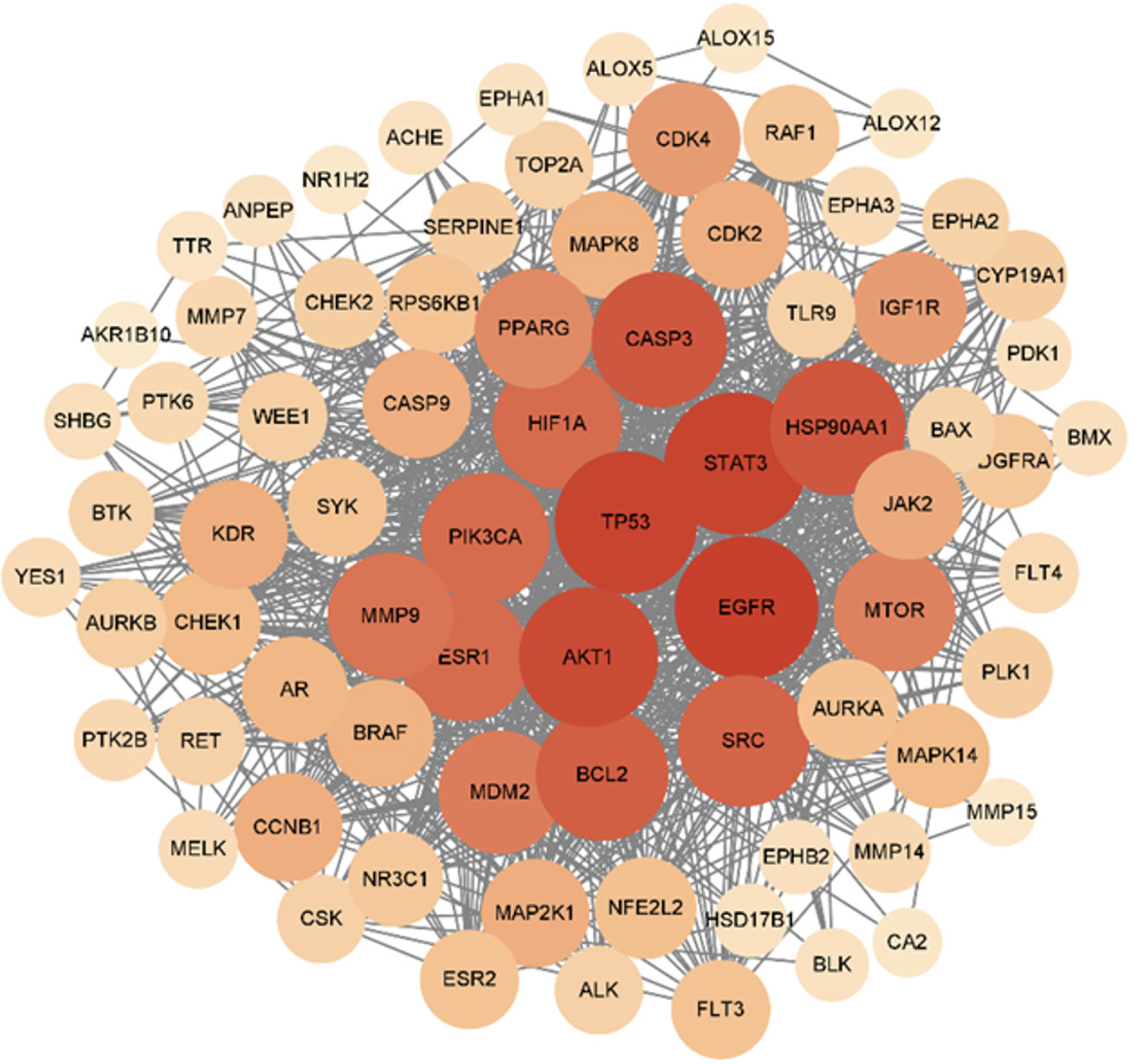
The 77 target protein data points were uploaded to the STRING platform and analyzed on the PPI network to determine the interactions between target proteins. This information was imported into Cytoscape 3.9.1 for visualization, with targets represented by nodes and interactions between targets represented by edges. After removing two isolated nodes, the PPI network consisted of 77 nodes and 767 interactive connections (Figure 3). Using the Centiscape plug-in for network topology analysis, we obtained degree, betweenness, and closeness values and used them as screening criteria to select 16 core targets (Table 1). Among them, the degree of the core target, P53, was the largest.
| Name | Betweenness centrality | Closeness centrality | Average degree value |
| TP53 | 537.5688911 | 0.011111111 | 58 |
| HSP90AA1 | 411.5735117 | 0.010416667 | 53 |
| STAT3 | 280.5350034 | 0.010309278 | 52 |
| AKT1 | 451.5531513 | 0.010204082 | 50 |
| SRC | 297.0466707 | 0.010000000 | 49 |
| EGFR | 222.7036191 | 0.00990099 | 48 |
| CASP3 | 233.6425731 | 0.009803922 | 47 |
| PIK3CA | 159.1340985 | 0.00952381 | 46 |
| MTOR | 126.8320074 | 0.009433962 | 43 |
| ESR1 | 202.0774342 | 0.009345794 | 43 |
| HIF1A | 109.3920337 | 0.009259259 | 41 |
| MDM2 | 66.2593338 | 0.008928571 | 37 |
| MMP9 | 230.2669059 | 0.00877193 | 34 |
| PPARG | 269.6527851 | 0.008695652 | 33 |
| KDR | 65.5729245 | 0.008130081 | 26 |
| AR | 63.40344608 | 0.008064516 | 26 |
GO enrichment analysis revealed 495 biological processes, 19 cell components, and 39 molecular functions. The top 10 were selected from the results to create a bubble diagram (Figure 4A). The biological processes related to cancer involved in the core targets mainly included the enzyme-linked receptor protein signaling pathway and transmembrane receptor protein tyrosine kinase signaling pathway.
Enrichment analysis of the KEGG pathways revealed 97 pathways related to the core targets. The top 30 were selected to make the bubble map (Figure 4B), with larger P values presented as a redder color, indicating that the level of enrichment was higher. As shown in Figure 4B, the signaling pathway involving P53 was related to nine directions of cancer, including central carbon metabolism, endocrine resistance, human cytomegalovirus infection, lipids and atherosclerosis, EGFR tyrosine kinase inhibitor resistance, advanced cancer, and protein glycosides in cancer.
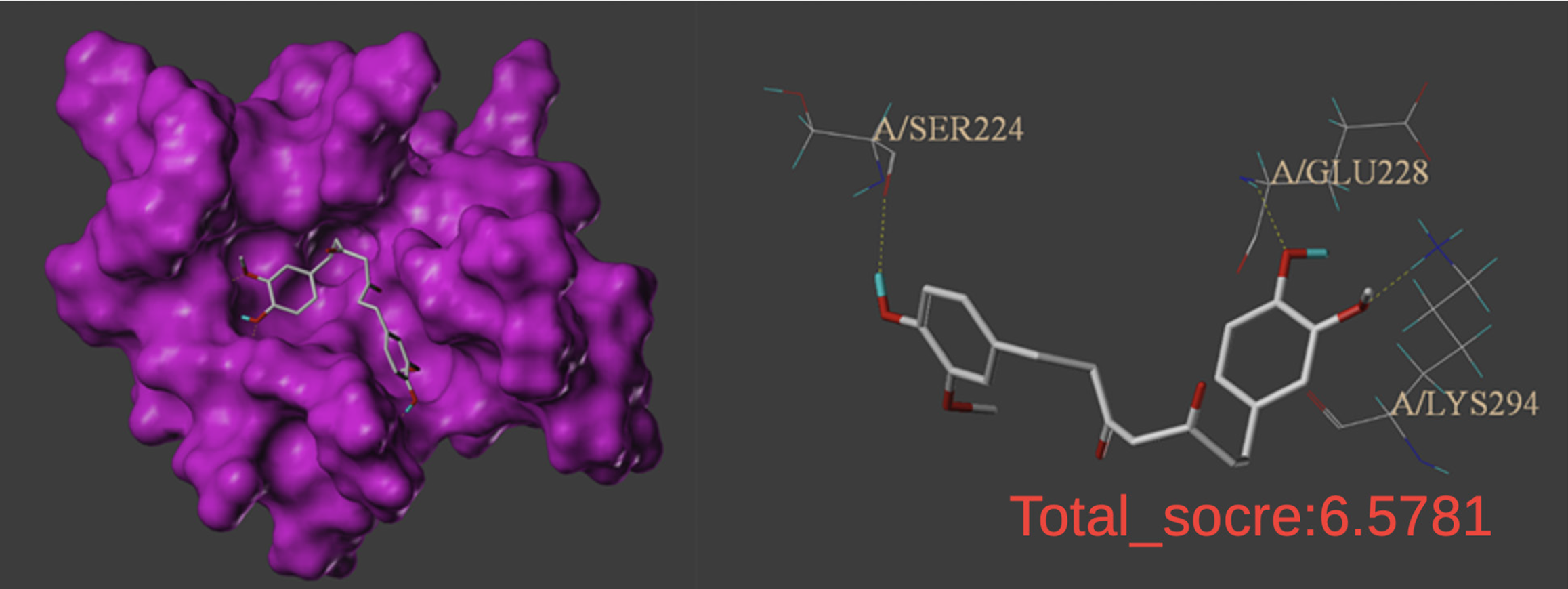
The TP53 gene is a key tumor suppressor gene in humans, and the P53 protein it encodes controls cell growth, repairs DNA damage, and induces cell stress to promote cell cycle arrest, apoptosis, or senescence, thereby inhibiting tumors. Molecular docking was used to verify the binding ability of TCM components to 16 key protein targets. Figure 5 shows a diagram of the core molecular docking results. THC showed certain binding activity by forming three hydrogen bonds in the hydrophobic structure formed by the Ser224, Glu228, and Lys294 amino acid residues of P53. A total score ≥ 5 was used as the threshold for the docking results. A higher the total score indicated more stable binding between the ligand and its receptor. The target protein score for P53 was 6.5781, which indicated that the degree of binding of the active ingredients to P53 was good.
The CCK-8 assay was used to examine the effects of THC on HCC cell activity. The results showed that THC inhibited the proliferative activity of HepG2 and Huh7 cells at different time points (Figure 6). With increasing treatment time and drug concentration, the cell activity gradually decreased. The IC50 values of HepG2 cells treated with THC for 24, 48, and 72 hours were 95.30, 53.84, and 40.21 μM, respectively. The IC50 values for Huh7 cells at 24, 48, and 72 hours were 62.45, 40.53, and 25.15 μM.
We tested the effects of THC on the proliferative capacity of HCC cells using a plate cloning experiment. The drug was administered at different concentrations for 2 weeks, and the results showed that, compared to the blank control group, THC inhibited the colony formation of HepG2 and Huh7 cells (Figure 7). With a gradual increase in drug concentration, the number of HCC cells gradually decreased.
In the cell scratch experiment, the healing areas of HepG2 and Huh7 cells were observed after the cells were treated with 25, 50, and 100 μM THC for 48 hours, and the experimental results are shown in Figure 8. The migration ability of the two HCC cell lines decreased with increasing drug concentrations. The healing area was greater for cells treated with a low drug concentration for those treated with a high drug concentration. Thus, THC had an inhibitory effect on HCC cell migration. These results were verified using transwell experiments, which showed that the invasion and migration abilities of HCC cells decreased with increasing THC drug concentration (Figure 8).
Hoechst 33342 was used to stain HCC cells treated with different concentrations of THC. When apoptosis occurred, the nuclei of the apoptotic cells showed dense staining under a fluorescence microscope. The results showed that the number of cells decreased and the number of apoptotic bodies increased as the THC concentration increased, indicating that THC promoted the apoptosis of HCC cells (Figure 9).
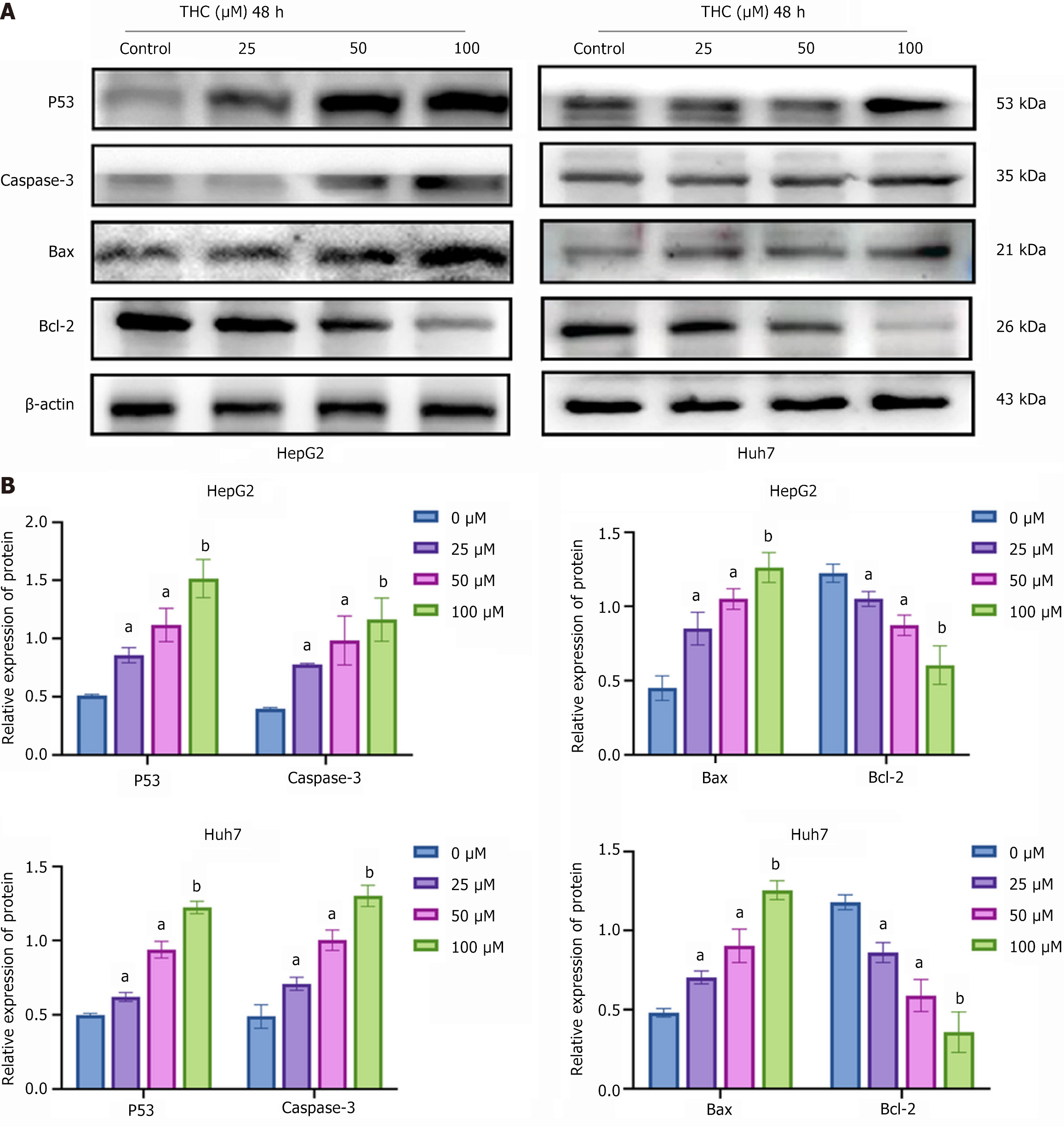
Western blotting was performed to analyze the expression of P53 signaling pathway-related proteins in HCC cells treated with different concentrations of THC for 48 hours. After THC treatment, the expression levels of pro-apoptotic proteins, P53, caspase-3, Bax, and Bcl-2 increased, thus promoting apoptosis (Figure 10).
Primary liver cancer is the second leading cause of death from malignant tumors in China[21], with 90% originating from HCC, which is the fifth most common cancer in the world and the second most common cause of cancer-related deaths. Currently, there are no effective treatments for these diseases at the advanced stage. Most clinical treatments for HCC include surgical resection, and postoperative liver failure is the main cause of death and morbidity after partial liver resection[22]. TCM anti-tumor drugs have the characteristics of multi-targeting, few side effects, and a medium price, and thus, they have become a new direction for anti-tumor drug research and development. Therefore, research and development of TCM anti-tumor drugs are of great value.
Previously, we used network pharmacology to analyze the targets of TCM through a combination of bioinformatics and in vitro experiments, and the mechanism of action was identified based on the biomolecular network of the drugs’ targets[23]. In this study, through the screening of relevant literature and previously published data, 114 targets of THC were successfully identified, 2361 targets related to HCC were identified, and 16 potential core targets for the treatment of HCC with THC were identified. In total, 77 intersecting targets related to THC and HCC were identified, including AKT1, CASP3, MDM2, KDR, PIK3CA, and P53. In addition, Cytoscape 3.9.1 was used to construct a compound target network and a PPI network to visualize common target proteins. The proteins with a high degree in the PPI network, indicating that they were more likely to be core targets of THC, were P53, HSP90AA1, and STAT3. The degree value of TP53 was the highest, indicating that the anti-HCC mechanism of THC was most closely related to the biological function of TP53. Notably, TP53 is an important tumor suppressor gene in humans, and its expression is upregulated in various tumors. The TP53 gene product is an important regulatory factor for cell growth, proliferation, and DNA damage repair, and its expression can be induced when cells are under stress, thus promoting cell cycle arrest, followed by apoptosis or senescence. This is the fundamental mechanism underlying the apoptosis induced by TP53. Thoenen et al[24] demonstrated that TP53 plays a key role as a tumor suppressor in nine types of bone and soft tissue sarcomas, including osteosarcoma. TP53 mutations are useful markers of tumor clonality, and the detection of TP53 mutations may also help identify early lesions with a high evolutionary risk[25]. TP53 mutations are also associated with poor prognosis in cancers such as breast cancer, colorectal cancer, and leukemia[26]. However, Mogi and Kuwano[27] found that the poor prognosis of small cell lung cancer was not associated with TP53 mutations. Tashakori et al[28] reported that mutant TP53 is an adverse risk factor for acute myeloid leukemia, and Zhu et al[29] found that P53 is involved in the therapeutic mechanism of trizolamide in the treatment of connective tissue diseases. Other mediators of TP53-related signaling pathways have also been extensively studied. For example, functional polymorphisms of caspase-9 have been shown to be associated with lung, bladder, pancreatic, colorectal, and gastric cancer[30]. Downregulation and mutations of Bax play important roles in tumor resistance to apoptosis[31]. In conclusion, TP53 is closely associated with the prevention and treatment of tumors and the prognosis of affected patients, providing a new direction for the study of the effects of novel drugs on HCC.
In this study, we combined network pharmacology to explore the role of the TP53 signaling pathway in the THC-induced apoptosis of HCC cells and showed that THC had effective anti-HCC activity by acting on TP53 target proteins and various upstream and downstream proteins; however, the specific mechanism of the anti-HCC effect of THC remains to be clarified. Although in vivo experiments can comprehensively evaluate the effects of drugs, there are limitations in controlling the dose and clarifying the mechanism of action[32]. By contrast, in vitro experiments, the dose can be controlled more precisely and it is possible to delve deeper into the molecular mechanisms of action of the drug. In this study, we investigated the effects of THC and CUR on tumor cells using in vitro experiments with accurately controlled drug concentrations. These experiments revealed the molecular mechanism whereby THC inhibits tumors through the mitochondrial apoptosis pathway, and provided a scientific basis for drug development and dose optimization. Previous studies have shown that THC can affect the metabolic pathway of the CYP450 enzyme; inhibit the expression of CYP1A1 and the activation of NF-κB pathway; and thus, inhibit the migration and invasion of breast cancer cells[33]. THC can reduce the expression levels of Ki67 and inhibit the proliferation of cervical cancer cells[34]. THC can inhibit the function of P-glycoprotein (a drug transporter associated with multi-drug resistance) in acute myeloid leukemia, and significantly increase the sensitivity of cancer cells to vinblastine, mitoxantrone, and etoposide[35]. Therefore, THC inhibits a variety of tumors and exertis anti-tumor effects through multiple targets and pathways.
Overall, TCM anti-tumor drugs have multiple targets, few side effects, and a medium price, and thus, they have become a new direction for the research and development of anti-tumor drugs. Using network pharmacological techniques combined with in vitro experiments, we clearly demonstrated that THC inhibited the proliferation, migration, and invasion and induced the apoptosis of HCC cells via TP53. These findings provide scientific evidence for the development of new and more effective anti-cancer drugs and a new reference for the prevention and treatment of HCC.
Through a combination of network pharmacology and in vitro experiments, we revealed the molecular mechanisms by which THC inhibits the proliferation, migration, and invasion and promotes the apoptosis of HCC cells by regulating the P53 signaling pathway. The results showed that THC has potential as a new therapeutic target for HCC and they provide a scientific basis for the development of novel anticancer drugs. This is of great significance for the clinical prevention and treatment of HCC.
| 1. | Ganesan P, Kulik LM. Hepatocellular Carcinoma: New Developments. Clin Liver Dis. 2023;27:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 294] [Article Influence: 98.0] [Reference Citation Analysis (1)] |
| 2. | Nia A, Dhanasekaran R. Genomic Landscape of HCC. Curr Hepatol Rep. 2020;19:448-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Yu YX, Wang S, Liu ZN, Zhang X, Hu ZX, Dong HJ, Lu XY, Zheng JB, Cui HJ. Traditional Chinese medicine in the era of immune checkpoint inhibitor: theory, development, and future directions. Chin Med. 2023;18:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 4. | Wu JC, Tsai ML, Lai CS, Wang YJ, Ho CT, Pan MH. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014;5:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Lai CS, Ho CT, Pan MH. The Cancer Chemopreventive and Therapeutic Potential of Tetrahydrocurcumin. Biomolecules. 2020;10:831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Lai CS, Wu JC, Yu SF, Badmaev V, Nagabhushanam K, Ho CT, Pan MH. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol Nutr Food Res. 2011;55:1819-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Yodkeeree S, Garbisa S, Limtrakul P. Tetrahydrocurcumin inhibits HT1080 cell migration and invasion via downregulation of MMPs and uPA. Acta Pharmacol Sin. 2008;29:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Wu JC, Lai CS, Badmaev V, Nagabhushanam K, Ho CT, Pan MH. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol Nutr Food Res. 2011;55:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Zhao L, Zhang H, Li N, Chen J, Xu H, Wang Y, Liang Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 546] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 10. | Gan D, Xu X, Chen D, Feng P, Xu Z. Network Pharmacology-Based Pharmacological Mechanism of the Chinese Medicine Rhizoma drynariae Against Osteoporosis. Med Sci Monit. 2019;25:5700-5716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Daina A, Zoete V. Application of the SwissDrugDesign Online Resources in Virtual Screening. Int J Mol Sci. 2019;20:4612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Fishilevich S, Nudel R, Rappaport N, Hadar R, Plaschkes I, Iny Stein T, Rosen N, Kohn A, Twik M, Safran M, Lancet D, Cohen D. GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database (Oxford). 2017;2017:bax028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 876] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 13. | Amberger JS, Hamosh A. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr Protoc Bioinformatics. 2017;58:1.2.1-1.2.12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 467] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 14. | Jiang G, Sun C, Wang X, Mei J, Li C, Zhan H, Liao Y, Zhu Y, Mao J. Hepatoprotective mechanism of Silybum marianum on nonalcoholic fatty liver disease based on network pharmacology and experimental verification. Bioengineered. 2022;13:5216-5235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, Jensen LJ, von Mering C. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605-D612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1211] [Cited by in RCA: 5263] [Article Influence: 1052.6] [Reference Citation Analysis (0)] |
| 16. | Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498-2504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24663] [Cited by in RCA: 35741] [Article Influence: 1624.6] [Reference Citation Analysis (7)] |
| 17. | Scardoni G, Petterlini M, Laudanna C. Analyzing biological network parameters with CentiScaPe. Bioinformatics. 2009;25:2857-2859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 18. | Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49:D1388-D1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2091] [Cited by in RCA: 2358] [Article Influence: 471.6] [Reference Citation Analysis (0)] |
| 19. | Shin JM, Cho DH. PDB-Ligand: a ligand database based on PDB for the automated and customized classification of ligand-binding structures. Nucleic Acids Res. 2005;33:D238-D241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Habeeb MM, Al-Attas AS, Al-Raimi DS. Spectroscopic studies and molecular orbital calculations of charge transfer complexation between 3,5-dimethylpyrazole with DDQ in acetonitrile. Spectrochim Acta A Mol Biomol Spectrosc. 2015;142:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Piantanida E, Ippolito S, Gallo D, Masiello E, Premoli P, Cusini C, Rosetti S, Sabatino J, Segato S, Trimarchi F, Bartalena L, Tanda ML. The interplay between thyroid and liver: implications for clinical practice. J Endocrinol Invest. 2020;43:885-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 23. | Wang X, Wang ZY, Zheng JH, Li S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. 2021;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 24. | Thoenen E, Curl A, Iwakuma T. TP53 in bone and soft tissue sarcomas. Pharmacol Ther. 2019;202:149-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1544] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 26. | Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 695] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 27. | Mogi A, Kuwano H. TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol. 2011;2011:583929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 28. | Tashakori M, Kadia T, Loghavi S, Daver N, Kanagal-Shamanna R, Pierce S, Sui D, Wei P, Khodakarami F, Tang Z, Routbort M, Bivins CA, Jabbour EJ, Medeiros LJ, Bhalla K, Kantarjian HM, Ravandi F, Khoury JD. TP53 copy number and protein expression inform mutation status across risk categories in acute myeloid leukemia. Blood. 2022;140:58-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 29. | Zhu W, Li Y, Zhao J, Wang Y, Li Y, Wang Y. The mechanism of triptolide in the treatment of connective tissue disease-related interstitial lung disease based on network pharmacology and molecular docking. Ann Med. 2022;54:541-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Li P, Zhou L, Zhao T, Liu X, Zhang P, Liu Y, Zheng X, Li Q. Caspase-9: structure, mechanisms and clinical application. Oncotarget. 2017;8:23996-24008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 31. | Liu Z, Ding Y, Ye N, Wild C, Chen H, Zhou J. Direct Activation of Bax Protein for Cancer Therapy. Med Res Rev. 2016;36:313-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 32. | Liu W, Zhang Z, Lin G, Luo D, Chen H, Yang H, Liang J, Liu Y, Xie J, Su Z, Cao H. Tetrahydrocurcumin is more effective than curcumin in inducing the apoptosis of H22 cells via regulation of a mitochondrial apoptosis pathway in ascites tumor-bearing mice. Food Funct. 2017;8:3120-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Zeng A, Yu X, Chen B, Hao L, Chen P, Chen X, Tian Y, Zeng J, Hua H, Dai Y, Zhao J. Tetrahydrocurcumin regulates the tumor immune microenvironment to inhibit breast cancer proliferation and metastasis via the CYP1A1/NF-κB signaling pathway. Cancer Cell Int. 2023;23:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 34. | Yoysungnoen B, Bhattarakosol P, Changtam C, Patumraj S. Effects of Tetrahydrocurcumin on Tumor Growth and Cellular Signaling in Cervical Cancer Xenografts in Nude Mice. Biomed Res Int. 2016;2016:1781208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Tseng YH, Chiou SS, Weng JP, Lin PC. Curcumin and tetrahydrocurcumin induce cell death in Ara-C-resistant acute myeloid leukemia. Phytother Res. 2019;33:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/