Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.98556
Revised: October 30, 2024
Accepted: November 18, 2024
Published online: February 15, 2025
Processing time: 203 Days and 4.4 Hours
Hepatocellular carcinoma (HCC) ranks as the fourth leading cause of cancer-related deaths in China, and the treatment options are limited. The cyclic gua
To investigate the dual-regulation mechanism of cGAS-STING in HCC.
This review was conducted according to the PRISMA guidelines. The study conducted a comprehensive search for articles related to HCC on PubMed and Web of Science databases. Through rigorous screening and meticulous analysis of the retrieved literature, the research aimed to summarize and elucidate the impact of the cGAS-STING pathway on HCC tumors.
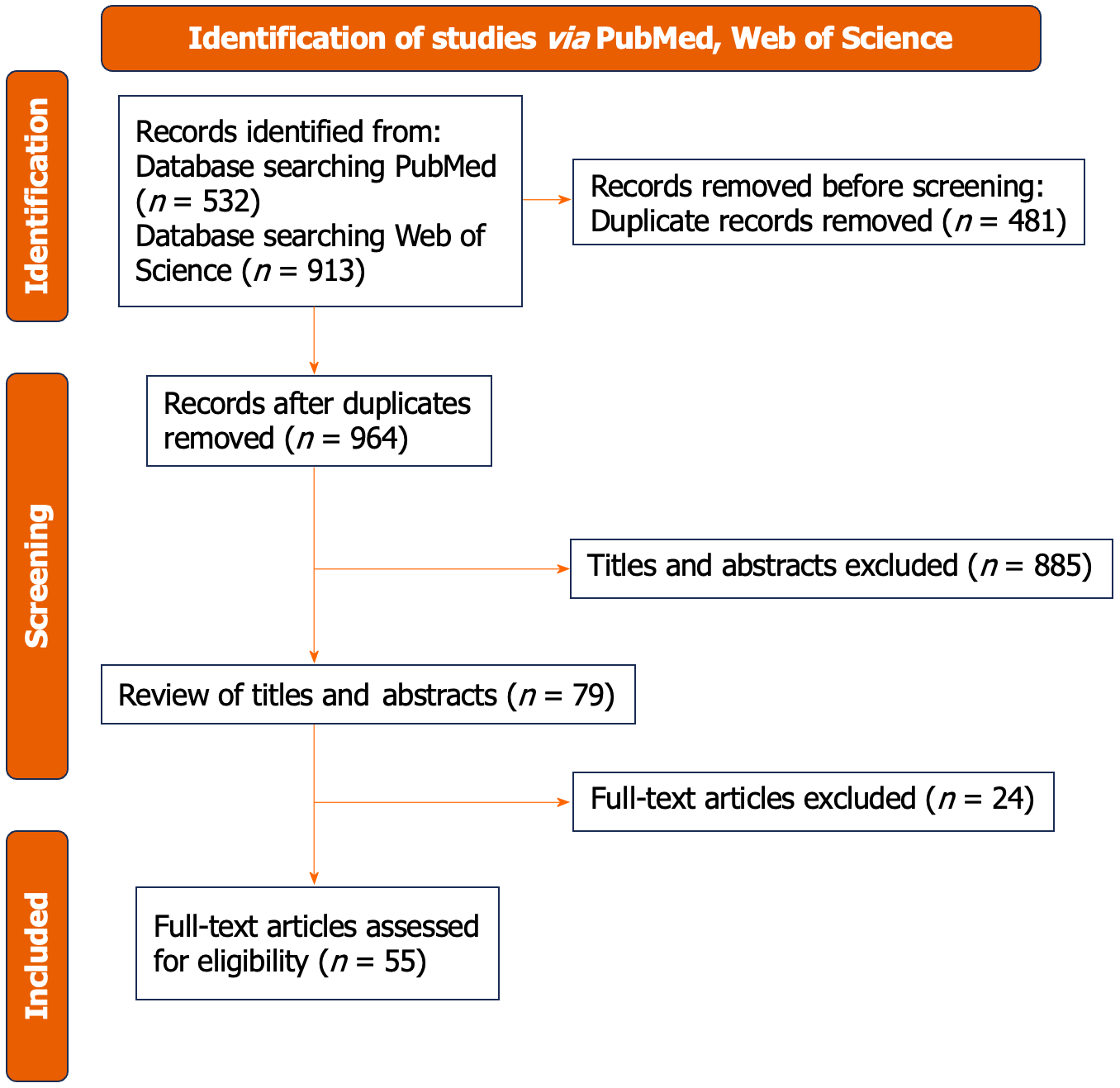
All authors collaboratively selected studies for inclusion, extracted data, and the initial search of online databases yielded 1445 studies. After removing duplicates, the remaining 964 records were screened. Ultimately, 55 articles met the inclusion criteria and were included in this review.
Acute inflammation can have a few inhibitory effects on cancer, while chronic inflammation generally promotes its progression. Extended cGAS-STING pathway activation will result in a suppressive tumor microenvironment.
Core Tip: As a crucial signaling pathway for both innate and adaptive immune responses, studies have demonstrated that cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)-stimulator of interferon gene (STING) pathway can be triggered by tumor DNA and genomic instability, thereby promoting or inhibiting tumor development and metastasis. A considerable amount of evidence indicates that the cGAS-STING pathway, as a potential therapeutic target, markedly inhibits tumor cell proliferation and metastasis, with its activation being particularly relevant in hepatocellular carcinoma. But at the same time, prolonged pathway activation may lead to an immunosuppressive tumor microenvironment, fostering liver damage, fibrosis, carcinogenesis, and the invasion or metastasis of liver tumor cells.
- Citation: Nie AY, Xiao ZH, Deng JL, Li N, Hao LY, Li SH, Hu XY. Bidirectional regulation of the cyclic guanosine monophosphate-adenosine monophosphate synthase-stimulator of interferon gene pathway and its impact on hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(2): 98556
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/98556.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.98556
Cancer remains a leading significant public health issue worldwide, and it ranks among the most common causes of death. Specifically, liver cancer, especially hepatocellular carcinoma (HCC), ranks fourth for its cause of death in China[1]. On the global scale, the death rate from this disease has been on the rise, having reached the third-highest by 2018 and second-highest by 2020[2]. HCC is one of the most common types of liver cancers, found all over the world[3]. Identifying HCC at an early stage enables patients to access a range of treatments, both curative and non-curative, that can be beneficial. Performing surgical resection for early-stage HCC can lead to significant improvements in survival rates, typically ranging from 50% to 70% over a 5-year period. Nevertheless, the risk of tumor recurrence and metastasis remains as high as 70%[4]. In addition, HCC can be quite elusive and has a tendency to rapidly spread. When patients are diagnosed, a significant number of them have already reached an advanced stage where surgery is no longer an option. Until recently, treatment options for individuals with advanced HCC were very limited. There are various factors at play here, such as HCC’s strong resistance to standard treatment plan and its limited tolerance for this treatment due to potential liver damage[5].
In 2007, sorafenib, a multikinase inhibitor that targets the vascular endothelial growth factor receptor family, became the first drug to demonstrate improved overall survival in advanced HCC, which was a major breakthrough in liver cancer treatment[6]. However, from 2007 to 2017, the range and effectiveness of available drugs remained limited. Indeed, the success of several clinical trials that have investigated targeted therapy in the management of a range of cancers has not even emulated or reached that of sorafenib in terms of its success at improving the prognosis or lengthening survival among HCC patients[7-10]. In 2018, Lenvatinib was approved as the first-line therapy for advanced HCC[11]. It has shown to be more effective compared to sorafenib[12].
An enormous work advancement has been observed in the understanding of the crossroads of immunology with oncology in recent years; many studies have been documented and concluded that immunology apparently plays an important role in defense mechanisms with the major purpose of preventing or protection from the process of tumorigenesis[13]. Some researchers have noted that when given to those cancer patients who do not respond to it in other ways, immunotherapy shows some of the best results, and that it does work the best for those patients who already have quite advanced stages of the disease[14]. This introduces a new perspective of cancer immunotherapy. More recent studies underline the opportunity to make the immune response of the body towards DNA, through the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)-stimulator of interferon gene (STING) pathway, effective and have high relevance for cancer as well[15]. Recognition of aberrant DNA species in the cytosol activates the STING signaling pathway through sensing of cytoplasmic DNA by cGAS[16]. Such activation further enhances the expression of type 1 interferon (IFN) genes[17], which is very essential for the immune response.
This review was conducted according to the PRISMA guidelines[18].
The study searched PubMed and Web of Science, employing combinations of the following search terms in titles, abstracts, and keywords: “cGAS-STING”, “hepatocellular carcinoma”, “cancer”, “HCC”, “liver”, and “tumor metastasis”. Relevant studies published from June 1, 2013, to May 1, 2024, and accessible online in full text were included. This review did not impose restrictions on research designs and included studies without language or publication date limits. In addition to automated searches, we manually examined the reference lists of selected articles to identify additional relevant studies.
The articles were imported into EndNote, which intelligently removed duplicates. We then alphabetically organized the titles and manually removed duplicates to ensure completeness.
Articles that met the eligibility criteria proceeded to the next phase of screening, where Xiao ZH and Deng JL assessed them for: The regulatory, promotional, and inhibitory effects of cGAS-STING on tumors; The correlation between HCC and cGAS-STING; and the impact of inhibitors or agonists on HCC metastasis and invasion. only studies published in English were considered for the meta-analysis.
All authors collaboratively selected studies for inclusion, extracted data, and the initial search of online databases yielded 1445 studies. After removing duplicates, the remaining 964 records were screened. Ultimately, 55 articles met the inclusion criteria and were included in this review. Figure 1 displays the PRISMA 2020 flowchart.
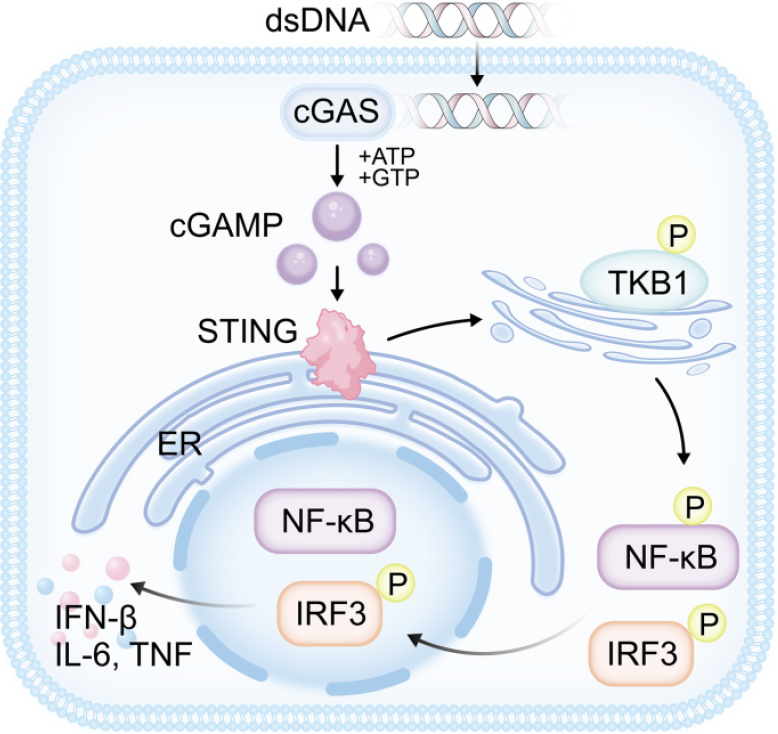
cGAS is a cytosolic sensor for diverse forms of double-strand DNA (dsDNA)[19]. Direct binding with dsDNA activates cGAS, which adopts a conformational change[20] that permits the catalysis of cGAS to produce cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) using adenosine triphosphate (ATP) and guanosine triphosphate (GTP)[21,22]. This way, it can identify exogenous DNA from pathogens and host endogenous DNA[23], such as mitochondrial DNA (mtDNA), micronucleus DNA, and cytoplasmic chromatin[24-27]. In its capacity as a second messenger, cGAMP interacts with the STING protein in the endoplasmic reticulum (ER)[28], inducing the oligomerization of STING into a tetramer and, eventually, a more complex structure[29]. This interaction also results in the movement of STING to the Golgi intermediate compartment and the Golgi apparatus[30]. The C-terminal tails of the STING dimer engage with cGAMP upon binding, leading to enhanced TANK-binding kinase 1 (TBK1) phosphorylation and activation of TBK1[29-33]. With the support of TBK1, STING is able to activate interferon regulatory factor 3 (IRF3) after it has been phosphorylated[34,35]. Once activated, IRF3 is involved in phosphorylation, dimer formation, and nuclear translocation to initiate IFN-I transcription[32,36]. Meanwhile, STING causes IκB kinase (IKK) to be activated, leading to the phosphorylation[37], ubiquitination[38] , and proteasomal destruction of proteins belonging to the IκB family[39,40]. When IκB degrades, nuclear factor kappa-B (NF-κB) is liberated and relocates to the nucleus. The process of transcribing different cytokines, like IFN-β, interleukin (IL)-6, and tumor necrosis factor (TNF), begins when it binds to the κB site on DNA[41-44]. Figure 2 shows the cGAS-STING pathway.
Antitumor mechanisms: Tumor cells, unlike normal cells, generally harbor abundant cytoplasmic dsDNA. Chromosomal instability (CIN) is widely recognized as a key contributor to cytoplasmic dsDNA, correlating with tumor progression, distant metastasis, and resistance to therapy[45]. When chromosomes are improperly segregated, chromosomal breaks may occur, potentially leading to oncogenic mutations. These lagging chromosomes often produce micronuclei in a cell cycle-dependent manner[46]. Approximately half of these micronuclei, with compromised membranes, rupture and release their genomic content into the cytoplasmic sol, thereby activating the cGAS-STING pathway[47]. Additionally, external factors like chemotherapy and radiotherapy can also induce DNA damage, which results in mtDNA leakage and further promotes a cGAS-STING-mediated antitumor response[48].
DNA damage can prompt acute STING signal transduction, resulting in cellular senescence[49]. Cellular senescence is characterized by a state of permanent growth arrest in damaged or aging cells and represents an irreversible halt in the cell cycle[50]. Activation of STING signaling impacts the release of pro-inflammatory cytokines, chemokines, proteases, and growth factors, collectively referred to as the senescence-associated secretory phenotype (SASP), which restricts tumor progression. SASP also promotes immune cell recruitment and the clearance of senescent cells[51]. The cGAS-STING pathway mainly regulates cellular aging through the secretion of soluble factors and other autocrine/paracrine signal transduction mechanisms[52]. Additionally, the downregulation of nuclear lamina protein B1 in senescent cells can lead to nuclear envelope rupture and the formation of cytoplasmic chromatin fragments, which further activates acute STING signaling. This evidence suggests that the cGAS-STING pathway plays a central role in regulating cellular senescence and activating SASP through endogenous DNA recognition[53,54]. Moreover, SASP signals to the immune system, regulating the tissue microenvironment. In a liver cancer model, senescent hepatocytes attract various immune cells through SASP, especially natural killer (NK) cells and neutrophils, which help eliminate tumor cells. The cGAS-STING pathway is critical for the antitumor effects mediated by SASP[55]. In STING-deficient mouse models, immune cell infiltration is reduced compared to controls, with observed reductions in the clearance of early senescent cells and substantial tumor growth in later stages in the experimental group[24].
The STING signaling pathway in cancer cells also promotes their own apoptosis. Activation of the cGAS-STING pathway generates substantial amounts of type I IFN, which plays a critical role in antitumor activity. Type I IFN recruits cytotoxic T cells to cancer cells and initiates a type I T helper cell response[56]. Furthermore, type I IFNs aid in the maturation of dendritic cells, which present tumor-specific antigens to cluster of differentiation (CD) 4 + and CD8 + T cells for protective immune responses[57]. CD4 + T cells serve not only as helper cells but also exhibit direct cytolytic activity[58], playing a crucial role in antiviral and antitumor immunity. Additionally, as the primary executors of acquired cellular immunity, CD8 + T cells possess significant cytotoxic potential when encountering cancer cells[59]. Upon phagocytosis of tumor cells by antigen-presenting cells, dendritic cells uptake tumor-associated antigens and migrate to tumor-draining lymph nodes via lymphatic vessels, where they present these antigens to T cells. This presentation activates CD8 + T cells, which then proliferate within the tumor-draining lymph nodes and subsequently travel through the bloodstream to target and eliminate cancer cells[60]. These findings suggest that type I IFN generation is closely linked to T cell activation and the antigenic response of cancer cells. Activation of the cGAS-STING pathway in cancer cells may inhibit tumor progression by upregulating various inflammatory genes, including type I IFN.
Tumor-promoting mechanism: While substantial evidence demonstrates that SASP functions as an antitumor component, it is crucial to recognize its potential role in promoting tumorigenesis[61]. Prolonged SASP activity can drive epithelial-mesenchymal transition and increase invasiveness, mainly through a paracrine mechanism involving SASP factors like IL-6 and IL-8. Simultaneously, it accelerates and intensifies the loss of function of the tumor suppressor protein p53 and increases expression of the oncogene RAS, contributing to genotoxic stress and cellular aging in normal cells[62]. RAS, a driver of cell proliferation in various cancers, can further exacerbate genotoxic stress and aging, causing senescent cells to exhibit unintended tumor-promoting effects[63]. Genotoxic stress activates both p53 and SASP; activation of p53 can halt cellular aging, thereby exerting antitumor effects. However, p53 also acts to inhibit SASP. When p53 function is compromised alongside cellular damage, SASP expansion is promoted. Furthermore, cells lacking p53 that escape senescence in response to genotoxic stress pose a significant threat, as they not only proliferate but also drive further SASP expansion, exacerbating tissue harm[62,64].
Additionally, senescent cells release cytokine receptors, which can assist neighboring precancerous or malignant cells in evading immune surveillance[65]. The persistent presence of senescent cells within tissues may create a pro-inflammatory environment, ultimately promoting tumor growth and metastasis[66].
Although the cancer surveillance function of the cGAS-STING pathway is well-documented, growing evidence suggests that cancer cells may deactivate cytoplasmic DNA-sensing pathways to evade immune detection. The chronic release of DNA activates the cGAS-STING-mediated IFN signaling pathway, posing a threat to cancer cells. Consequently, to counteract this inhibitory effect, cancer cells suppress this pathway to support tumor growth and metastasis. Studies have shown that in certain advanced cancer cells, the expression levels of cGAS and STING are downregulated[67,68]. This observation implies that cancer cells can downregulate cGAS and STING, promoting immune evasion in a manner dependent on the tumor microenvironment[69]. Micronuclei rupture, resulting from chromosomal segregation errors, releases genomic DNA into the cytoplasmic sol. This activates the cGAS-STING pathway and downstream non-canonical NF-κB signaling, with NF-κB known to drive inflammation, immune diseases, and tumor development and progression[70]. In highly aneuploid tumor models, genetic suppression of CIN significantly delays metastasis, while the induction of persistent chromosomal segregation errors promotes cell invasion and metastasis in a STING-dependent manner[71,72].
Autophagy in tumor: Autophagy is a cellular process in which the cytoplasmic sol and organelles are enclosed within double-membrane vesicles[73]. cGAMP-induced autophagy is crucial for the clearance of DNA or viruses within the cytoplasmic sol. Recent studies indicate that STING can induce autophagy in response to cGAMP stimulation without triggering IFN production. STING is also capable of activating non-canonical autophagy independently of IFN induction[74]. This activation of autophagy is vital for cell death and plays a central role in preventing cancer progression; hence, loss of autophagy is considered necessary for tumor development. Research by Nassour et al[75] has demonstrated that the terminal response to replication crisis in early cancer cells results in mitotic delay, unprotected telomere elongation, and cell death. Knocking out cellular cGAS and STING allows these cells to bypass crisis proliferation, showing decreased LC3-II levels and p62 accumulation. Accumulation of p62 leads to overactivation of the tumor necrosis factor receptor-associated factor 6-NF-κB pathway, promoting caspase-8 aggregation and interaction with the Cullin 3-type ubiquitin ligase Kelch like ECH associated protein 1 associated with nuclear factor-erythroid 2-related factor 2. This process facilitates the formation of intracellular inclusion bodies, potentially leading to pro-tumorigenic signaling[76]. Multiple preclinical studies suggest that autophagy supports late-stage tumor growth by activating various oncogenes and/or inactivating tumor suppressor genes, sustaining metabolic pathways within cancer cells, and promoting survival in challenging tumor microenvironments. Additionally, autophagy contributes to the effectiveness of various anticancer therapies[77-79].
Effect on HCC: The close association between DNA damage and cancer is well established. The initiation of the cGAS-STING signaling pathway, triggered by the recognition of abnormal dsDNA, leads to antigen presentation by dendritic cells, culminating in the activation and differentiation of T cells and the production of IFN-I with antitumor effects, significantly enhancing the host’s resistance to tumor cells. Interactions between dendritic (DC)-T cells also occur in the liver[80]. Targeted strategies to enhance the function of DC cells, such as the injection of DC vaccines, have been used to bolster antitumor responses[81]. DC-based immunotherapies can yield better results, improving the CD4 + T/CD8 + T cell ratio and ensuring patient safety. In HCC, CD8 + and CD4 + T cells are enriched within the tumor and the peritumoral area[82]. Flecken et al[83] found that the occurrence of tumor-related antigen-specific CD8 + T cell responses in HCC patients is associated with improved progression-free survival, observable in over 50% of patients. It is hypothesized that tumor-related antigen-specific CD8 + T cell responses may inhibit tumor growth and prolong the duration of early-stage disease, thereby benefiting patient survival. In addition, experiments have shown that lipid metabolism disorders in nonalcoholic fatty liver disease lead to a selective loss of CD4 + T cells in the liver, where CD4 + T lymphocytes have a greater mitochondrial mass than CD8 + T lymphocytes and produce higher levels of mitochondrial reactive oxygen species (ROS). Reversing lipid metabolism disorder-induced reductions in hepatic CD4 + T lymphocytes can also delay HCC[84]. Treatment of HCC with tetrathiomolybdate has shown that it induces the accumulation of ROS and activates various cell death pathways, including necroptosis and ferroptosis. This results in mitochondrial damage and the release of mtDNA into the cytoplasm, which subsequently activates the cGAS-STING-IFN axis, promoting the infiltration and activation of CD8 + T cells[85].
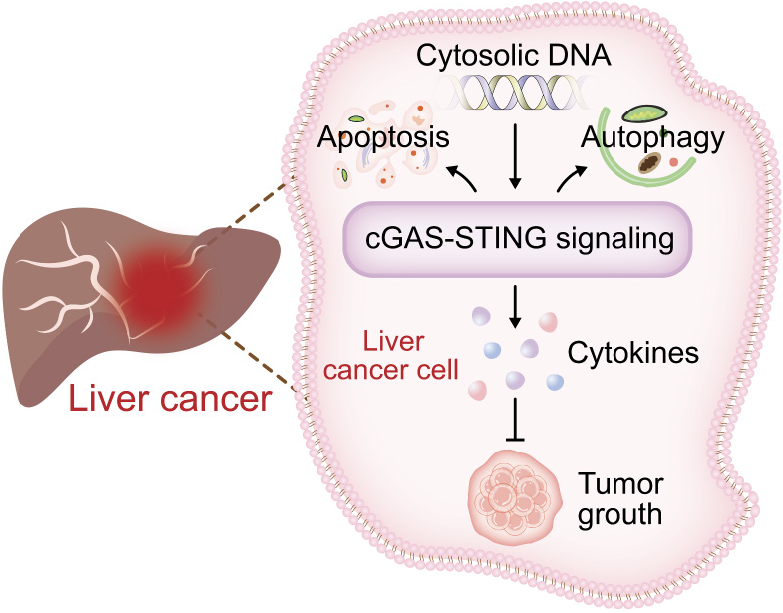
However, the impact of cGAS-STING on HCC has both benefits and drawbacks. Trials involving the treatment of HCC with tetrathiomolybdate have also found that the IFN pathway induces the expression of Programmed cell death 1 ligand 1 (PD-L1) in liver cancer cells, facilitating immune evasion[85]. Chromosome instability and the presence of tumor byproducts in the form of self-DNA can activate the cGAS-STING pathway, thereby either promoting or inhibiting tumor development. Ma et al’s team demonstrated that disruption of the BRCA1 and PALB2 interaction in mice leads to persistent high levels of DNA damage in HCC cells, activating the cGAS-STING pathway in malignant hepatocytes and M1 macrophages in the tumor microenvironment[86]. This activation, through the STING-IRF3-signal transducer and activator of transcription 1 pathway, induces PD-L1 expression, causing immunosuppression and promoting tumor occurrence and progression while recruiting T lymphocytes, leading to their infiltration into the tumor[86]. Consequently, blocking the PD-L1 pathway has shown significant antitumor effects in advanced cancer patients. Activating the cGAS pathway promotes IFN expression and downregulates PD-L1 expression, effectively preventing the progression and metastasis of HCC[87]. Numerous studies have confirmed that blocking PD-L1 enhances the anti-HCC properties[88-90]. Figure 3 shows the role of the cGAS-STING signaling pathway in HCC.
Facilitate tumor growth: Ongoing cGAS-STING pathway activation has the ability to enhance tumor development and metastasis. By maintaining an immune-suppressing tumor microenvironment, persistent activation of this signaling pathway can aid tumor development, metastasis, and treatment resistance[15,91]. In fact, higher STING expression levels have been associated with a poor outcome in certain colorectal cancer cases[60], again hinting that this pathway may actually be taking part in driving tumor growth and thus preventing its elimination by the immune system. In fact, it has been demonstrated that the stimulation of STING has the power to inhibit the immune response against the tumor in NK cells because it produces IL-35, which further potentiates the Breg cell function[92]. In addition, the activity of STING within the tumor microenvironment enhances indoleamine 2,3-dioxygenase (IDO) activity, which stimulates the advancement of Lewis lung cancer[93]. In this line, the observation by Cheng et al[94] showed that enhanced levels of mitochondrial Lon resulted in an increase in the production of ROS and oxidative damage to mtDNA. mtDNA, when damaged and released in the cytosol, stimulates an IFN signaling pathway in cancer cells via the STING-TBK1 pathway, followed by the upregulation of the expression of PD-L1 and IDO-1. This upregulation process inhibits the T-cell activation, thus resulting in weak T-cell immunity within the tumor microenvironment, an environment that ultimately may contribute to promoting the tumor.
Facilitate tumor spread: Finally, the STING signaling pathway has been found to be upregulated by IDO activity, known to assist in facilitating tumor growth[93], as it catalyzes the first, rate-limiting enzymatic step in the conversion of L-tryptophan to N-formylkynurenine in an effort to evade immune detection and T-cell growth[95]. Studies implicate that sustained activation of the cGAS-STING pathway and type I IFN responses could be a mechanism of immune evasion of the cancer cells from being eliminated[96,97]. Moreover, the presence of CIN is very likely to bring about continuous cGAS-STING activation that would otherwise modify signaling pathways in cancer cells, supporting the building of a metastatic tumor microenvironment[98]. When cells have unstable chromosomes, most of the DNA fragments from the cells are emitted to the cytoplasm through micronuclei. This, in turn, triggers the cGAS-STING pathway not reliant on the conventional NF-κB pathway. Aneuploidy, the appearance of a variety of karyotypes, and the occurrence of micronuclei can all be grouped under the genetic abnormalities, among many others, which may thus be expected to arise out of such instability[69]. This causes a rise in the chromosomal content in these micronuclei, and when the membrane is disrupted[71], the chromosomes are released into the cytoplasm. This leads to the activation of the cGAS-STING pathway, among other signaling pathways[99,100], and raises the expression level of the p52 gene. This, in turn, causes the cells to be killed by T-cells[101].
Effect on tumor growth: Extensive research has been done on the cGAS-STING pathway in tumor cells and its potential to boost the antitumor effect[102-106]. Several clinical trials have demonstrated their promising potential in enhancing the body’s ability to fight against tumors through this pathway. Its activation is very crucial in breaking immunological tolerance and enhancing tumor-specific immunity by increasing tumor cell death, autophagy, and the IFN response. Activation of the cGAS-STING pathway leads to the induction of the type I IFN response and T cell activation to cause the regression of the tumor[69,101]. In their study, Ka et al[107], they found that nuclear receptor NR1D1 can be activated by NR1D1. This activation leads to enhanced body immunity against tumors, with decreased growth of breast cancer and lung metastasis. In a colon cancer study, the higher levels of cGAS and STING were associated with a higher expression of effector CD8 and mature CD4 gene markers compared to the lower ones[108]. In addition, research in living organisms and laboratory settings has shown considerable effects of Disitamab Vedotin, the new antibody-drug conjugate, on the reduction of human epidermal growth factor receptor 2-positive colon cancer[109]. Furthermore, it can enhance the efficacy of anti-PD-1 therapy in tumors through the cGAS-STING pathway[109]. The research by the team of Shen et al[110] reported that metformin mediates activation of the cGAS-STING signaling axis in gastric cancer by repression of SOX2/protein kinase B and further augments its antitumor effects. Further investigation revealed that Raddeanin A directly interacted with transactive responsive DNA-binding protein 43 and caused mitochondrial instability, which allowed the release of mtDNA[111]. Such processes, therefore, allowed Raddeanin A to activate cGAS and type I IFN signaling. These processes increase the antigen-presenting ability of dendritic cells and also activate T cells. These processes have the result of tumor suppression[111].
Activation of the cGAS-STING pathway by cytoplasmic DNA results in the induction of cell senescence. Pathways activated by cytoplasmic DNA promote autophagy, induced by inflammatory cytokines[75,112], and have an important role in tumor suppression. This identifies DNA in cells, activates the production of type I IFNs and SASP factors[113], together with the increase in the release of a number of cytokines and chemokines. These attract immune cells to the site, where senescent cells have also produced cytokines, leading to a permanent halt in cell division[52]. All of these processes driven by oncogene activation may normally lead to the promotion of cell senescence and, through this action, reduce cancer risk.
The damage to the tumor cells created is triggering the production of dsDNA, following which their death will also be triggered[15,114]. Similar to normal proliferative cells, mostly, if not exclusively, cancerous cells will execute aerobic glycolysis as the mode of glucose catabolism[115,116]. This change in metabolic activity during aerobic glycolysis increases ROS production in glycolytically active tumor cells. This causes cell death, which in turn releases mtDNA[117,118]. Upon detecting either endogenous or foreign DNA, immune cells initiate the cGAS-STING signaling cascade. Other recent findings suggest that cGAS can increase the body’s immune response to cancers by targeting free DNA produced from dying tumor cells[119-121].
Furthermore, the cGAS-STING pathway has been shown to activate a number of cellular events, such as apoptosis[122], autophagy[123], pyroptosis[124], ferroptosis[125], and necroptosis[126]. Therefore, new cancer treatments that target the immunopathology of the cGAS-STING pathway have a lot of promise.
The suppressive impact of cGAS-STING on HCC: Liver cancer progression is aided by the cGAS-STING pathway, which allows for tumor immunosuppression and T lymphocyte infiltration in HCC[86,127]. Pu et al[128] highlighted the significance of specific genes in the cGAS-STING pathway in relation to HCC in their investigation. Researchers discovered that individuals with HCC had a worse prognosis when tumor tissues had lower levels of STING expression. Furthermore, research in animal models of HCC has shown that teniposide can improve the efficacy of anti-cancer therapies[129]. In order to accomplish this, certain pathways within the immune response are activated, and dendritic cells, which are essential for delivering antigens to T cells, are encouraged to become activated[129,130]. Also, cancer cells can be made to die when STING agonists are given to them directly. Tang et al[131] discovered that Eμ-TCL1 mice with chronic lymphocytic leukemia undergo apoptosis when exposed to the STING agonist 3’3’-cGAMP. Researchers Song et al[132] found that injecting SHR1032 directly into MC38 mice syngeneic tumors significantly reduced tumor growth. Vitamin C enhances the efficacy of immunotherapy by activating a particular route in tumors, which in turn normalizes blood vessel function. Furthermore, tumor cells are little affected by Sting deficiency caused by its ablation, but cells with high cGAS expression are unaffected[133]. Improving combination immunotherapy for liver cancer may be as simple as encouraging tumor vascular normalization, as these results highlight the tumor immune environment’s interaction with vascular endothelial cells. According to research on HCC models, cyclic dinucleotides (CDN) STING agonists can activate the TBK1/IRF3 and NF-κB signaling pathways, which in turn cause the release of type I IFN and pro-inflammatory cytokines. This, in turn, stimulates an immune response and successfully decreases the size of tumors[134,135]. By administering the DNA damage response inhibitor AZD6738 to HCC mice, Sheng et al[136] found that cGAS, phosphorylated STING (p-STING), and p-TBK1 levels were elevated. A notable impact on PD-L1 expression in HCC cells was the elevation of p-STING levels[136]. Additionally, it enhanced the CD8/CD3 T lymphocyte ratio, increased the population of activated CD8 T lymphocytes via the cGAS-STING pathway, and led to an upregulation of regulatory T cells. This led to its beneficial effect of reducing tumor growth[96]. What is more, injectable hydrogels integrated with cleaved OK-432 and doxorubicin have shown good promise for the treatment of remaining liver cancer. These hydrogels reactivate the cGAS-STING-IFN-I signaling pathway, leading to good outcomes[137]. These findings have pointed out the cGAS-STING axis as a potential therapeutic target for HCC. This pathway has been described to be capable of inducing tumor cell regression and, at the same time, to activate an immune response in diverse tumor models[131,135,138,139]. This vast collection of experimental evidence highlights promising molecular targets for further advancing HCC immunotherapy.
The role of cGAS-STING in promoting HCC: Study has found a correlation between high expression of cGAS in patients and poor survival outcomes[140,141]. Neutrophil extracellular traps (NETs) derived from diabetic patients might support the spread and invasiveness of HCC[142]. Inhibiting cGAS would, therefore, result in effectively suppressing the activation of the non-classical NF-κB pathway caused by NETs DNA and thus result in inhibiting the translocation of NF-κB RELB that causes a reduction in invasiveness into HCC cells. This study therefore uncovers the mechanism by which cGAS activation leads to the translocation of STING near the nucleus in HCC cells and thereby facilitates local invasion or metastasis. In addition, DNA damage caused by hepatocyte ferroptosis can result in liver damage, fibrosis, and the progression of cancer. On the other hand, the inhibitor C-176 has been shown to reduce tumor burden in TAK1 models by suppressing STING activity[143]. The cytoplasmic dsDNA sensing cascade of the cGAS-STING pathway plays a crucial role in initiating innate immune responses, which can lead to liver damage and autoimmune disorders[45,144]. In a study conducted by Du et al[141], it was found that the liver experiences significant DNA accumulation, leading to the production of IFN-I and activation of cGAS-STING. This, in turn, exacerbates liver cell damage. It should be appreciated that an interdependent IFN regulation in the tumors is implicated. First, the IFN signaling augments the anti-tumor activity[111,145]. Secondly, the mechanism discussed above may enhance tumor cell resistance to the immune checkpoint inhibition effect[146]. This emphasizes a subtle role of the cGAS-STING pathway in HCC.
STING agonists: Activation of the cGAS-STING pathway within the tumor microenvironment can induce effective cross-priming of tumor-specific antigens and promote effector T cell infiltration. Consequently, targeting the cGAS-STING pathway has become a high priority in drug development. To date, various STING agonists have been identified, primarily classified into the following categories: CDNs and their derivatives, 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and its analogs, and small-molecule agonists.
CDNs are the first class of STING agonists to enter drug development, serving as natural ligands of STING by directly binding to and activating it. This class includes cyclic di-GMP (c-di-GMP), cyclic di-AMP, and cGAMP[147], which have shown considerable potential in cancer therapy[148,149]. CDN agonists efficiently activate the STING pathway by mimicking the structure of natural ligands, thereby triggering immune responses. The antitumor effect of this activation was first observed with c-di-GMP[150]. In a melanoma mouse model, peptide nanotubes loaded with c-di-GMP promoted the expression of INF-β, TNF-α, IL-6, and IL-1β, upregulating the activity of CD4 and CD8 T cells to kill tumor cells and intensify the immune response within tumor tissues, significantly inhibiting tumor growth in tumor-bearing mice[151]. In a mouse liver cancer model, intratumoral injection of the STING agonist c-di-GMP significantly increased the expression of TNF-α and IFN-γ in CD4 + and CD8 + T cells, along with a notable rise in the percentage of IFN-γ-producing NK cells. These findings suggest that intratumoral administration of c-di-GMP supports a pro-inflammatory environment conducive to T and NK cell activation[134]. In malignant B cell tumors, 3’3’-cGAMP selectively triggers mitochondria-mediated apoptosis in both normal and malignant B cells. It induces the prolonged presence of STING within the ER or Golgi, forming protein complexes that activate apoptosis. This indicates that beyond their established role in promoting antitumor immune responses, STING agonists can directly eradicate malignant B cells, potentially providing therapeutic benefits for B cell malignancies such as chronic lymphocytic leukemia and multiple myeloma[131]. When cGAMP was used to treat mice with colorectal adenocarcinoma, data indicated that the cGAMP-cGAS-STING-IRF3 pathway stimulates and strengthens innate immune responses, increases antitumor cytokine production, and activates DCs to inhibit tumor growth. Additionally, cGAMP may activate other STING-independent pathways to suppress colorectal cancer tumor growth[152].
In cancer treatment, DMXAA primarily exerts antitumor effects by activating the body’s immune response, particularly through the activation and recruitment of immune cells such as DCs, macrophages, and NK cells at the tumor site[153]. DMXAA can also inhibit tumor angiogenesis, thereby reducing the blood supply and nutrient flow to the tumor. This restriction of resources limits tumor growth and spread while making it easier for immune cells to reach the tumor site[154]. In mesothelioma treatment, DMXAA has been observed to promote tumor antigen presentation in tumor-draining lymph nodes and reduce the size of tumor blood vessels. Despite its effectiveness in treating mesothelioma, it led to a decrease in the proportion of CD4 + and CD8 + T cells in tumors, with no sustained T cell response, which may ultimately allow tumor cells to evade immune attack and resume growth. Notably, combining DMXAA with agonistic anti-CD40 antibodies or IL-2 reduced antitumor efficacy[155]. DMXAA treatment also showed significant antitumor effects and a tumor-specific CD8 + T cell immune response against TC-1 tumors, though it did not enhance the antigen-specific immune response in tumor-bearing mice[156]. Positive results were also achieved in melanoma treatment[157]. However, clinical studies on DMXAA in patients did not confirm the expected outcomes, likely due to structural differences in the STING protein between mice and humans. After 7 days of DMXAA administration, upregulation of hypoxia inducible factor (HIF)-1α levels and an increase in the number of blood vessels in the tumor were observed, leading to tumor regrowth. Tumor regeneration occurred at the periphery, while the central area was largely necrotic, with an increased density of blood vessels in the marginal zone[158]. This outcome is thought to result from DMXAA-induced necrosis around damaged vessels and hypoxic regions within the tumor, leading to activation of the HIF-1α transcription factor. Additionally, it stimulates new blood vessel formation, enhancing survival, proliferative potential, and invasiveness of tumor cells, and ultimately triggering immune suppression[159,160]. While the antitumor effect of DMXAA is undeniable, further research is required to understand its impact on the tumor microenvironment and its combined use with immunotherapies.
Currently, small molecule-based cancer immunotherapy represents a breakthrough in cancer treatment. These small molecule compounds can activate STING by binding to different sites on the protein or by influencing conformational changes in STING[161]. Amide benzoimidazole is a novel STING agonist, and intravenous delivery of this small molecule in immunocompetent mice has resulted in pronounced tumor regression. Ramanjulu et al[162] have demonstrated that this new compound has significantly enhanced binding affinity, leading to an adaptive CD8 + T cell response in vivo, showing substantial potential for advancing immunotherapy in human cancers.
STING inhibitors: With the deepening understanding of the cGAS-STING pathway, it has become clear that excessive activation of this pathway can prolong the expression and secretion of inflammatory cytokines, promoting tumor growth and metastasis. Therefore, the development of STING inhibitors to intervene in such disease processes is also necessary. Existing inhibitors can be classified by their inhibition mechanisms into palmitoylation inhibitors and competitive antagonists targeting the CDN binding site[163]. STING activation requires palmitoylation at the Golgi apparatus[164]. Blocking STING palmitoylation is an effective strategy for inhibiting STING activation. STING inhibitors act by covalently binding to the Cys88 or Cys91 residues within the transmembrane domain of the STING protein, thereby disrupting palmitoylation. The 3-acylamino indole derivative indole ureas (H-151) can form a covalent bond with Cys91. In a mouse model of STING activation, administration of H-151 achieved effective systemic levels and significantly reduced serum levels of type I IFN and IL-6. This indicates that H-151 is an effective small molecule antagonist of STING, capable of inhibiting type I IFN response, TBK1 phosphorylation, and STING palmitoylation[165]. In human cells, nitro-fatty acids (NO2-FAs), effective inhibitors of STING signaling, are formed after herpes simplex virus-2 infection and mediate anti-inflammatory, antioxidative, and cytoprotective effects. Derivatives of NO2-FAs also covalently modify the Cys88 and Cys91 residues of STING, inhibiting its palmitoylation and thereby suppressing IFN-I production in fibroblasts associated with STING-related vascular diseases that onset in infancy[166].
Competitive antagonists targeting the CDN binding site interact with the C-terminal ligand-binding domain of STING, competing with endogenous STING ligands. Additionally, they promote STING protein degradation and downregulate the STING pathway. Before binding with cGAMP, the STING apoprotein adopts an “open” conformation, which shifts to a “closed” conformation upon binding. Tetrahydroisoquinolone compounds can bind to this “open” site, resulting in protein activation. By taking advantage of the natural symmetry of the STING protein and using a 2:1 binding stoichiometry, these compounds occupy the binding site fully, effectively competing with cGAMP, while maintaining favorable physicochemical properties for oral administration[167]. Astin C, a cyclic peptide isolated from the medicinal plant Aster tataricus, binds to the C-terminal of STING, blocking IRF3 recruitment and inhibiting STING pathway activation. Studies indicate that Astin C can significantly reduce autoinflammatory responses, displaying low cytotoxic side effects in cellular and animal models. This suggests that Astin C may be applicable in treating STING-mediated cancers and autoimmune diseases[168].
Innate immunity plays a pivotal role in the pathogenesis of cancer. Beyond the canonical role of cGAS-STING, recent evidence has emerged that expands the functional roles of cGAS-STING to cancer[15]. The cGAS-STING pathway serves as a direct sensor for cytoplasmic DNA within the innate immune system[19,20]. While the self-DNA sensor cGAS can detect cellular or tissue damage, excessive activation of the cGAS-STING pathway can incite inflammation and subsequent complications[61].
Investigating the cGAS-STING pathway in relation to HCC enhances our understanding of how innate immune responses contribute to the development of liver inflammation and injury. Recent discoveries regarding the regulation of this pathway will facilitate the identification of critical molecules that could serve as potential therapeutic targets for liver disease.
DNA damage leads to the formation of dsDNA in cancer cells, and under its stimulation, the cGAS STING signaling pathway is activated and promotes the release of type I IFN, which is crucial for DC maturation[56,57]. The activation of the cGAS STING signaling pathway in DCs is a core step in the entire cancer immune cycle, which can be initiated through phagocytosis of dead or damaged cancer cells, exosome metastasis, and cGAMP gap junctions. Then, with the help of type I IFN, DCs migrate to tumor draining lymph nodes and cross activate tumor specific CD8 +T cells, inducing systemic anti-tumor immunity to control local and distant tumor growth[60].
However, sustained activation of the cGAS-STING pathway may facilitate inflammation-driven tumorigenesis[61]. CIN within tumor cells can lead to cGAS-STING-dependent DNA sensing of micronuclei, which has the potential to stimulate tumor metastasis[71,72]. Although STING agonists and inhibitors have demonstrated efficacy in tumor treatment, the clinical and molecular guidelines for their application are still being established. Recognizing the dual, or dichotomous, roles of cGAS-STING in cancer progression is of utmost importance for the advancement of cancer therapeutic strategies.
In aggregate, these studies suggest the cGAS-STING pathway as a critical therapeutic target for tumors. The cGAS-STING pathway has been shown to potently inhibit the proliferation and metastasis of tumor cells, including HCC. The cGAS-STING pathway activation within HCC augments the body’s immunity against the tumor by provoking cellular senescence and inducing cell death. The combined effects additively result in an effective inhibition of tumor growth. Research in this pathway opens an exciting opportunity for the development of HCC therapeutics. On the other hand, the cGAS-STING pathway also contributes to HCC development and progression. If acute inflammation can have a few inhibitory effects on cancer, chronic inflammation generally promotes its progression. Extended pathway activation results in a suppressive tumor microenvironment; this may be contributory to the promotion of liver damage, fibrosis, and the growth of cancer and the outspread of tumor cells to other portions of the liver. Prior to using this pathway for HCC treatment, it is essential to evaluate the possible adverse effects, taking into account the different effects on tumors. The dosing and timing of STING agonists and inhibitors in cancer treatment must be therefore carefully considered. An innovative therapeutic strategy that has the ability to significantly improve HCC treatments is the cGAS-STING pathway.
The authors are grateful to the dedicated and committed participants in the study.
| 1. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2503] [Article Influence: 625.8] [Reference Citation Analysis (2)] |
| 2. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 2004] [Article Influence: 400.8] [Reference Citation Analysis (3)] |
| 3. | Berkemeyer S. Primary Liver Cancers: Connecting the Dots of Cellular Studies and Epidemiology with Metabolomics. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Niu ZS, Wang WH, Niu XJ. Recent progress in molecular mechanisms of postoperative recurrence and metastasis of hepatocellular carcinoma. World J Gastroenterol. 2022;28:6433-6477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (3)] |
| 5. | Cho JY, Paik YH, Lim HY, Kim YG, Lim HK, Min YW, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int. 2013;33:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10531] [Article Influence: 585.1] [Reference Citation Analysis (9)] |
| 7. | Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, Chung HC, Song X, Xu J, Poggi G, Omata M, Pitman Lowenthal S, Lanzalone S, Yang L, Lechuga MJ, Raymond E. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 8. | Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, Ying J, Lu Y, Meng Z, Pan H, Yang P, Zhang H, Chen X, Xu A, Cui C, Zhu B, Wu J, Xin X, Wang J, Shan J, Chen J, Zheng Z, Xu L, Wen X, You Z, Ren Z, Liu X, Qiu M, Wu L, Chen F. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol. 2021;39:3002-3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 297] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 9. | Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC, Gorbunova V, Eskens FA, Qian J, McKee MD, Ricker JL, Carlson DM, El-Nowiem S. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 489] [Article Influence: 40.8] [Reference Citation Analysis (1)] |
| 10. | Han Y, Zhi WH, Xu F, Zhang CB, Huang XQ, Luo JF. Selection of first-line systemic therapies for advanced hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. World J Gastroenterol. 2021;27:2415-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 11. | Lee MMP, Chan LL, Chan SL. The role of lenvatinib in the era of immunotherapy of hepatocellular carcinoma. J Liver Cancer. 2023;23:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 12. | Lee J, Sung PS, Yang H, Lee SK, Nam HC, Yoo SH, Lee HL, Kim HY, Lee SW, Kwon JH, Jang JW, Kim CW, Nam SW, Bae SH, Choi JY, Yoon SK. A Real-World Comparative Analysis of Lenvatinib and Sorafenib as a Salvage Therapy for Transarterial Treatments in Unresectable HCC. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 13. | Miller JF, Sadelain M. The journey from discoveries in fundamental immunology to cancer immunotherapy. Cancer Cell. 2015;27:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Makkouk A, Weiner GJ. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res. 2015;75:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 232] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Kwon J, Bakhoum SF. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020;10:26-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 891] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 16. | Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1305] [Cited by in RCA: 2005] [Article Influence: 143.2] [Reference Citation Analysis (0)] |
| 17. | Gan Y, Li X, Han S, Liang Q, Ma X, Rong P, Wang W, Li W. The cGAS/STING Pathway: A Novel Target for Cancer Therapy. Front Immunol. 2021;12:795401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 18. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7062] [Cited by in RCA: 5639] [Article Influence: 1127.8] [Reference Citation Analysis (33)] |
| 19. | Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3162] [Cited by in RCA: 3881] [Article Influence: 298.5] [Reference Citation Analysis (0)] |
| 20. | Xia P, Wang S, Gao P, Gao G, Fan Z. DNA sensor cGAS-mediated immune recognition. Protein Cell. 2016;7:777-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, Hornung V, Hopfner KP. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 441] [Cited by in RCA: 711] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 22. | Zhang X, Wu J, Du F, Xu H, Sun L, Chen Z, Brautigam CA, Zhang X, Chen ZJ. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Rep. 2014;6:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 23. | Kumar V. The Trinity of cGAS, TLR9, and ALRs Guardians of the Cellular Galaxy Against Host-Derived Self-DNA. Front Immunol. 2020;11:624597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, Simithy J, Lan Y, Lin Y, Zhou Z, Capell BC, Xu C, Xu M, Kieckhaefer JE, Jiang T, Shoshkes-Carmel M, Tanim KMAA, Barber GN, Seykora JT, Millar SE, Kaestner KH, Garcia BA, Adams PD, Berger SL. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 1053] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 25. | Xu L, Li M, Yang Y, Zhang C, Xie Z, Tang J, Shi Z, Chen S, Li G, Gu Y, Wang X, Zhang F, Wang Y, Shen X. Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release. mBio. 2022;13:e0363221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 26. | Ren H, Ma C, Peng H, Zhang B, Zhou L, Su Y, Gao X, Huang H. Micronucleus production, activation of DNA damage response and cGAS-STING signaling in syncytia induced by SARS-CoV-2 infection. Biol Direct. 2021;16:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Willemsen J, Neuhoff MT, Hoyler T, Noir E, Tessier C, Sarret S, Thorsen TN, Littlewood-Evans A, Zhang J, Hasan M, Rush JS, Guerini D, Siegel RM. TNF leads to mtDNA release and cGAS/STING-dependent interferon responses that support inflammatory arthritis. Cell Rep. 2021;37:109977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 155] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 28. | Taguchi T, Mukai K, Takaya E, Shindo R. STING Operation at the ER/Golgi Interface. Front Immunol. 2021;12:646304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Zhao B, Du F, Xu P, Shu C, Sankaran B, Bell SL, Liu M, Lei Y, Gao X, Fu X, Zhu F, Liu Y, Laganowsky A, Zheng X, Ji JY, West AP, Watson RO, Li P. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature. 2019;569:718-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 30. | Prabakaran T, Troldborg A, Kumpunya S, Alee I, Marinković E, Windross SJ, Nandakumar R, Narita R, Zhang BC, Carstensen M, Vejvisithsakul P, Marqvorsen MHS, Iversen MB, Holm CK, Østergaard LJ, Pedersen FS, Pisitkun T, Behrendt R, Pisitkun P, Paludan SR. A STING antagonist modulating the interaction with STIM1 blocks ER-to-Golgi trafficking and inhibits lupus pathology. EBioMedicine. 2021;66:103314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Shang G, Zhang C, Chen ZJ, Bai XC, Zhang X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature. 2019;567:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 575] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 32. | Zhang C, Shang G, Gui X, Zhang X, Bai XC, Chen ZJ. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 816] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 33. | Jiang M, Jia K, Wang L, Li W, Chen B, Liu Y, Wang H, Zhao S, He Y, Zhou C. Alterations of DNA damage response pathway: Biomarker and therapeutic strategy for cancer immunotherapy. Acta Pharm Sin B. 2021;11:2983-2994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 34. | Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5:ra20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1059] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 35. | Yum S, Li M, Fang Y, Chen ZJ. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 418] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 36. | Zhang X, Bai XC, Chen ZJ. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity. 2020;53:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 593] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 37. | Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2812] [Cited by in RCA: 2868] [Article Influence: 159.3] [Reference Citation Analysis (1)] |
| 38. | Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1241] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 39. | Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 790] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 40. | Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2497] [Cited by in RCA: 3867] [Article Influence: 227.5] [Reference Citation Analysis (0)] |
| 41. | Ko R, Seo J, Park H, Lee N, Lee SY. Pim1 promotes IFN-β production by interacting with IRF3. Exp Mol Med. 2022;54:2092-2103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 42. | Guan J, Han S, Wu J, Zhang Y, Bai M, Abdullah SW, Sun S, Guo H. Ribosomal Protein L13 Participates in Innate Immune Response Induced by Foot-and-Mouth Disease Virus. Front Immunol. 2021;12:616402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 1000] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 44. | Werner SL, Kearns JD, Zadorozhnaya V, Lynch C, O'Dea E, Boldin MP, Ma A, Baltimore D, Hoffmann A. Encoding NF-kappaB temporal control in response to TNF: distinct roles for the negative regulators IkappaBalpha and A20. Genes Dev. 2008;22:2093-2101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | McClelland SE. Role of chromosomal instability in cancer progression. Endocr Relat Cancer. 2017;24:T23-T31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D. DNA breaks and chromosome pulverization from errors in mitosis. Nature. 2012;482:53-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 971] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 47. | Hatch EM, Fischer AH, Deerinck TJ, Hetzer MW. Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell. 2013;154:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 587] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 48. | Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1288] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 49. | Saeed AFUH, Ruan X, Guan H, Su J, Ouyang S. Regulation of cGAS-Mediated Immune Responses and Immunotherapy. Adv Sci (Weinh). 2020;7:1902599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Chandeck C, Mooi WJ. Oncogene-induced cellular senescence. Adv Anat Pathol. 2010;17:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Lopes-Paciencia S, Saint-Germain E, Rowell MC, Ruiz AF, Kalegari P, Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 421] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 52. | Glück S, Guey B, Gulen MF, Wolter K, Kang TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol. 2017;19:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 757] [Cited by in RCA: 912] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 53. | Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 802] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 54. | Ivanov A, Pawlikowski J, Manoharan I, van Tuyn J, Nelson DM, Rai TS, Shah PP, Hewitt G, Korolchuk VI, Passos JF, Wu H, Berger SL, Adams PD. Lysosome-mediated processing of chromatin in senescence. J Cell Biol. 2013;202:129-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 435] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 55. | Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1709] [Cited by in RCA: 1963] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 56. | Tough DF. Modulation of T-cell function by type I interferon. Immunol Cell Biol. 2012;90:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Hemann EA, Green R, Turnbull JB, Langlois RA, Savan R, Gale M Jr. Interferon-λ modulates dendritic cells to facilitate T cell immunity during infection with influenza A virus. Nat Immunol. 2019;20:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 58. | Malyshkina A, Brüggemann A, Paschen A, Dittmer U. Cytotoxic CD4(+) T cells in chronic viral infections and cancer. Front Immunol. 2023;14:1271236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 59. | Giles JR, Globig AM, Kaech SM, Wherry EJ. CD8(+) T cells in the cancer-immunity cycle. Immunity. 2023;56:2231-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 258] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 60. | An X, Zhu Y, Zheng T, Wang G, Zhang M, Li J, Ji H, Li S, Yang S, Xu D, Li Z, Wang T, He Y, Zhang L, Yang W, Zhao R, Hao D, Li X. An Analysis of the Expression and Association with Immune Cell Infiltration of the cGAS/STING Pathway in Pan-Cancer. Mol Ther Nucleic Acids. 2019;14:80-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 61. | Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 4004] [Article Influence: 250.3] [Reference Citation Analysis (2)] |
| 62. | Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853-2868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2346] [Cited by in RCA: 3167] [Article Influence: 186.3] [Reference Citation Analysis (4)] |
| 63. | Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 328] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 64. | Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212-4222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1077] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 65. | Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 364] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 66. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11513] [Article Influence: 479.7] [Reference Citation Analysis (2)] |
| 67. | Song S, Peng P, Tang Z, Zhao J, Wu W, Li H, Shao M, Li L, Yang C, Duan F, Zhang M, Zhang J, Wu H, Li C, Wang X, Wang H, Ruan Y, Gu J. Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep. 2017;7:39858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 68. | Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates With Tumorigenesis. Cell Rep. 2016;14:282-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 482] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 69. | Bakhoum SF, Cantley LC. The Multifaceted Role of Chromosomal Instability in Cancer and Its Microenvironment. Cell. 2018;174:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 534] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 70. | Hou Y, Liang H, Rao E, Zheng W, Huang X, Deng L, Zhang Y, Yu X, Xu M, Mauceri H, Arina A, Weichselbaum RR, Fu YX. Non-canonical NF-κB Antagonizes STING Sensor-Mediated DNA Sensing in Radiotherapy. Immunity. 2018;49:490-503.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 215] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 71. | Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK, Duran M, Pauli C, Shaw C, Chadalavada K, Rajasekhar VK, Genovese G, Venkatesan S, Birkbak NJ, McGranahan N, Lundquist M, LaPlant Q, Healey JH, Elemento O, Chung CH, Lee NY, Imielenski M, Nanjangud G, Pe'er D, Cleveland DW, Powell SN, Lammerding J, Swanton C, Cantley LC. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 962] [Cited by in RCA: 1199] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 72. | Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 378] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 73. | Klionsky DJ. The molecular machinery of autophagy: unanswered questions. J Cell Sci. 2005;118:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 689] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 74. | Gui X, Yang H, Li T, Tan X, Shi P, Li M, Du F, Chen ZJ. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 992] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 75. | Nassour J, Radford R, Correia A, Fusté JM, Schoell B, Jauch A, Shaw RJ, Karlseder J. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 349] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 76. | Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, Kim M, Nishito Y, Iemura S, Natsume T, Ueno T, Kominami E, Motohashi H, Tanaka K, Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1546] [Cited by in RCA: 1984] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 77. | Liu J, Debnath J. The Evolving, Multifaceted Roles of Autophagy in Cancer. Adv Cancer Res. 2016;130:1-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | Münz C. Autophagy in cellular transformation, survival and communication with the tumor microenvironment. Semin Cancer Biol. 2013;23:299-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Viry E, Paggetti J, Baginska J, Mgrditchian T, Berchem G, Moussay E, Janji B. Autophagy: an adaptive metabolic response to stress shaping the antitumor immunity. Biochem Pharmacol. 2014;92:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 809] [Article Influence: 62.2] [Reference Citation Analysis (1)] |
| 81. | Cao J, Kong FH, Liu X, Wang XB. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: A meta-analysis. World J Gastroenterol. 2019;25:3649-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 82. | Kasper HU, Drebber U, Stippel DL, Dienes HP, Gillessen A. Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World J Gastroenterol. 2009;15:5053-5057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, Blum HE, Neumann-Haefelin C, Thimme R. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 303] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 84. | Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, Han M, Thornton AM, Zhang H, Egger M, Luo J, Felsher DW, McVicar DW, Weber A, Heikenwalder M, Greten TF. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 626] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 85. | Li X, Pan YF, Chen YB, Wan QQ, Lin YK, Shang TY, Xu MY, Jiang TY, Pei MM, Tan YX, Dong LW, Wan XY. Arsenic trioxide augments immunogenic cell death and induces cGAS-STING-IFN pathway activation in hepatocellular carcinoma. Cell Death Dis. 2024;15:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 86. | Ma H, Kang Z, Foo TK, Shen Z, Xia B. Disrupted BRCA1-PALB2 interaction induces tumor immunosuppression and T-lymphocyte infiltration in HCC through cGAS-STING pathway. Hepatology. 2023;77:33-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 87. | Lv T, Xiong X, Yan W, Liu M, Xu H, He Q. Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 88. | Lu LG, Zhou ZL, Wang XY, Liu BY, Lu JY, Liu S, Zhang GB, Zhan MX, Chen Y. PD-L1 blockade liberates intrinsic antitumourigenic properties of glycolytic macrophages in hepatocellular carcinoma. Gut. 2022;71:2551-2560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 89. | Han R, Ling C, Wang Y, Lu L. Enhancing HCC Treatment: innovatively combining HDAC2 inhibitor with PD-1/PD-L1 inhibition. Cancer Cell Int. 2023;23:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 90. | Zhang R, Wang J, Du Y, Yu Z, Wang Y, Jiang Y, Wu Y, Le T, Li Z, Zhang G, Lv L, Ma H. CDK5 destabilizes PD-L1 via chaperon-mediated autophagy to control cancer immune surveillance in hepatocellular carcinoma. J Immunother Cancer. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 91. | Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1467] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 92. | Li S, Mirlekar B, Johnson BM, Brickey WJ, Wrobel JA, Yang N, Song D, Entwistle S, Tan X, Deng M, Cui Y, Li W, Vincent BG, Gale M Jr, Pylayeva-Gupta Y, Ting JP. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature. 2022;610:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 93. | Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, Munn D, Mellor AL. STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res. 2016;76:2076-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 94. | Cheng AN, Cheng LC, Kuo CL, Lo YK, Chou HY, Chen CH, Wang YH, Chuang TH, Cheng SJ, Lee AY. Mitochondrial Lon-induced mtDNA leakage contributes to PD-L1-mediated immunoescape via STING-IFN signaling and extracellular vesicles. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 95. | Schlichtner S, Yasinska IM, Klenova E, Abooali M, Lall GS, Berger SM, Ruggiero S, Cholewa D, Milošević M, Gibbs BF, Fasler-Kan E, Sumbayev VV. L-Kynurenine participates in cancer immune evasion by downregulating hypoxic signaling in T lymphocytes. Oncoimmunology. 2023;12:2244330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 96. | Boukhaled GM, Harding S, Brooks DG. Opposing Roles of Type I Interferons in Cancer Immunity. Annu Rev Pathol. 2021;16:167-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 97. | Rai KR, Shrestha P, Yang B, Chen Y, Liu S, Maarouf M, Chen JL. Acute Infection of Viral Pathogens and Their Innate Immune Escape. Front Microbiol. 2021;12:672026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 98. | Li J, Hubisz MJ, Earlie EM, Duran MA, Hong C, Varela AA, Lettera E, Deyell M, Tavora B, Havel JJ, Phyu SM, Amin AD, Budre K, Kamiya E, Cavallo JA, Garris C, Powell S, Reis-Filho JS, Wen H, Bettigole S, Khan AJ, Izar B, Parkes EE, Laughney AM, Bakhoum SF. Non-cell-autonomous cancer progression from chromosomal instability. Nature. 2023;620:1080-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 199] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 99. | Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J Virol. 2014;88:5328-5341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 628] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 100. | Tegowski M, Baldwin A. Noncanonical NF-κB in Cancer. Biomedicines. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 101. | Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 932] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 102. | Zhao Q, Manohar M, Wei Y, Pandol SJ, Habtezion A. STING signalling protects against chronic pancreatitis by modulating Th17 response. Gut. 2019;68:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 103. | Lv M, Chen M, Zhang R, Zhang W, Wang C, Zhang Y, Wei X, Guan Y, Liu J, Feng K, Jing M, Wang X, Liu YC, Mei Q, Han W, Jiang Z. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 640] [Article Influence: 106.7] [Reference Citation Analysis (1)] |
| 104. | Yan X, Yao C, Fang C, Han M, Gong C, Hu D, Shen W, Wang L, Li S, Zhu S. Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer. Int J Biol Sci. 2022;18:585-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 105. | Wang X, Liu Y, Xue C, Hu Y, Zhao Y, Cai K, Li M, Luo Z. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat Commun. 2022;13:5685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 147] [Reference Citation Analysis (0)] |
| 106. | Cao L, Tian H, Fang M, Xu Z, Tang D, Chen J, Yin J, Xiao H, Shang K, Han H, Li X. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials. 2022;290:121856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 106] [Reference Citation Analysis (0)] |
| 107. | Ka NL, Park MK, Kim SS, Jeon Y, Hwang S, Kim SM, Lim GY, Lee H, Lee MO. NR1D1 Stimulates Antitumor Immune Responses in Breast Cancer by Activating cGAS-STING Signaling. Cancer Res. 2023;83:3045-3058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 108. | Kaneta A, Nakajima S, Okayama H, Matsumoto T, Saito K, Kikuchi T, Endo E, Ito M, Mimura K, Kanke Y, Saito M, Saze Z, Fujita S, Sakamoto W, Onozawa H, Momma T, Ohki S, Kono K. Role of the cGAS-STING pathway in regulating the tumor-immune microenvironment in dMMR/MSI colorectal cancer. Cancer Immunol Immunother. 2022;71:2765-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 109. | Wu X, Xu L, Li X, Zhou Y, Han X, Zhang W, Wang W, Guo W, Liu W, Xu Q, Gu Y. A HER2-targeting antibody-MMAE conjugate RC48 sensitizes immunotherapy in HER2-positive colon cancer by triggering the cGAS-STING pathway. Cell Death Dis. 2023;14:550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 110. | Shen Q, Yang L, Li C, Wang T, Lv J, Liu W, Lin Y, Yin Y, Tao K. Metformin promotes cGAS/STING signaling pathway activation by blocking AKT phosphorylation in gastric cancer. Heliyon. 2023;9:e18954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 111. | Yin M, Dong J, Sun C, Liu X, Liu Z, Liu L, Kuang Z, Zhang N, Xiao D, Zhou X, Deng H. Raddeanin A Enhances Mitochondrial DNA-cGAS/STING Axis-Mediated Antitumor Immunity by Targeting Transactive Responsive DNA-Binding Protein 43. Adv Sci (Weinh). 2023;10:e2206737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 112. | Luo K, Li N, Ye W, Gao H, Luo X, Cheng B. Activation of Stimulation of Interferon Genes (STING) Signal and Cancer Immunotherapy. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 113. | Loo TM, Miyata K, Tanaka Y, Takahashi A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020;111:304-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 114. | Chen R, Du J, Zhu H, Ling Q. The role of cGAS-STING signalling in liver diseases. JHEP Rep. 2021;3:100324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 115. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 12322] [Article Influence: 724.8] [Reference Citation Analysis (0)] |
| 116. | Lee HY, Nga HT, Tian J, Yi HS. Mitochondrial Metabolic Signatures in Hepatocellular Carcinoma. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 117. | Yan C, Duanmu X, Zeng L, Liu B, Song Z. Mitochondrial DNA: Distribution, Mutations, and Elimination. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 118. | Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W, Li L, Zhou H, Lu L. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022;52:102305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 249] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 119. | Snow A, Chen D, Lang JE. The current status of the clinical utility of liquid biopsies in cancer. Expert Rev Mol Diagn. 2019;19:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 120. | Ueda K, Sakai C, Ishida T, Morita K, Kobayashi Y, Horikoshi Y, Baba A, Okazaki Y, Yoshizumi M, Tashiro S, Ishida M. Cigarette smoke induces mitochondrial DNA damage and activates cGAS-STING pathway: application to a biomarker for atherosclerosis. Clin Sci (Lond). 2023;137:163-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 121. | Zhou S, Cheng F, Zhang Y, Su T, Zhu G. Engineering and Delivery of cGAS-STING Immunomodulators for the Immunotherapy of Cancer and Autoimmune Diseases. Acc Chem Res. 2023;56:2933-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 122. | Zheng W, Liu A, Xia N, Chen N, Meurens F, Zhu J. How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Apoptosis. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 123. | Lu Q, Chen Y, Li J, Zhu F, Zheng Z. Crosstalk between cGAS-STING pathway and autophagy in cancer immunity. Front Immunol. 2023;14:1139595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 124. | Liu J, Zhou J, Luan Y, Li X, Meng X, Liao W, Tang J, Wang Z. cGAS-STING, inflammasomes and pyroptosis: an overview of crosstalk mechanism of activation and regulation. Cell Commun Signal. 2024;22:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 86] [Reference Citation Analysis (0)] |
| 125. | Wu X, Jiang Y, Li R, Xia Y, Li F, Zhao M, Li G, Tan X. Ficolin B secreted by alveolar macrophage exosomes exacerbates bleomycin-induced lung injury via ferroptosis through the cGAS-STING signaling pathway. Cell Death Dis. 2023;14:577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 126. | Brault M, Olsen TM, Martinez J, Stetson DB, Oberst A. Intracellular Nucleic Acid Sensing Triggers Necroptosis through Synergistic Type I IFN and TNF Signaling. J Immunol. 2018;200:2748-2756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 127. | Sun F, Xu Y, Deng Z, Yang P. A recombinant oncolytic influenza virus expressing a PD-L1 antibody induces CD8(+) T-cell activation via the cGas-STING pathway in mice with hepatocellular carcinoma. Int Immunopharmacol. 2023;120:110323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 128. | Pu Z, Liu J, Liu Z, Peng F, Zhu Y, Wang X, He J, Yi P, Hu X, Fan X, Chen J. STING pathway contributes to the prognosis of hepatocellular carcinoma and identification of prognostic gene signatures correlated to tumor microenvironment. Cancer Cell Int. 2022;22:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 129. | Yang KS, Xu CQ, Lv J. Hyperbaric oxygen facilitates teniposide-induced cGAS-STING activation to enhance the antitumor efficacy of PD-1 antibody in HCC. J Immunother Cancer. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Wang Z, Chen J, Hu J, Zhang H, Xu F, He W, Wang X, Li M, Lu W, Zeng G, Zhou P, Huang P, Chen S, Li W, Xia LP, Xia X. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J Clin Invest. 2019;129:4850-4862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 131. | Tang CH, Zundell JA, Ranatunga S, Lin C, Nefedova Y, Del Valle JR, Hu CC. Agonist-Mediated Activation of STING Induces Apoptosis in Malignant B Cells. Cancer Res. 2016;76:2137-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 132. | Song C, Liu D, Liu S, Li D, Horecny I, Zhang X, Li P, Chen L, Miller M, Chowdhury R, Issa M, Shen R, Yan Y, Zhang F, Zhang L, Zhang L, Bai C, Feng J, Zhuang L, Zhang R, Li J, Wilkinson H, Liu J, Tao W. SHR1032, a novel STING agonist, stimulates anti-tumor immunity and directly induces AML apoptosis. Sci Rep. 2022;12:8579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 133. | Lv H, Zong Q, Chen C, Lv G, Xiang W, Xing F, Jiang G, Yan B, Sun X, Ma Y, Wang L, Wu Z, Cui X, Wang H, Yang W. TET2-mediated tumor cGAS triggers endothelial STING activation to regulate vasculature remodeling and anti-tumor immunity in liver cancer. Nat Commun. 2024;15:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 61] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 134. | Lasarte-Cia A, Lozano T, Cano D, Martín-Otal C, Navarro F, Gorraiz M, Casares N, Vivas I, Lasarte JJ. Intratumoral STING Agonist Injection Combined with Irreversible Electroporation Delays Tumor Growth in a Model of Hepatocarcinoma. Biomed Res Int. 2021;2021:8852233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 135. | Thomsen MK, Skouboe MK, Boularan C, Vernejoul F, Lioux T, Leknes SL, Berthelsen MF, Riedel M, Cai H, Joseph JV, Perouzel E, Tiraby M, Vendelbo MH, Paludan SR. The cGAS-STING pathway is a therapeutic target in a preclinical model of hepatocellular carcinoma. Oncogene. 2020;39:1652-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 136. | Sheng H, Huang Y, Xiao Y, Zhu Z, Shen M, Zhou P, Guo Z, Wang J, Wang H, Dai W, Zhang W, Sun J, Cao C. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 137. | Cao Y, Sun T, Sun B, Zhang G, Liu J, Liang B, Zheng C, Kan X. Injectable hydrogel loaded with lysed OK-432 and doxorubicin for residual liver cancer after incomplete radiofrequency ablation. J Nanobiotechnology. 2023;21:404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 138. | Du H, Xu T, Cui M. cGAS-STING signaling in cancer immunity and immunotherapy. Biomed Pharmacother. 2021;133:110972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 139. | Hu M, Zhou M, Bao X, Pan D, Jiao M, Liu X, Li F, Li CY. ATM inhibition enhances cancer immunotherapy by promoting mtDNA leakage and cGAS/STING activation. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 140. | Gao D, Li T, Li XD, Chen X, Li QZ, Wight-Carter M, Chen ZJ. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci U S A. 2015;112:E5699-E5705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 557] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 141. | Du S, Chen G, Yuan B, Hu Y, Yang P, Chen Y, Zhao Q, Zhou J, Fan J, Zeng Z. DNA sensing and associated type 1 interferon signaling contributes to progression of radiation-induced liver injury. Cell Mol Immunol. 2021;18:1718-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 142. | Li N, Zheng X, Chen M, Huang L, Chen L, Huo R, Li X, Huang Y, Sun M, Mai S, Wu Z, Zhang H, Liu J, Yang CT. Deficient DNASE1L3 facilitates neutrophil extracellular traps-induced invasion via cyclic GMP-AMP synthase and the non-canonical NF-κB pathway in diabetic hepatocellular carcinoma. Clin Transl Immunology. 2022;11:e1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 143. | Su W, Gao W, Zhang R, Wang Q, Li L, Bu Q, Xu Z, Liu Z, Wang M, Zhu Y, Wu G, Zhou H, Wang X, Lu L. TAK1 deficiency promotes liver injury and tumorigenesis via ferroptosis and macrophage cGAS-STING signalling. JHEP Rep. 2023;5:100695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 144. | Qi Z, Yan F, Chen D, Xing W, Li Q, Zeng W, Bi B, Xie J. Identification of prognostic biomarkers and correlations with immune infiltrates among cGAS-STING in hepatocellular carcinoma. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 145. | Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005-2016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 759] [Cited by in RCA: 1013] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 146. | Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167:1540-1554.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 923] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 147. | Wang Y, Luo J, Alu A, Han X, Wei Y, Wei X. cGAS-STING pathway in cancer biotherapy. Mol Cancer. 2020;19:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 148. | Li X, Shu C, Yi G, Chaton CT, Shelton CL, Diao J, Zuo X, Kao CC, Herr AB, Li P. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39:1019-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 537] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 149. | Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW Jr, Gajewski TF. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015;11:1018-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1206] [Article Influence: 109.6] [Reference Citation Analysis (0)] |
| 150. | Ross P, Mayer R, Weinhouse H, Amikam D, Huggirat Y, Benziman M, de Vroom E, Fidder A, de Paus P, Sliedregt L. The cyclic diguanylic acid regulatory system of cellulose synthesis in Acetobacter xylinum. Chemical synthesis and biological activity of cyclic nucleotide dimer, trimer, and phosphothioate derivatives. J Biol Chem. 1990;265:18933-18943. [RCA] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 149] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 151. | Zhang Z, Liu J, Xiao M, Zhang Q, Liu Z, Liu M, Zhang P, Zeng Y. Peptide nanotube loaded with a STING agonist, c-di-GMP, enhance cancer immunotherapy against melanoma. Nano Res. 2023;16:5206-5215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 152. | Li T, Cheng H, Yuan H, Xu Q, Shu C, Zhang Y, Xu P, Tan J, Rui Y, Li P, Tan X. Antitumor Activity of cGAMP via Stimulation of cGAS-cGAMP-STING-IRF3 Mediated Innate Immune Response. Sci Rep. 2016;6:19049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 153. | Temizoz B, Shibahara T, Hioki K, Hayashi T, Kobiyama K, Lee MSJ, Surucu N, Sag E, Kumanogoh A, Yamamoto M, Gursel M, Ozen S, Kuroda E, Coban C, Ishii KJ. 5,6-dimethylxanthenone-4-acetic acid (DMXAA), a partial STING agonist, competes for human STING activation. Front Immunol. 2024;15:1353336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 154. | Buchanan CM, Shih JH, Astin JW, Rewcastle GW, Flanagan JU, Crosier PS, Shepherd PR. DMXAA (Vadimezan, ASA404) is a multi-kinase inhibitor targeting VEGFR2 in particular. Clin Sci (Lond). 2012;122:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 155. | Graham PT, Nowak AK, Cornwall SMJ, Larma I, Nelson DJ. The STING agonist, DMXAA, reduces tumor vessels and enhances mesothelioma tumor antigen presentation yet blunts cytotoxic T cell function in a murine model. Front Immunol. 2022;13:969678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 156. | Peng S, Monie A, Pang X, Hung CF, Wu TC. Vascular disrupting agent DMXAA enhances the antitumor effects generated by therapeutic HPV DNA vaccines. J Biomed Sci. 2011;18:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 157. | Henare K, Wang L, Wang LC, Thomsen L, Tijono S, Chen CJ, Winkler S, Dunbar PR, Print C, Ching LM. Dissection of stromal and cancer cell-derived signals in melanoma xenografts before and after treatment with DMXAA. Br J Cancer. 2012;106:1134-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 158. | Smolarczyk R, Cichoń T, Pilny E, Jarosz-Biej M, Poczkaj A, Kułach N, Szala S. Combination of anti-vascular agent - DMXAA and HIF-1α inhibitor - digoxin inhibits the growth of melanoma tumors. Sci Rep. 2018;8:7355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 159. | Wigerup C, Påhlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 512] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 160. | Schito L, Semenza GL. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer. 2016;2:758-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 721] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 161. | Ding C, Song Z, Shen A, Chen T, Zhang A. Small molecules targeting the innate immune cGAS‒STING‒TBK1 signaling pathway. Acta Pharm Sin B. 2020;10:2272-2298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 162. | Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, Tran JL, Moore P, Lehmann S, Eberl HC, Muelbaier M, Schneck JL, Clemens J, Adam M, Mehlmann J, Romano J, Morales A, Kang J, Leister L, Graybill TL, Charnley AK, Ye G, Nevins N, Behnia K, Wolf AI, Kasparcova V, Nurse K, Wang L, Puhl AC, Li Y, Klein M, Hopson CB, Guss J, Bantscheff M, Bergamini G, Reilly MA, Lian Y, Duffy KJ, Adams J, Foley KP, Gough PJ, Marquis RW, Smothers J, Hoos A, Bertin J. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 665] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 163. | Yu X, Cai L, Yao J, Li C, Wang X. Agonists and Inhibitors of the cGAS-STING Pathway. Molecules. 2024;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 164. | Mukai K, Konno H, Akiba T, Uemura T, Waguri S, Kobayashi T, Barber GN, Arai H, Taguchi T. Activation of STING requires palmitoylation at the Golgi. Nat Commun. 2016;7:11932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 552] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 165. | Haag SM, Gulen MF, Reymond L, Gibelin A, Abrami L, Decout A, Heymann M, van der Goot FG, Turcatti G, Behrendt R, Ablasser A. Targeting STING with covalent small-molecule inhibitors. Nature. 2018;559:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 885] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 166. | Hansen AL, Buchan GJ, Rühl M, Mukai K, Salvatore SR, Ogawa E, Andersen SD, Iversen MB, Thielke AL, Gunderstofte C, Motwani M, Møller CT, Jakobsen AS, Fitzgerald KA, Roos J, Lin R, Maier TJ, Goldbach-Mansky R, Miner CA, Qian W, Miner JJ, Rigby RE, Rehwinkel J, Jakobsen MR, Arai H, Taguchi T, Schopfer FJ, Olagnier D, Holm CK. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc Natl Acad Sci U S A. 2018;115:E7768-E7775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 167. | Siu T, Altman MD, Baltus GA, Childers M, Ellis JM, Gunaydin H, Hatch H, Ho T, Jewell J, Lacey BM, Lesburg CA, Pan BS, Sauvagnat B, Schroeder GK, Xu S. Discovery of a Novel cGAMP Competitive Ligand of the Inactive Form of STING. ACS Med Chem Lett. 2019;10:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 168. | Li S, Hong Z, Wang Z, Li F, Mei J, Huang L, Lou X, Zhao S, Song L, Chen W, Wang Q, Liu H, Cai Y, Yu H, Xu H, Zeng G, Wang Q, Zhu J, Liu X, Tan N, Wang C. The Cyclopeptide Astin C Specifically Inhibits the Innate Immune CDN Sensor STING. Cell Rep. 2018;25:3405-3421.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/