Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.100861
Revised: December 29, 2024
Accepted: January 13, 2025
Published online: March 15, 2025
Processing time: 169 Days and 22.4 Hours
Hepatocellular carcinoma (HCC) has become a growing health concern globally. Microvascular invasion and high tumor burden are key factors limiting the cura
This case study reports a 49-year-old woman who was diagnosed with China Liver Cancer Staging (CNLC) IIIa HCC and > 15 cm tumor diameter. Initially, due to insufficient future liver remnant and vascular invasion, the tumor was unresectable; however, radical hepatectomy was performed after successful conversion therapy with SIRT using yttrium-90 (90Y) resin microspheres followed by hepatic arterial infusion chemotherapy (HAIC) with tyrosine kinase inhibitor (TKI) and anti-programmed death-1 (PD-1) antibody. SIRT using 90Y resin microspheres was given by the right hepatic artery and chemoembolization was simultaneously performed in the tumor’s feeding vessels from the right diaphragmatic artery. HAIC was followed every three weeks with lenvatinib and tislelizumab. At 4 months post-SIRT, the tumor was downstaged to CNLC Ib and the patient successfully under
This case study provides evidence for an integrated treatment strategy combining SIRT and HAIC with TKI and anti-PD-1 antibodies for patients with large HCC and microvascular invasion. Further confirmatory trials are re
Core Tip: Although selective internal radiation therapy (SIRT) has been used to treat unresectable hepatic cancers for more than 20 years, it is mainly employed to treat patients with ≤ 8 cm tumor size. This research reports a hepatocellular carcinoma patient with > 15 cm hepatic mass, microvascular invasion, and China Liver Cancer Staging (CNLC) IIIa who received radical hepatectomy after successful conversion therapy with SIRT using yttrium-90 resin microspheres followed by hepatic arterial infusion chemotherapy using anti-programmed death-1 antibody and tyrosine kinase inhibitor. After 4 months of SIRT, the tumor was downstaged to CNLC Ib and the future liver remnant increased from 434 mL to 802 mL.
- Citation: Hao MZ, Lin HL, Hu YB, Chen QZ, Chen ZX, Qiu LB, Lin DY, Zhang H, Zheng DC, Fang ZT, Liu JF. Combination therapy strategy based on selective internal radiation therapy as conversion therapy for inoperable giant hepatocellular carcinoma: A case report. World J Gastrointest Oncol 2025; 17(3): 100861
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/100861.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.100861
Hepatocellular carcinoma (HCC) is a growing global health concern and > 80% of the patients are inoperable[1]. Insufficient future liver remnant (FLR) and microvascular invasion are the key limiting factors for surgical intervention[2,3]. Using yttrium-90 (90Y) resin microspheres in selective internal radiation therapy (SIRT) can induce contralateral liver lobe enlargement and shrink the affected liver lobe, thereby effectively increasing FLR while controlling the tumor[4]. A comprehensive German study reviewed 11014 liver cancer cases treated with 90Y-SIRT between 2012 and 2019. Of 11014 patients, 39.7% had primary liver cancer with a maximum tumor diameter of ≤ 8.0 cm[5]. However, HCC in China typi
Conversion therapy might provide greater opportunities for patients with inoperable HCC to undergoing a surgical resection. The currently available conversion therapy methods include systemic (drug) therapy and locoregional thera
This research reports an HCC patient with a giant tumor (15 cm), which was initially unresectable; however, after SIRT followed by HAIC with anti- programmed death-1 (PD-1) antibody and TKI treatment, the patient underwent successful tumor resection.
Due to recurrent discomfort in area of liver for 1 month.
A 49-year-old woman was admitted on 24 October 2023.
She had a history of chronic hepatitis B for > 40 years.
No history of drinking alcohol.
She had mild tenderness in the upper right abdomen.
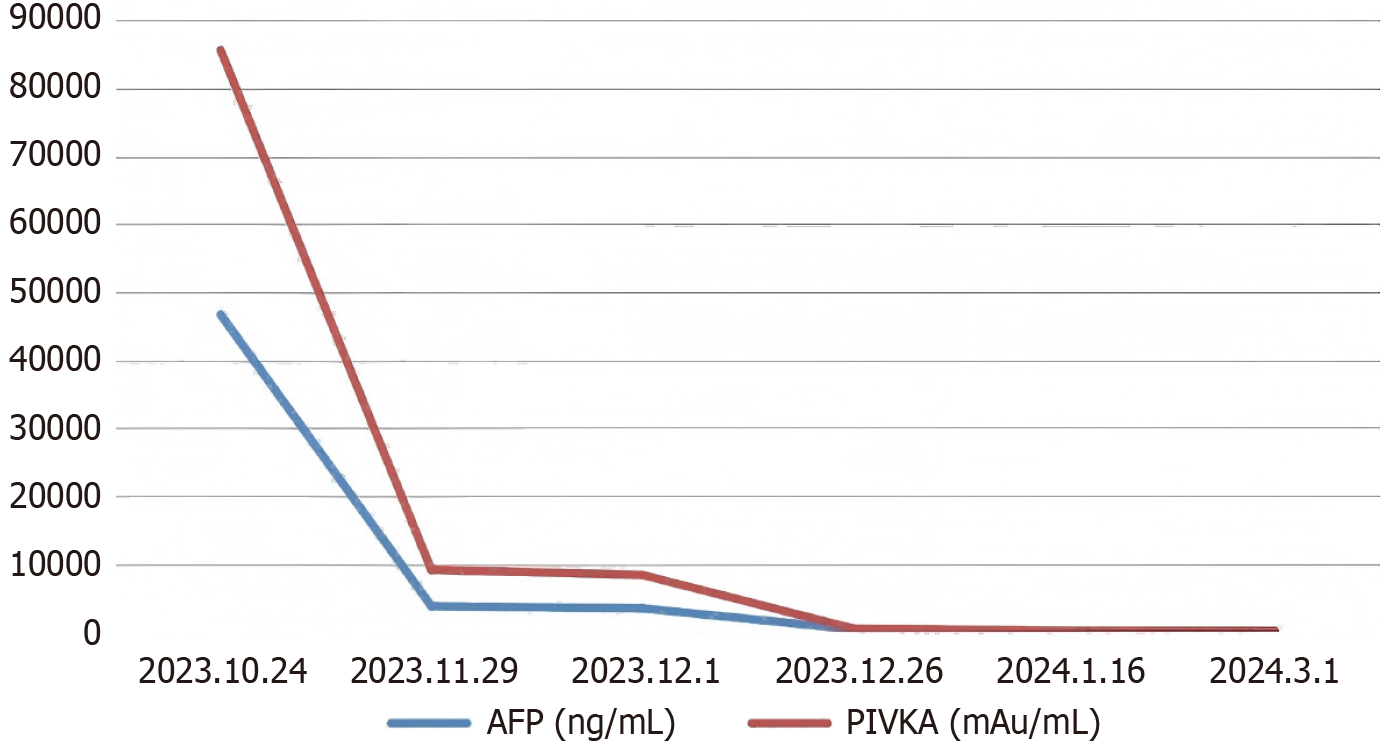
Serum alpha-fetoprotein (AFP) and antagonist-II (PIVKA-II) were 46699 ng/mL and 38949 mAU/mL, respectively. In addition, with a height of 168 cm and weight of 65 kg, the FLR of this case was 434 mL.
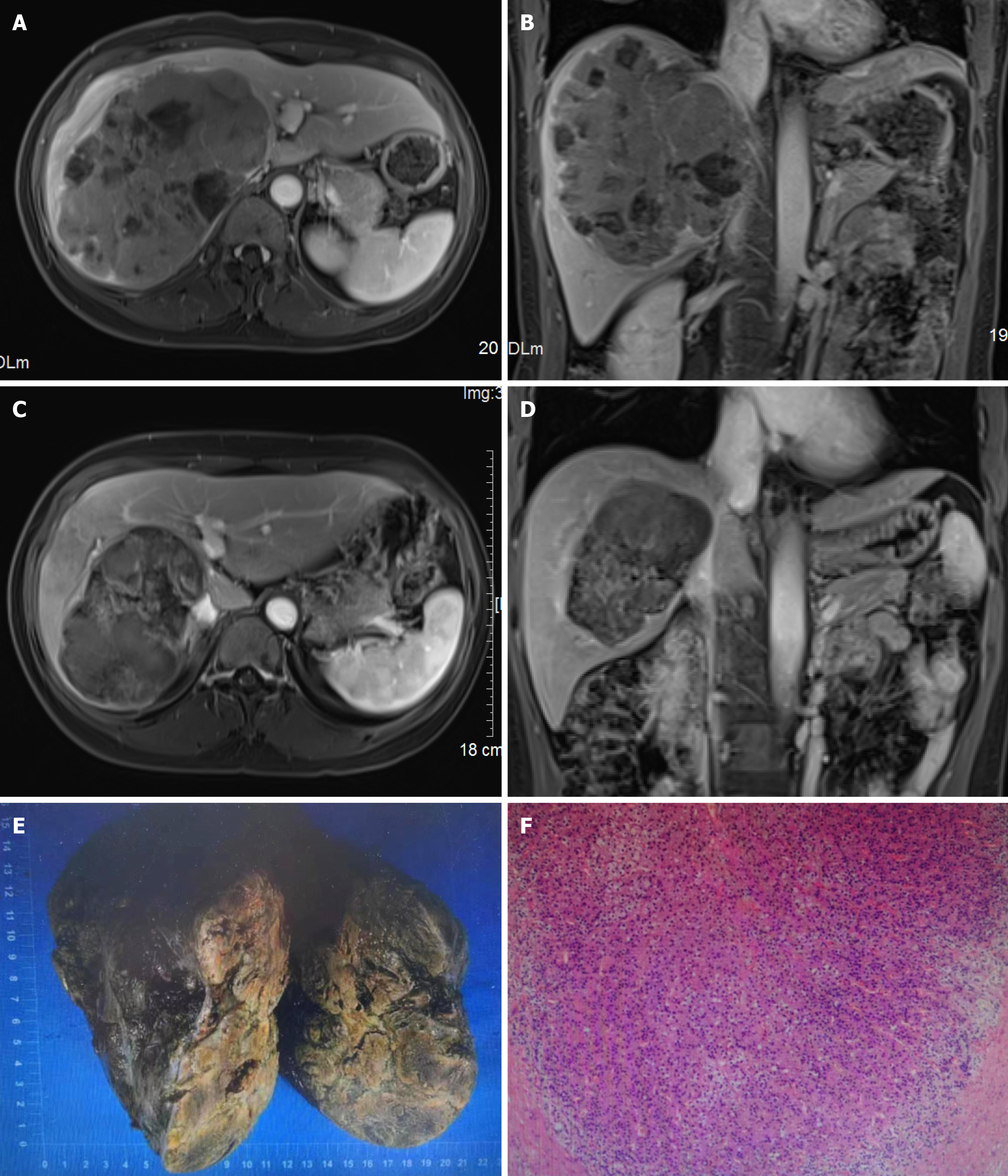
Her magnetic resonance imaging results showed a giant mass (15.8 cm × 11.4 cm × 14.8 cm) in the right hepatic lobe. The tumor indicated arterial hyperenhancement, was washed out in the portal vein, and was in delayed phases. Furthermore, the tumor was accompanied by invasion of the right portal vein as well as right and middle hepatic veins (Figure 1A and B).
After the multidisciplinary liver tumor board consenses, SIRT was recommended to the patient for conversion therapy. Because of the reduced efficacy of SIRT alone in the transformation therapy of giant HCC, HAIC with TKI and anti-PD-1 antibody were recommended as subsequent combination therapy methods.
China Liver Cancer Staging (CNLC) IIIa stage HCC.
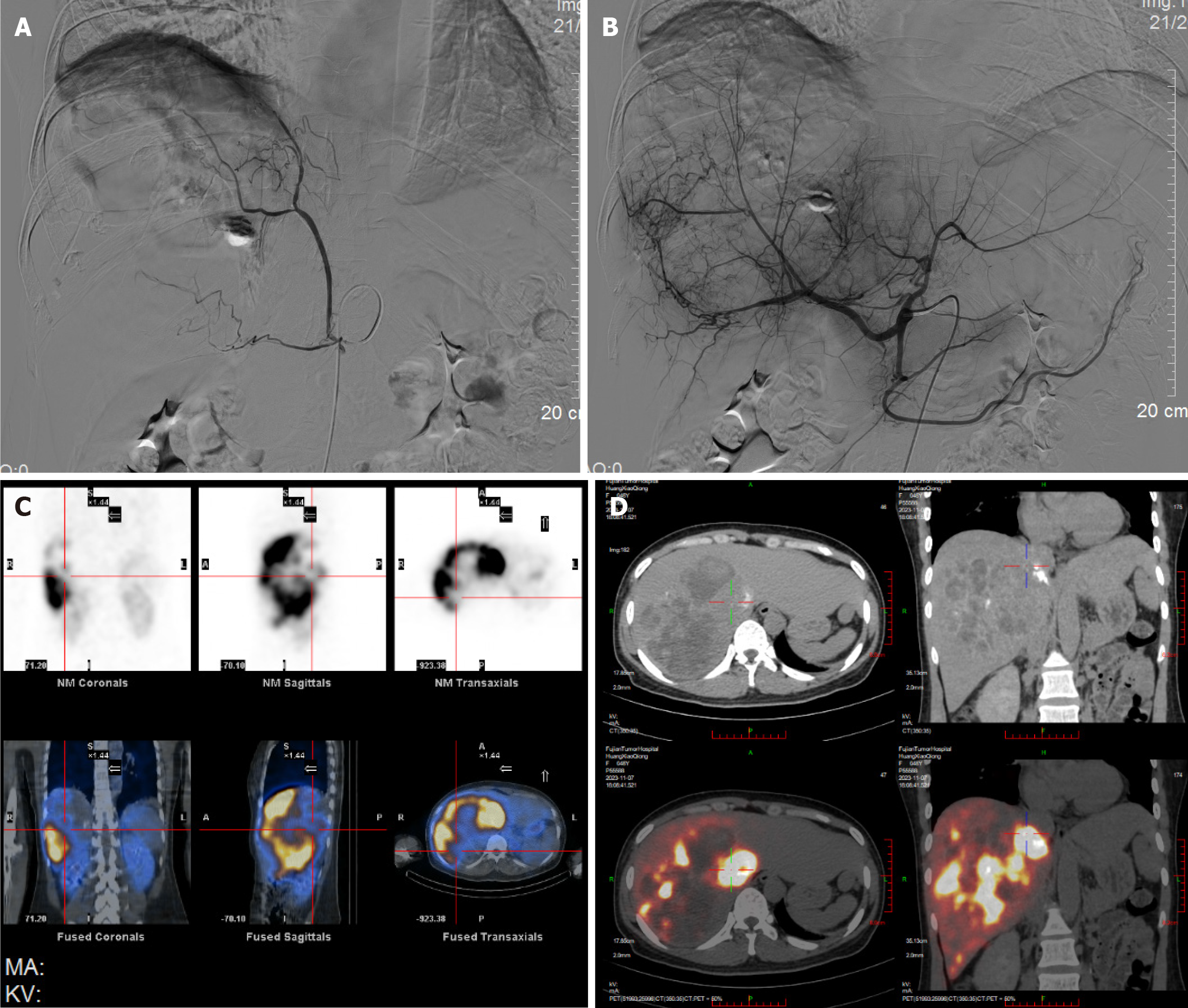
On 31 October 2023, before SIRT, a mapping procedure was carried out (Figure 2A and B). The angiography showed that an aberrant blood vessel originates from the right diaphragmatic artery and is involved in blood supply of some tumors. And 5 mL temperature-sensitive liquid embolic agent loaded with 30 mg Epirubicin and 1 g polyvinyl alcohol embolic microspheres (500-700 µm) was embolized by selectively inserting into this artery. After embolization, the tumor vessel’s blood flow was obstructed and the peripheral tumor staining disappeared. The main artery supplying blood to the tumor came from the right hepatic artery. The prescription dose was 110 Gy, calculated through a partition model. SIRT was carried out on 7 November. After a 2.6 Fr microcatheter was superselectively inserted into the right hepatic artery, and 90Y resin microspheres (3.8 GBq; SIR-Spheres®, Sirtex Medical) were given under fluoroscopic guidance. The dose delivery was verified through liver SPET/CT scanning after 1 hour of injection (Figure 2C and D).
On the second day post-SIRT, the patient was treated with lenvatinib (8 mg/day; Levima®, Eisai, Tokyo, Japan) and 200 mg of tislelizumab (every 3 weeks). On the third day post-SIRT, the patient was discharged.
HAIC was scheduled to be administered every 3 weeks for 4 cycles, with the first session occurring on day 21 after SIRT. The HAIC procedure was conducted under local anesthesia. Briefly, the right femoral artery was punctured, a catheter sheath was inserted, and a 4F rehabilitation catheter was placed for the celiac artery’s selective angiography. Then, a microcatheter was inserted and securely fixed in the proper hepatic artery. The patient was then transferred back to the ward for chemotherapy drugs infusion. The chemotherapy regimen consisted of lobaplatin 30 mg administered for 2 hours and raltitrexed 4 mg for 2 hours on day 1. The interventional procedures were carried out by physicians with over 15 years of surgical and clinical experience.
After SIRT, mild anorexia was observed on the first day, which disappeared on the second day. No other adverse events were observed. After HAIC, no adverse events was observed. Laboratory tests of liver and renal function showed no significant abnormalities on the third and tenth day respectively.
After 2 months of SIRT, the tumor decreased to 13.2 cm and then further reduced to 11.6 cm, there was no vascular invasion, indicating a complete response according to mRECIST. On 1 March 2024, it was observed that the tumor was downstaged to CNLC Ib, serum AFP was reduced to 14 ng/mL, and PIVKA-II levels decreased to 55 mAU/mL, and FLR increased to 802 mL (Figure 1C and D). Right hepatectomy and caudate lobectomy were performed 4 months after SIRT. The histopathological analysis after the surgery revealed a tumor bed measuring 13.5 cm × 12 cm × 9.5 cm with extensive necrosis (≥ 85%) (Figure 1E and F). One month after the hepatectomy, serum AFP and PIVKA-II reduced to 5 ng/mL and 16 mAU/mL, respectively (Figure 3). Currently, the patient is under standardized follow-up.
For more than 20 years, SIRT (using 90Y resin microspheres) has been used to treat unresectable hepatic cancers with tumors ≤ 8 cm in size[5]. Much literature supports the application of SIRT for HCC conversion therapy. The rates of successful downstaging to curative therapy range from 32% to 78%[8-10]. Furthermore, when the tumor burden is high (median size = 8.75 cm) or the tumor is complicated by portal vein tumor thrombus, the surgical conversion rate is only 22%[11].
The principal surgical conversion therapies for patients with insufficient FLR volume include associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) and portal vein embolization (PVE). However, PVE is primarily limited by the risk of tumor progression during the contralateral liver lobe enlargement[12,13]. Furthermore, although ALPPS is effective in rapidly increasing liver volume, it is constrained by the high incidence of serious complications and mortality risks during the perioperative period[14]. Conversely, SIRT stimulates liver regeneration by constantly enhan
A multicenter retrospective research indicated that for unresectable HCC patients with high tumor burden and portal vein tumor thrombus, HAIC combined with lenvatinib and tislelizumab resulted in a high ORR[16]. In 2001, the results of a phase III randomized controlled study that enrolled 74 patients with colorectal cancer with liver metastases (CRLM) showed that patients who received a single dose of 90Y resin microspheres in combination with once-monthly fluorouri
HAIC can promote or regulate the release of tumor antigens and enhance the response of immunotherapy. The combi
In this case, due to tumor downsizing and liver hypertrophy, FLR increased from 434 mL in baseline to 802 mL post-SIRT, making the patient eligible for resection. Futhermore, the complexity of the surgery did not increase after SIRT, as confirmed in our case.
The giant tumor with invasion of large blood vessels was downstaged from CNLC IIIa at diagnosis to CNLC Ib after SIRT followed by HAIC with TKIs and anti-PD-1 antibody. The initially inoperable patient was converted to a candidate for surgical resection, and underwent a radical right hepatectomy and caudate lobectomy successfully. Thus, an combi
There are several limitations in this report: (1) The study is based on a single case, which limits the generalizability of the findings; (2) Long-term follow-up data are needed to assess the overall survival benefits; and (3) In the future, further research will be conducted to explore the timing and optimal application population of SIRT combined with HAIC, and to explore mechanisms underlying the therapy, such as the regulatory effects of SIRT combined with HAIC on the tumor microenvironment and immune response. Therefore, in the future, it is necessary to conduct larger, prospective clinical trials to validate the efficacy and safety of this combination therapy in a broader patient population and investigate the molecular mechanisms underlying the synergistic effects of SIRT, HAIC, TKIs, and anti-PD-1 antibodies to optimize the treatment regimen further.
In summary, this case study provided evidence for the surgical resection of large HCC with microvascular invasion via an integrated treatment strategy combining SIRT and HAIC with TKI and anti-PD-1 antibody. Further clinical trials are required to validate these results for future application.
| 1. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 1013] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 2. | Wang K, Guo WX, Chen MS, Mao YL, Sun BC, Shi J, Zhang YJ, Meng Y, Yang YF, Cong WM, Wu MC, Lau WY, Cheng SQ. Multimodality Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Large-Scale, Multicenter, Propensity Mathching Score Analysis. Medicine (Baltimore). 2016;95:e3015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, Zhao M, Bi X, Li G, Bai X, Ji Y, Xu L, Zhu XD, Bai D, Chen Y, Chen Y, Dai C, Guo R, Guo W, Hao C, Huang T, Huang Z, Li D, Li G, Li T, Li X, Li G, Liang X, Liu J, Liu F, Lu S, Lu Z, Lv W, Mao Y, Shao G, Shi Y, Song T, Tan G, Tang Y, Tao K, Wan C, Wang G, Wang L, Wang S, Wen T, Xing B, Xiang B, Yan S, Yang D, Yin G, Yin T, Yin Z, Yu Z, Zhang B, Zhang J, Zhang S, Zhang T, Zhang Y, Zhang Y, Zhang A, Zhao H, Zhou L, Zhang W, Zhu Z, Qin S, Shen F, Cai X, Teng G, Cai J, Chen M, Li Q, Liu L, Wang W, Liang T, Dong J, Chen X, Wang X, Zheng S, Fan J; Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11:227-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 4. | Fernández-Ros N, Silva N, Bilbao JI, Iñarrairaegui M, Benito A, D'Avola D, Rodriguez M, Rotellar F, Pardo F, Sangro B. Partial liver volume radioembolization induces hypertrophy in the spared hemiliver and no major signs of portal hypertension. HPB (Oxford). 2014;16:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Mertens A, Essing T, Minko P, Möllenhoff K, Mattes-György K, Giesel FL, Antoch G, Luedde T, Roderburg C, Loosen SH. Selective internal radiotherapy in Germany: a review of indications and hospital mortality from 2012 to 2019. J Clin Transl Res. 2023;9:123-132. [PubMed] |
| 6. | Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, Ding WB, Li WH, Huang JH, Mu W, Gu SZ, Li JP, Zhao H, Wen SW, Lei YM, Song YS, Yuan CW, Wang WD, Huang M, Zhao W, Wu JB, Wang S, Zhu X, Han JJ, Ren WX, Lu ZM, Xing WG, Fan Y, Lin HL, Zhang ZS, Xu GH, Hu WH, Tu Q, Su HY, Zheng CS, Chen Y, Zhao XY, Fang ZT, Wang Q, Zhao JW, Xu AB, Xu J, Wu QH, Niu HZ, Wang J, Dai F, Feng DP, Li QD, Shi RS, Li JR, Yang G, Shi HB, Ji JS, Liu YE, Cai Z, Yang P, Zhao Y, Zhu XL, Lu LG, Teng GJ; CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (1)] |
| 7. | Lencioni R, Kudo M, Erinjeri J, Qin S, Ren Z, Chan S, Arai Y, Heo J, Mai A, Escobar J, Lopez Chuken YA, Yoon J, Tak WY, Suttichaimongkol T, Bouattour M, Lin S, Żotkiewicz M, Udoye S, Cohen G, Sangro B. EMERALD-1: A phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization. J Clin Oncol. 2024;42:LBA432-LBA432. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 113] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 8. | Vouche M, Habib A, Ward TJ, Kim E, Kulik L, Ganger D, Mulcahy M, Baker T, Abecassis M, Sato KT, Caicedo JC, Fryer J, Hickey R, Hohlastos E, Lewandowski RJ, Salem R. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 9. | Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, Hunter RD, Nemcek AA Jr, Abecassis MM, Haines KG 3rd, Salem R. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 10. | Ettorre GM, Levi Sandri GB, Laurenzi A, Colasanti M, Meniconi RL, Lionetti R, Santoro R, Lepiane P, Sciuto R, Pizzi G, Cianni R, Golfieri R, D'Offizi G, Pellicelli AM, Antonini M, Vennarecci G. Yttrium-90 Radioembolization for Hepatocellular Carcinoma Prior to Liver Transplantation. World J Surg. 2017;41:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Pracht M, Edeline J, Lenoir L, Latournerie M, Mesbah H, Audrain O, Rolland Y, Clément B, Raoul JL, Garin E, Boucher E. Lobar hepatocellular carcinoma with ipsilateral portal vein tumor thrombosis treated with yttrium-90 glass microsphere radioembolization: preliminary results. Int J Hepatol. 2013;2013:827649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 957] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 13. | Imamura H, Seyama Y, Makuuchi M, Kokudo N. Sequential transcatheter arterial chemoembolization and portal vein embolization for hepatocellular carcinoma: the university of Tokyo experience. Semin Intervent Radiol. 2008;25:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | D'Haese JG, Neumann J, Weniger M, Pratschke S, Björnsson B, Ardiles V, Chapman W, Hernandez-Alejandro R, Soubrane O, Robles-Campos R, Stojanovic M, Dalla Valle R, Chan AC, Coenen M, Guba M, Werner J, Schadde E, Angele MK. Should ALPPS be Used for Liver Resection in Intermediate-Stage HCC? Ann Surg Oncol. 2016;23:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Fernandez-Ros N, Iñarrairaegui M, Paramo JA, Berasain C, Avila MA, Chopitea A, Varo N, Sarobe P, Bilbao JI, Dominguez I, D'Avola D, Herrero JI, Quiroga J, Sangro B. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int. 2015;35:1590-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Chen S, Shi F, Wu Z, Wang L, Cai H, Ma P, Zhou Y, Mai Q, Wang F, Tang S, Zhuang W, Lai J, Chen X, Chen H, Guo W. Hepatic Arterial Infusion Chemotherapy Plus Lenvatinib and Tislelizumab with or Without Transhepatic Arterial Embolization for Unresectable Hepatocellular Carcinoma with Portal Vein Tumor Thrombus and High Tumor Burden: A Multicenter Retrospective Study. J Hepatocell Carcinoma. 2023;10:1209-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 17. | Gray B, Van Hazel G, Hope M, Burton M, Moroz P, Anderson J, Gebski V. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 370] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Liang J, Bai Y, Ha FS, Luo Y, Deng HT, Gao YT. Combining local regional therapy and systemic therapy: Expected changes in the treatment landscape of recurrent hepatocellular carcinoma. World J Gastrointest Oncol. 2023;15:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Wang W, Li R, Li H, Wang M, Wang J, Wang X, Zhou Q. Addition of Immune Checkpoint Inhibitor Showed Better Efficacy for Infiltrative Hepatocellular Carcinoma Receiving Hepatic Arterial Infusion Chemotherapy and Lenvatinib: A Multicenter Retrospective Study. Immunotargets Ther. 2024;13:399-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/