Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.94956
Revised: October 2, 2024
Accepted: October 25, 2024
Published online: February 15, 2025
Processing time: 295 Days and 17.3 Hours
There are currently no relevant studies at home or abroad that combine inflammatory indicators and nomograms to predict the prognosis of gastrointestinal stromal tumor (GIST) patients after surgery. The purpose of this study was to investigate the predictive value of related inflammatory indicators [systemic immune-inflammation index (SII), neutrophil/lymphocyte ratio (NLR), plate
To explore the relationships between the SII, NLR, PLR, and MLR and posto
This study retrospectively included patients who underwent GIST surgery from January 2014 to January 2017 and analyzed the potential relationships between the preoperative SII, NLR, PLR, and MLR and clinicopathological features. The independent risk factors influencing the prognosis of GIST patients were obtained via multivariate regression analysis, and a nomogram model based on the in
Among the 124 GIST patients included in the present study, 31 (25%) experienced recurrence within 5 years. Kaplan-Meier survival analysis revealed a correlation between the MLR and PLR and tumor size (P = 0.016 and P = 0.002, respectively). The preoperative SII, MLR, NLR, and PLR were significantly associated with recurrence-free survival (RFS) (P < 0.05). The multivariate analysis results identified the PLR, MLR, and targeted therapy as independent prognostic factors for patient outcomes.
Preoperative MLR and PLR, which are independent risk factors for GIST recurrence, were correlated with RFS. Nomograms based on the PLR, MLR and targeted therapy can be used for clinical treatment.
Core Tip: This study revealed significant correlations between preoperative inflammatory markers [systemic immune-inflammation index, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio (PLR) and monocyte/Lymphocyte ratio (MLR)] and recurrence-free survival in gastrointestinal stromal tumor (GIST) patients. The MLR and PLR were identified as independent risk factors for GIST recurrence. Research has highlighted the prognostic relevance of inflammatory markers in various cancers. Nomograms have been utilized in predicting survival and recurrence in different cancers. This study pioneered the construction of a nomogram based on inflammatory markers for assessing the recurrence risk prognosis in GIST patients. The nomogram demonstrated utility in predicting GIST patient recurrence risk, emphasizing the ongoing sensitivity of inflammatory markers in patients receiving postoperative targeted therapy.
- Citation: Zhao JL, Wang MY, Lv YZ, Zhou YJ. Prognostic value of inflammatory markers in predicting recurrence-free survival in gastrointestinal stromal tumor patients: A nomogram-based approach. World J Gastrointest Oncol 2025; 17(2): 94956
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/94956.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.94956
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract and are most common in the stomach (60%) and the small bowel (30%)[1]. GISTs exhibit different degrees of malignant potential and can rapidly progress from essentially benign tumors to malignant tumors. The reported incidence of GISTs has significantly increased over the past 20 years[2,3]. While complete surgical resection is the currently endorsed treatment for GISTs, postoperative recurrence is the primary factor contributing to decreased survival rates[4]. Thus, identification of recurrence risk following GIST surgery is urgently needed.
Inflammation-driven tumorigenesis and progression play crucial roles in malignant diseases[5]. Immune and inflammatory cells in the peripheral blood, including monocytes, neutrophils, platelets, and lymphocytes, intricately contribute to the progression of diverse tumors and act as key effectors of tumor cell invasion and metastasis[6,7]. Some indices of the above inflammatory cells, such as the systemic immune-inflammation index (SII), neutrophil/Lymphocyte ratio (NLR), platelet/Lymphocyte ratio (PLR), and monocyte/lymphocyte ratio (MLR), have become prognostic factors for various cancers. They are used to predict the prognosis of cancers and have achieved significant results in many types of cancer[8], such as liver cancer[9], pancreatic cancer[10], and other malignant diseases.
While existing research underscores the prognostic relevance of MLR, PLR, and NLR in specific cancers, there have been limited studies elucidating the prognostic relationship between SII and GISTs. In recent years, nomograms have been widely used to predict survival and recurrence after cancer surgery, but their value in GISTs remains unclear. This study aimed to evaluate the prognostic value of SII, MLR, PLR, and NLR for GIST patients. Additionally, a nomogram prediction model has been developed to predict recurrence-free survival (RFS) in postoperative GIST patients. This research contributes valuable insights toward refining prognostic assessments and therapeutic strategies for individuals undergoing GIST surgery.
This study collected the clinical data of GIST patients who underwent radical surgery at the Affiliated Hospital of Southwest Medical University from January 2014 to January 2017. The inclusion criteria were as follows: (1) Patients who received radical surgery for GIST; (2) Patients who were diagnosed with GIST based on pathological examination after radical surgery; and (3) Patients with comprehensive clinicopathological data and complete follow-up information. The exclusion criteria were as follows: (1) Patients who had received chemotherapy and/or radiotherapy before surgery; and (2) Patients with a history of autoimmune diseases, hematological diseases, or other malignancies.
All included patients provided written informed consent before surgery. In addition, this retrospective study was approved by the ethics committee of Affiliated Hospital of Southwest Medical University. Subsequently, all patients underwent regular follow-ups, including physical examinations, tumor marker assessments, and computed tomography scans every 3 months for the first 2 years, followed by assessments every 6 months until 5 years postsurgery. The cutoff date for follow-up in this study was January 31, 2022.
Clinicopathological data were collected from medical records, including name, sex, age, body mass index (BMI), tumor size, tumor site, whether the tumor had ruptured, and preoperative neutrophils, platelets, lymphocytes, and monocytes. The use of targeted therapy after surgery and the time to diagnosis of recurrence and metastasis were collected.
Blood samples were obtained within 7 days prior to surgery by professional nurses. MLR, NLR, PLR, and SII were calculated as previously described: MLR = monocytes/lymphocytes, NLR = neutrophils/lymphocytes, PLR = platelet/lymphocytes, and SII = platelets × neutrophils/lymphocytes[11]. Where M, L, N, and P represent monocytes, lym
Data analysis was performed with IBM SPSS Statistics 25.0 (IBM Corporation). The optimal cutoff values for prognostic factors were determined by receiver operating characteristic (ROC) curve analysis. The correlation between the SII and characteristics was assessed by χ2 test. Survival differences were compared by the Kaplan-Meier method and a log-rank test. Multivariate prognostic analysis was performed using a Cox regression model with time-dependent covariates. Time-dependent ROC curves were generated using R software version 3.2.0 (http://www.r-project.org/) with the "rms", "car" and "pROC" packages. P < 0.05 was considered statistically significant.
A total of 124 patients completed follow-up and were included in the study. Of these, 48 were men, and 76 were women, with a mean age of 58 ± 12.836 years. This included 66 cases (53.23%) of tumor diameter < 5 cm and 58 cases (46.77%) of tumor diameter ≥ 5 cm. Of these, there were 76 cases (61.29%) of gastric tumors and 48 cases (38.7%) of nongastric tumors. Thirty-one patients (25.0%) underwent targeted therapy postoperatively. During the follow-up period, the 5-year RFS was 75% (Table 1).
| Variable | n | mean ± SD |
| Age (years) | 58 ± 12.836 | |
| BMI | 22.10 ± 3.692 | |
| Sex | ||
| Male | 48 (38.70) | |
| Female | 76 (61.30) | |
| Anatomic site of GIST | ||
| Gastric tumors | 76 (61.30) | |
| Non-gastric tumors | 48 (38.70) | |
| Tumor size (cm) | ||
| < 5 | 66 (53.23) | |
| ≥ 5 | 58 (46.77) | |
| Targeted therapy | ||
| Yes | 31 (25) | |
| No | 93 (75) | |
| 5-years RFS | 93 (75) |
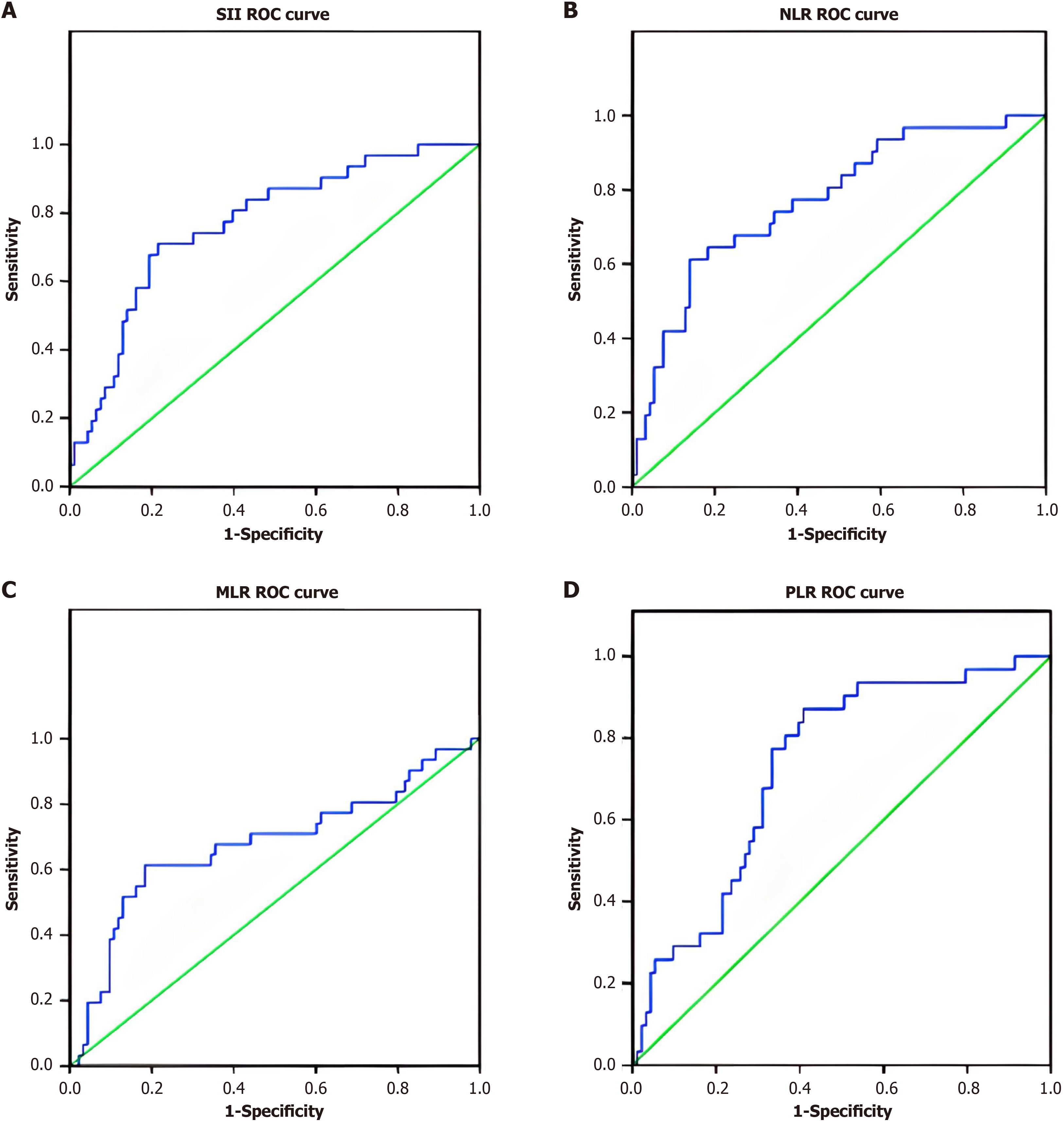
The optimal cutoff point of inflammation-related indices was determined by using ROC curve analysis (Figure 1). The optimal cutoff points were 1079, 5.79, 0.43, and 181.33 for SII, NLR, MLR, and PLR, respectively. The areas under the curve for RFS were 0.768, 0.771, 0.677, and 0.730 for SII, NLR, MLR, and PLR, respectively (Table 2). As shown in Table 3 and Table 4, 42 patients (33.87%) had SII ≥ 1079, 34 patients (27.42%) had NLR ≥ 5.79, 36 patients (29.03%) had MLR ≥ 0.430, and 65 patients (52.42%) had PLR ≥ 181.33 (Figure 1, Table 2).
| Object | Cut-off | AUC | P value | 95%CI |
| SII | 1097 | 0.768 | < 0.001 | 0.674-0.863 |
| NLR | 5.79 | 0.771 | < 0.001 | 0.676-0.867 |
| MLR | 0.43 | 0.677 | 0.003 | 0.556-0.798 |
| PLR | 181.33 | 0.730 | < 0.001 | 0.634-0.826 |
| Variable | SII | P value | χ2 | NLR | P value | χ2 | ||
| < 1079 (n = 82) | ≥ 1079 (n = 42) | < 5.79 (n = 90) | ≥ 5.79 (n = 34) | |||||
| Gender | 0.920 | 0.010 | 0.447 | 0.577 | ||||
| Male | 32 (39.0) | 16 (38.1) | 33 (36.7) | 15 (44.1) | ||||
| Female | 50 (61.0) | 26 (61.9) | 57 (63.3) | 19 (55.9) | ||||
| Age | 56.12 ± 12.796 | 56.14 ± 12.493 | 0.968 | 0.002 | 56.04 ± 12.556 | 56.59 ± 13.738 | 0.807 | 0.60 |
| BMI | 22.13 ± 3.643 | 22.49 ± 3.819 | 0.914 | 0.12 | 22.35 ± 3.970 | 22.00 ± 2.863 | 0.268 | 1.239 |
| Anatomic site of GIST | 0.497 | 0.460 | 0.098 | 2.742 | ||||
| Stomach | 52 (63.4) | 24 (57.1) | 62 (68.9) | 18 (52.9) | ||||
| Non-Stomach | 30 (36.6) | 18 (42.9) | 28 (31.1) | 16 (47.1) | ||||
| Targeted therapy | 0.511 | 0.432 | 0.816 | 0.054 | ||||
| Yes | 19 (23.2) | 12 (28.6) | 22 (24.4) | 9 (26.5) | ||||
| No | 63 (76.8) | 30 (71.4) | 68 (75.6) | 25 (73.5) | ||||
| Tumor size, cm | 5.88 ± 3.395 | 7.24 ± 4.057 | 0.078 | 3.160 | 6.15 ± 3.657 | 6.85 ± 3.723 | 0.308 | 1.047 |
| Size of GIST, cm | 0.920 | 0.10 | 0.111 | 2.536 | ||||
| < 5 cm | 32 (39.0) | 16 (38.1) | 23 (28.8) | 15 (44.1) | ||||
| ≥ 5 cm | 50 (61.0) | 26 (61.9) | 57 (71.3) | 19 (55.9) | ||||
| Variable | MLR | P value | χ2 | PLR | P value | χ2 | ||
| < 0.430 (n = 88) | ≥ 0.43 (n = 36) | < 181.33 (n = 59) | ≥ 181.33 (n = 65) | |||||
| Gender | 0.213 | 1.549 | 0.497 | 0.461 | ||||
| Male | 31 (35.2) | 17 (47.2) | 21 (35.6) | 27 (41.5) | ||||
| Female | 57 (64.8) | 19 (52.8) | 38 (64.4) | 38 (58.5) | ||||
| Age | 56.22 ± 13.045 | 56.14 ± 12.493 | 0.746 | 0.105 | 56.42 ± 55.98 | 56.98 ± 14.318 | 0.083 | 3.059 |
| BMI | 22.1 ± 3.687 | 22.43 ± 3.750 | 0.852 | 0.035 | 22.3 ± 3.967 | 22.20 ± 3.453 | 0.262 | 1.272 |
| Anatomic site of GIST | 0.40 | 4.232 | 0.953 | 0.004 | ||||
| Stomach | 59 (67.0) | 17 (47.2) | 36 (61.0) | 40 (61.5) | ||||
| Non-stomach | 29 (33.0) | 19 (52.8) | 23 (39.0) | 25 (38.5) | ||||
| Targeted therapy | 0.170 | 1.879 | 0.917 | 0.011 | ||||
| Yes | 19 (21.6) | 12 (33.3) | 15 (25.4) | 16 (24.6) | ||||
| No | 69 (78.4) | 24 (66.7) | 44 (74.6) | 49 (75.4) | ||||
| Tumor size, cm | 5.77 ± 3.243 | 7.74 ± 4.296 | 0.016 | 5.928 | 5.40 ± 2.746 | 7.19 ± 4.191 | 0.002 | 10.508 |
| Size of GIST, cm | 0.704 | 0.144 | 0.425 | 0.637 | ||||
| < 5 cm | 35 (39.8) | 13 (36.1) | 25 (42.4) | 23 (35.4) | ||||
| ≥ 5 cm | 53 (60.2) | 23 (63.9) | 34 (57.6) | 42 (64.6) | ||||
| Variable | n | RFS (month) | RFS (95%CI) | P value | χ2 |
| Gender | 0.697 | 0.152 | |||
| Male | 48 | 77.646 | (69.98-85.31) | ||
| Female | 76 | 79.395 | (73.40-85.39) | ||
| Age | 0.377 | 0.780 | |||
| ≤ 58 | 64 | 76.547 | (69.617-83.477) | ||
| >58 | 60 | 81.033 | (74.707-87.360) | ||
| BMI (kg/m2) | 0.351 | 0.870 | |||
| ≤ 22 | 62 | 81.417 | (75.325-87.599) | ||
| >22 | 62 | 76.188 | (69.159-83.216) | ||
| Anatomic site of GIST | 0.679 | 0.171 | |||
| Stomach | 76 | 79.513 | (73.570-85.456) | ||
| Non-stomach | 48 | 77.458 | (69.699-85.218) | ||
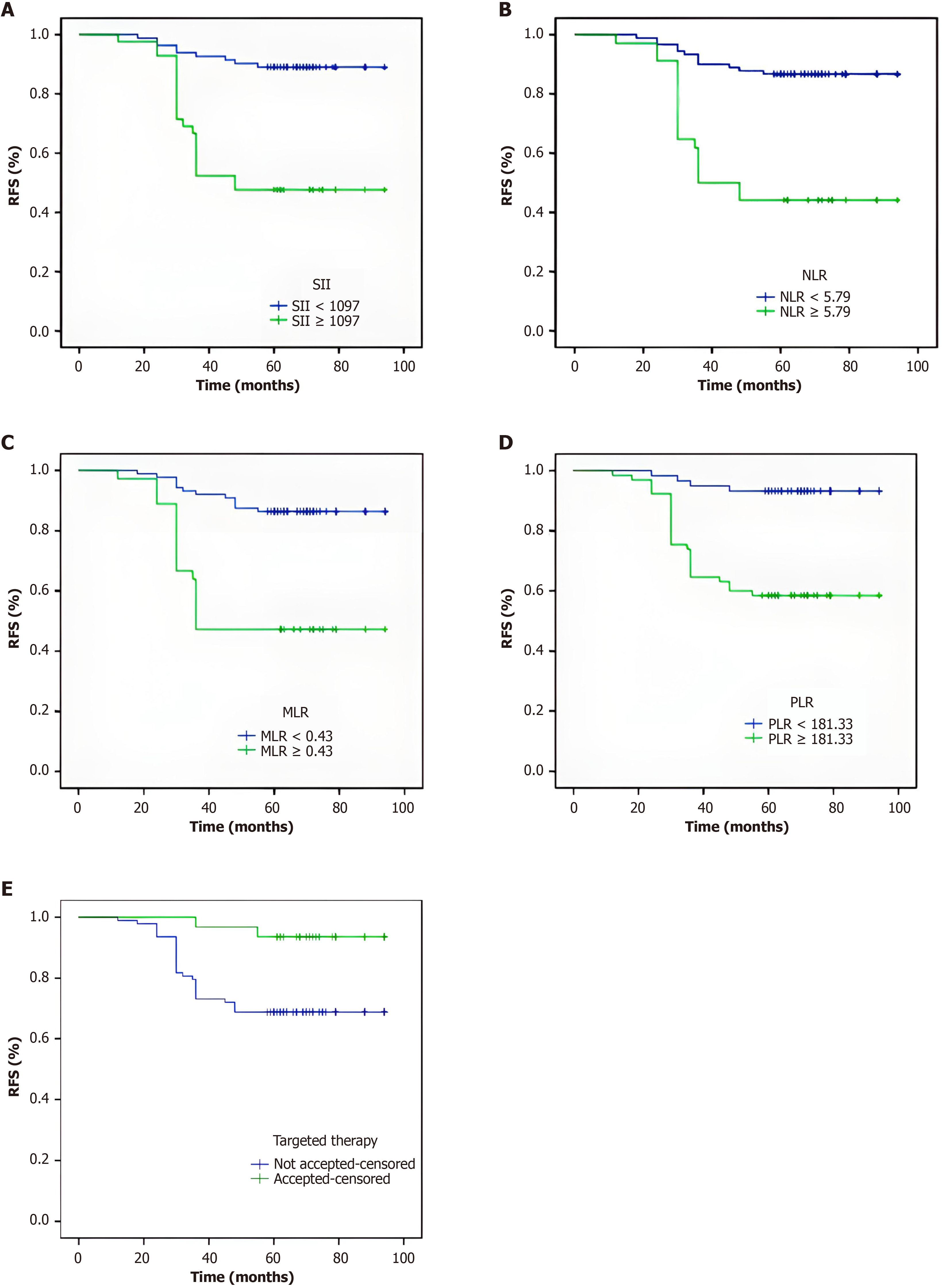
| Targeted therapy | 0.007 | 7.398 | |||
| Yes | 31 | 74.667 | (68.756-80.577) | ||
| No | 93 | 90.871 | (86.591-95.151) | ||
| Tumor size, cm | 0.604 | 0.269 | |||
| <5 | 52 | 80.308 | (73.454-87.162) | ||
| ≥ 5 | 72 | 77.569 | (71.125- 84.014) | ||
| SII | < 0.001 | 26.946 | |||
| <1079 | 84 | 87.463 | (83.346-91.580) | ||
| ≥ 1079 | 42 | 61.643 | (52.176-71.110) | ||
| NLR | < 0.001 | 26.845 | |||
| <5.79 | 90 | 86.067 | (81.817-90.316) | ||
| ≥ 5.79 | 34 | 59.265 | (48.708-69.821) | ||
| MLR | < 0.001 | 24.242 | |||
| <0.430 | 88 | 86.227 | (82.053-90.402) | ||
| ≥ 0.430 | 36 | 60.361 | (49.872, 70.851) | ||
| PLR | |||||
| < 181.33 | 59 | 90 | (86.172-93.828) | < 0.001 | 19.871 |
| ≥ 181.33 | 65 | 68.477 | (60.985-75.969) |
| Variable | RFS | |
| P-value | HR (95%CI) | |
| PLR | 0.008 | 4.556 (1.496-13.878) |
| MLR | 0.000 | 4.063 (1.855-8.901) |
| Targeted therapy | 0.004 | 0.119 (0.028-0.505) |
As shown in Table 1, we considered gender, age, BMI, tumor site, tumor size, and treatment strategy as important clinicopathological features and analyzed their correlation with SII, NLR, MLR, and PLR. Preoperative MLR and PLR were significantly correlated with tumor size (P = 0.016 and P = 0.002), while the other parameters did not show statistical significance with SII, NLR, MLR, and PLR (Table 3 and Table 4).
To explore whether SII, NLR, MLR and PLR affect the prognosis of GIST, we used Kaplan-Meier methodology to assess the 5-year RFS of the 124 patients. In the univariate regression analysis, the RFS values of patients in the SII high-level group (P < 0.001), NLR high-level group (P < 0.001), MLR high-level group (P < 0.001) and PLR high-level group (P < 0.001) were shorter than those in the corresponding low-level groups, and the patients who underwent postoperative targeted therapy had a lower RFS compared with those who did not receive it. Log-rank tests indicated that the difference was statistically significant (Table 5). Then, we plotted the RFS survival curves for MLR, NLR, PLR, SII and targeted therapy (Figure 2).
Prognostic SII, NLR, MLR, PLR and targeted therapy factors were included in the multivariate regression analysis. The results showed that PLR (P = 0.008), MLR (P < 0.001) and postoperative targeted therapy (P = 0.004) were independent risk factors for prognosis (Table 6).
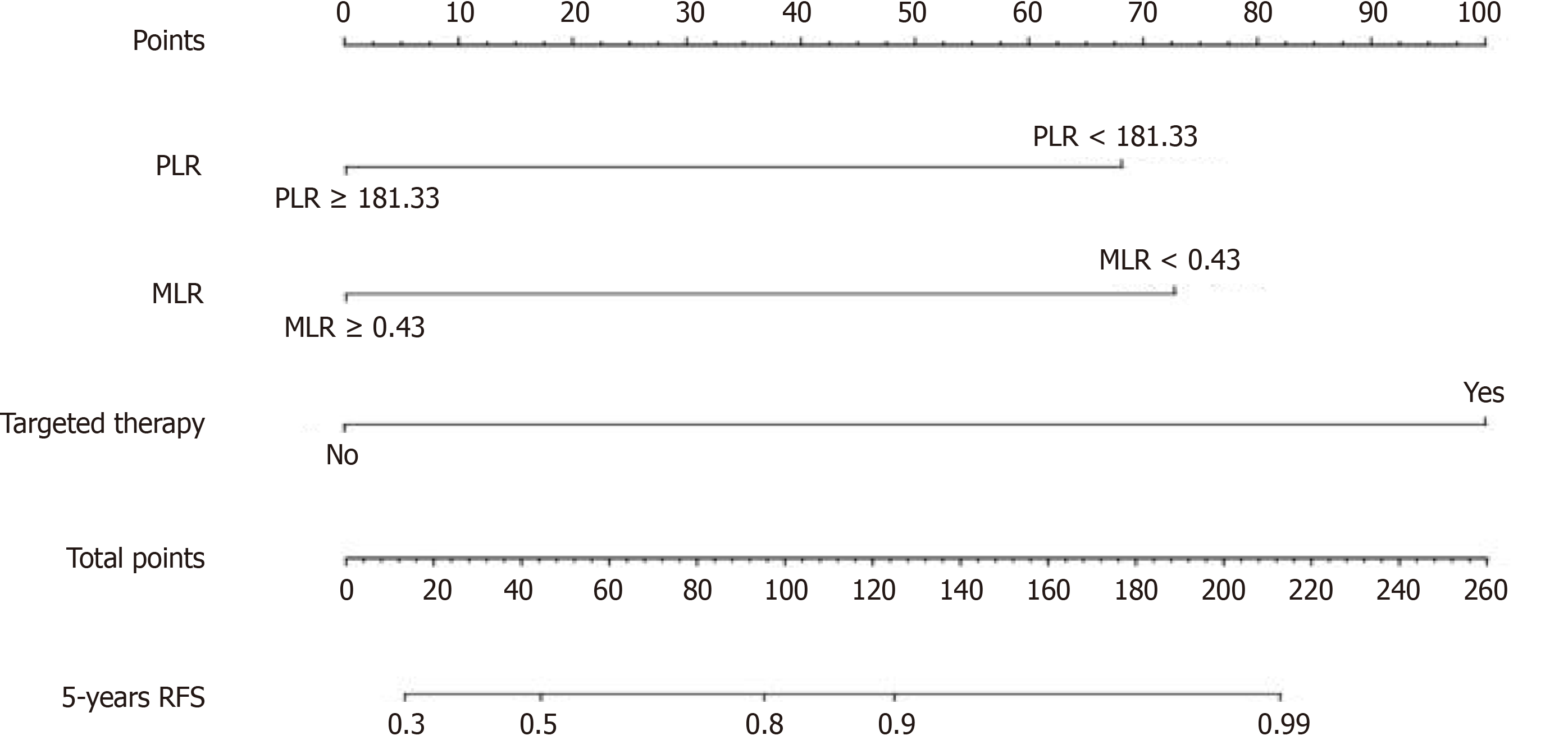
The nomogram used to predict the 5-year RFS eventually integrated the following variables based on the results of the multivariate analysis: PLR, MLR, and postoperative targeted therapy (Figure 3).
The primary strength of our study lies in the integration of inflammatory markers such as MLR and PLR as independent predictors of RFS in patients with GIST. By analyzing both clinical and inflammatory parameters, we were able to construct a nomogram that incorporates these markers alongside targeted therapy, providing a practical tool for predicting postoperative recurrence and guiding personalized treatment strategies. This study's novelty and clinical relevance are reinforced by its focus on the prognostic utility of preoperative inflammatory indicators in GIST patients.
Inflammation is recognized for its role in elevating the risk of tumor development, instigating genetic mutations, and serving as a pivotal mechanism in tumor progression and metastasis[12]. Within the tumor microenvironment, the rapid proliferation of tumor cells induces hypoxia, necrosis, or local tissue damage, triggering a nonspecific inflammatory response that manifests as systemic inflammation throughout the body[13]. Prolonged chronic inflammatory responses play a crucial role in tumor occurrence and development, significantly influencing treatment outcomes. Inflammatory cells secrete a cascade of cytokines and chemokines, fostering angiogenesis and inducing immunosuppression in tumor cells, thereby facilitating invasion and metastasis[9]. Consequently, accumulating research suggests that inflammatory parameters can serve as robust indicators of cancer prognosis.
This study encompassed four inflammatory biomarkers derived from neutrophils, platelets, monocytes, and lymphocytes: SII, NLR, MLR and PLR. Widely regarded as the seventh predictor, these inflammatory indicators have been progressively explored for their potential value and application scenarios through extensive studies. In a large prospective cohort study involving 440000 participants[14], positive associations were observed between SII, NLR, PLR, and the risk of seven out of 17 types of cancer. Moreover, the literature supports the association of inflammatory markers with the prognosis of various tumors, including distal cholangiocarcinoma[15], pancreatic cancer[16], gastric cancer[17], and breast cancer[18]. In our study, inflammatory indicators yielded similar conclusions regarding the prediction of RFS in GIST patients, specifically identifying PLR, MLR, and postoperative targeted therapy as independent influencing factors.
The study revealed a substantial correlation between PLR and RFS, a finding consistent with previous research. Goh et al[19] and Stotz et al[20] independently established high preoperative PLR as a risk factor for GIST recurrence. This correlation is attributed to the tumor microenvironment's secretion of inflammatory factors, including interleukin IL-6 and tumor necrosis factor-α, stimulating megakaryocyte proliferation, platelet production, and increased platelet cir
Cananzi et al[21] confirmed the prognostic value of MLR in GIST as an independent risk factor for GIST recurrence, and our study reached the same conclusion. Feng et al[22] included 274 patients with surgically treated GIST. Univariate analysis revealed that MLR was associated with RFS, but tumor size was the only prognostic factor in the multivariate analysis. In our study, MLR and PLR were found to only be associated with tumor size at the time of surgery, which may indicate that MLR and PLR are independent risk factors for recurrence in GIST patients. Further research is essential to elucidate the predictive value of MLR.
Our study revealed that targeted therapy is also an independent risk factor for postoperative recurrence in GIST patients. Some scholars[23] have suggested that regular follow-up and the utilization of targeted drugs may transform GIST treatment into a chronic disease management approach. The patients included in this study underwent targeted therapy based on tumor size and location, which could impact patients' RFS. Therefore, the study did not conclude that tumor size was an independent risk factor for postoperative recurrence survival in patients. Of course, the risk of GIST recurrence in patients who have already received postoperative adjuvant targeted therapy should not be overlooked. Thus, for high-risk patients, inflammatory markers such as MLR and PLR are not only prognostic factors but can also guide the adjustment of the duration of targeted therapy and the frequency of follow-up.
This study revealed that NLR and SII were not independent risk factors for RFS in GIST patients. Rutkowski et al[24] found that patients with NLR values below 2.7 had relatively long OS and RFS. Our study yielded the same conclusion as previous studies. The RFS in the NLR < 5.79 group was longer, but NLR was not an independent risk factor for GIST. This may be related to the slower chronic inflammatory response of neutrophil granulocytes to cancer compared with platelets. Similarly, SII is not an independent risk factor for GIST. It is possible that the regular postoperative targeted therapy of some patients included in the study has an impact on postoperative RFS of GIST patients. Nevertheless, patients receiving targeted therapy were included in this study. First, this was a retrospective study, and it is possible that drug factors may have affected RFS after surgery. In addition, no studies have shown that the predictive value of preoperative inflammatory indicators such as SII and MLR can be significantly affected by targeted drugs in tumor patients, and large-scale prospective studies can be conducted in the future to yield better data.
A recent study compared the nomogram with the traditional staging system to evaluate the prognosis of GISTs and revealed that the nomogram, with full inclusion of prognostic factors, had better accuracy and discriminability[25]. Nomograms, using two or more known variables to assess clinical events, have been widely utilized for predicting prognosis in various cancers[26]. This study pioneers the construction of a nomogram based on inflammatory indicators for assessing the recurrence risk prognosis in GIST patients. It demonstrated the nomogram's utility in predicting GIST patient recurrence risk, emphasizing the ongoing sensitivity of inflammatory markers in patients receiving postoperative targeted therapy.
As with every study, our work had some limitations. First, in our investigation of the nomogram, we only performed internal validation and lacked external or multicenter data verification. Second, due to the intervention of targeted drugs after surgery, the prognostic data may not fully reflect the operative situation. Finally, the number of GIST patients in our study was relatively small. Conducting a prospective cohort study with a larger sample size in the future is necessary to provide more reliable research evidence for GIST patients.
The preoperative MLR and PLR, which are independent risk factors for GIST recurrence and can be used as important predictors of GIST recurrence, were correlated with RFS. Nomograms based on the PLR, MLR and targeted therapy can be used for clinical treatment.
The authors thank all the patients who participated in the study at Affiliated Hospital of Southwest Medical University. All their help is truly appreciated.
| 1. | Patel N, Benipal B. Incidence of Gastrointestinal Stromal Tumors in the United States from 2001-2015: A United States Cancer Statistics Analysis of 50 States. Cureus. 2019;11:e4120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 933] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 3. | Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 4. | Gatto L, Nannini M, Saponara M, Di Scioscio V, Beltramo G, Frezza GP, Ercolani G, Pinna AD, Astolfi A, Urbini M, Brandi G, Biasco G, Pantaleo MA. Radiotherapy in the management of gist: state of the art and new potential scenarios. Clin Sarcoma Res. 2017;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8407] [Article Influence: 525.4] [Reference Citation Analysis (7)] |
| 6. | Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1457] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 7. | Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446-3458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1085] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 8. | Shi H, Jiang Y, Cao H, Zhu H, Chen B, Ji W. Nomogram Based on Systemic Immune-Inflammation Index to Predict Overall Survival in Gastric Cancer Patients. Dis Markers. 2018;2018:1787424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Yang J, Bao Y, Chen W, Duan Y, Sun D. Nomogram Based on Systemic Immune Inflammation Index and Prognostic Nutrition Index Predicts Recurrence of Hepatocellular Carcinoma After Surgery. Front Oncol. 2020;10:551668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Jomrich G, Gruber ES, Winkler D, Hollenstein M, Gnant M, Sahora K, Schindl M. Systemic Immune-Inflammation Index (SII) Predicts Poor Survival in Pancreatic Cancer Patients Undergoing Resection. J Gastrointest Surg. 2020;24:610-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 11. | Feng JF, Chen S, Yang X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine (Baltimore). 2017;96:e5886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Ostan R, Lanzarini C, Pini E, Scurti M, Vianello D, Bertarelli C, Fabbri C, Izzi M, Palmas G, Biondi F, Martucci M, Bellavista E, Salvioli S, Capri M, Franceschi C, Santoro A. Inflammaging and cancer: a challenge for the Mediterranean diet. Nutrients. 2015;7:2589-2621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 13. | Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1713] [Article Influence: 155.7] [Reference Citation Analysis (0)] |
| 14. | Nøst TH, Alcala K, Urbarova I, Byrne KS, Guida F, Sandanger TM, Johansson M. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36:841-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 379] [Article Influence: 75.8] [Reference Citation Analysis (1)] |
| 15. | Terasaki F, Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Ohgi K, Uesaka K. Systemic immune-inflammation index as a prognostic marker for distal cholangiocarcinoma. Surg Today. 2021;51:1602-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Shui Y, Li M, Su J, Chen M, Gu X, Guo W. Prognostic and clinicopathological significance of systemic immune-inflammation index in pancreatic cancer: a meta-analysis of 2,365 patients. Aging (Albany NY). 2021;13:20585-20597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Qiu Y, Zhang Z, Chen Y. Prognostic Value of Pretreatment Systemic Immune-Inflammation Index in Gastric Cancer: A Meta-Analysis. Front Oncol. 2021;11:537140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Li W, Ma G, Deng Y, Chen W, Liu Z, Chen F, Wu Q. Systemic Immune-Inflammation Index Is a Prognostic Factor for Breast Cancer Patients After Curative Resection. Front Oncol. 2021;11:570208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Goh BK, Chok AY, Allen JC Jr, Quek R, Teo MC, Chow PK, Chung AY, Ong HS, Wong WK. Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery. 2016;159:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Stotz M, Liegl-Atzwanger B, Posch F, Mrsic E, Thalhammer M, Stojakovic T, Bezan A, Pichler M, Gerger A, Szkandera J. Blood-Based Biomarkers Are Associated with Disease Recurrence and Survival in Gastrointestinal Stroma Tumor Patients after Surgical Resection. PLoS One. 2016;11:e0159448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Cananzi FCM, Minerva EM, Samà L, Ruspi L, Sicoli F, Conti L, Fumagalli Romario U, Quagliuolo VL. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J Surg Oncol. 2019;119:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Feng F, Tian Y, Liu S, Zheng G, Liu Z, Xu G, Guo M, Lian X, Fan D, Zhang H. Combination of PLR, MLR, MWR, and Tumor Size Could Significantly Increase the Prognostic Value for Gastrointestinal Stromal Tumors. Medicine (Baltimore). 2016;95:e3248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Stamatakos M, Douzinas E, Stefanaki C, Safioleas P, Polyzou E, Levidou G, Safioleas M. Gastrointestinal stromal tumor. World J Surg Oncol. 2009;7:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 24. | Rutkowski P, Teterycz P, Klimczak A, Bylina E, Szamotulska K, Lugowska I. Blood neutrophil-to-lymphocyte ratio is associated with prognosis in advanced gastrointestinal stromal tumors treated with imatinib. Tumori. 2018;104:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Zhang W, Wang R, Ma W, Wu Y, Maskey N, Guo Y, Liu J, Mao S, Zhang J, Yao X, Liu Y. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Transl Med. 2019;7:431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2573] [Article Influence: 233.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/