Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.100094
Revised: October 23, 2024
Accepted: November 5, 2024
Published online: February 15, 2025
Processing time: 164 Days and 6.1 Hours
The nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3) inflammasome is a critical modulator in inflammatory disease. Activation and mutation of NLRP3 can cause severe inflammation in diseases such as chronic infantile neurologic cutaneous and articular syndrome, Muckle-Wells syndrome, and familial cold autoinflammatory syndrome 1. To date, a great effort has been made to decode the underlying mechanisms of NLRP3 activation. The priming and activation of NLRP3 drive the maturation and release of active interleukin (IL)-18 and IL-1β to cause inflammation and pyroptosis, which can significantly trigger many diseases including inflammatory diseases, immune disorders, metabolic diseases, and neurodegenerative diseases. The investigation of NLRP3 as a therapeutic target for disease treatment is a hot topic in both preclinical studies and clinical trials. Developing potent NLRP3 inhibitors and downstream IL-1 inhibitors attracts wide-spectrum attention in both research and pharmaceutical fields. In this minireview, we first updated the molecular me
Core Tip: The nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3) inflammasome plays a pivotal role in many diseases such as inflammatory diseases, metabolic disorders, and neurodegenerative diseases. The investigation of NLRP3 as a therapeutic target is involved in many preclinical studies and clinical trials. Among these studies, NLRP3 inhibitors and downstream interleukin-1 inhibitors attract wide-spectrum attention. In addition, NLRP3 activation also impacts cancer development and immunotherapy, serving as a potential therapeutic target for cancer treatment.
- Citation: Zhang CY, Liu S, Sui YX, Yang M. Nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 inflammasome: From action mechanism to therapeutic target in clinical trials. World J Gastrointest Oncol 2025; 17(2): 100094
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/100094.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.100094
Nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3 (NLRP3) is a protein encoded by the gene Nlrp3, which is mainly expressed in macrophages, monocytes, T cells, myeloid dendritic cells, basophils, Langerhans cells, Hoffbauer cells, and Kupffer cells[1,2]. NLRP3 serves as an intracellular sensor and a key mediator of inflammasome activation, which can cause cell inflammation and pyroptosis[3]. NLRP3 also serves as a regulator for T helper 2 cell differentiation, and its deficiency in T helper 2 cells can promote the pathogenesis of asthma and melanoma tumor proliferation[4].
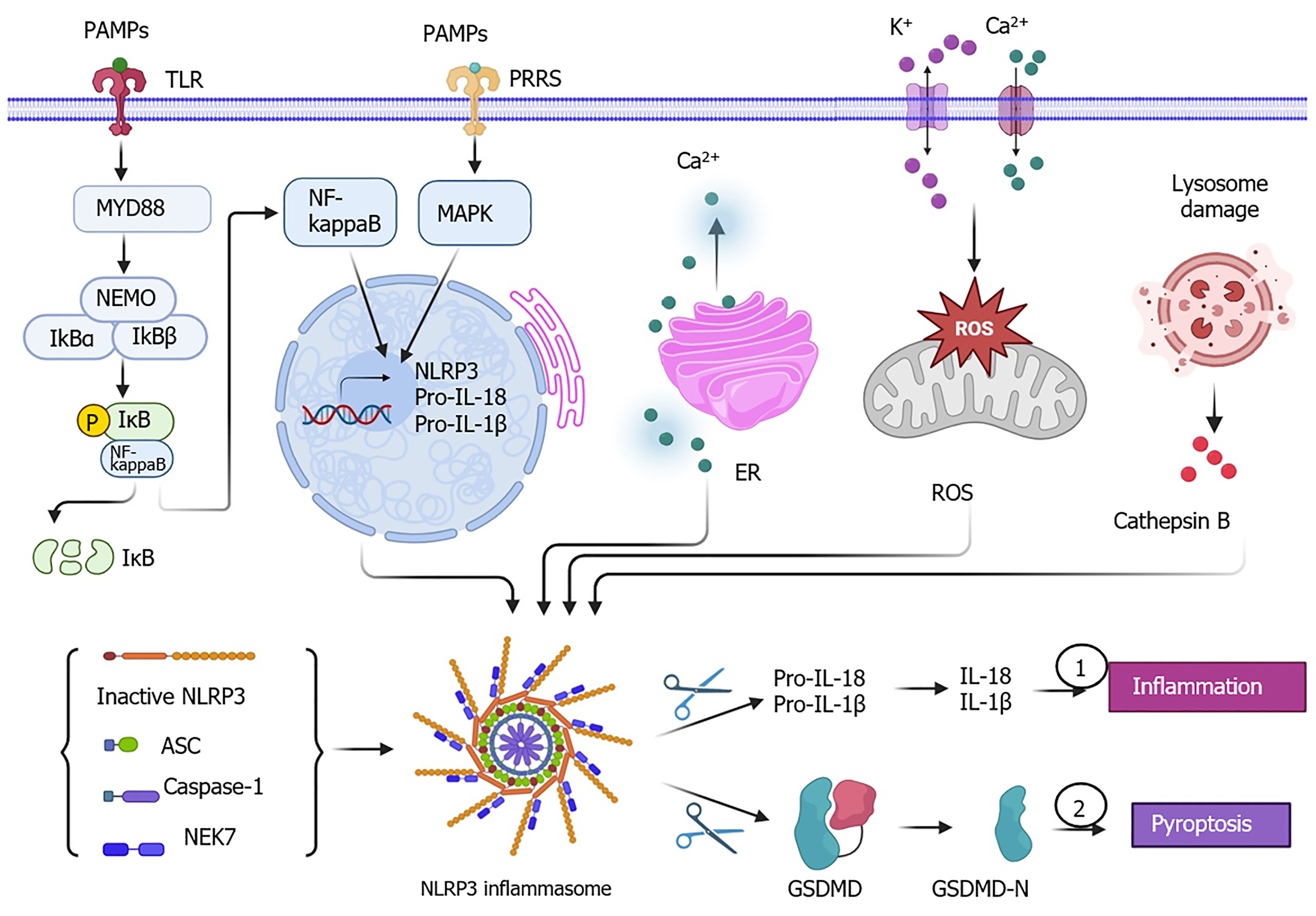
The triggering stimuli of NLRP3 activation include pathogen-associated molecular patterns (PAMPs), such as molecules associated with viral or bacterial infection, and damage-associated molecular patterns (DAMPs), such as molecules associated with cell death and damage[1]. Upon exposure to the stimuli, NLRP3 together with caspase-1 and adaptor apoptosis-associated speck-like protein containing a caspase recruitment domain form a complex named the NLRP3 inflammasome, followed by the activation process that is mediated by never in mitosis A-related kinase 7 (NEK7) to trigger the cleavage of pro-interleukin (IL)-18 and pro-IL-1β, resulting in the secretion of active mature IL-1β and IL-18 to induce inflammation. Simultaneously, gasdermin D (GSDMD) also goes through the cleavage process by the NLRP3 inflammasome via disconnecting the C-terminal to produce GSDMD-N that can cause the formation of a pore in the plasma membrane and eventually induce pyroptosis[5,6].
NLRP3 plays an essential role in health and disease. The mutation of NLRP3 can cause inflammatory diseases, such as chronic infantile neurologic cutaneous and articular syndrome, Muckle-Wells syndrome, and familial cold autoinflammatory syndrome 1[7,8]. Accumulating studies have also demonstrated that NLRP3 affects the development of many diseases, such as inflammatory bowel disease[9], metabolic disorders (obesity and diabetes)[10-12], cardiovascular diseases[13,14], and neurologic-related diseases [Alzheimer’s disease and Parkinson’s disease (PD)][15,16]. In the following sections, some examples are given to discuss the specific roles of NLRP3 in diseases.
The underlying molecular mechanism of NLRP3 activation includes two steps: priming and activation (Figure 1). First, NLRP3 serves as a sensor, which is triggered by the binding of stimuli, including PAMPs/DAMPs and pattern recognition receptors such as toll-like receptors (TLR) and IL-1R on cellular membrane surface[17]. Then, this leads to the activation of either nuclear factor kappa B (NF-κB) or mitogen-activated protein kinase signaling pathway or both to increase the expression levels of NLRP3, pro-IL-18, and pro-IL-1β and their translocation from the nucleus to the cytoplasm. In the cytoplasm, the assembling of NLRP3 complex consisting of the components of inactive NLRP3, adaptor apoptosis-associated speck-like protein containing a caspase recruitment domain, and caspase-1 is initiated[18,19]. The activation of NLRP3 signaling is initiated by NEK7 and a variety of activators, such as PAMPs, DAMPs, endoplasmic reticulum stress-induced release of Ca2+, mitochondrial reactive oxygen species (ROS)-related K+ efflux and Ca2+ signaling, and cathepsin B released by damaged lysosomes[20-22]. The catalytic domain of NEK7 can bind with NLRP3 and disrupt the NLRP3 oligomers, which results in the formation of the NEK7/NLRP3 complex. The NEK7/NLRP3 complex undergoes the process of NLRP3 inflammasome assembly and pro-caspase-1 activation leading to the release of mature caspase-1. Caspase-1 (also known as IL-1β-converting enzyme) is a protease that can cleave inactive pro-IL-18 and pro-IL-1β into their active forms IL-18 and IL-1β. Upon activation, the NLRP3 inflammasome contributes to inflammation that is mediated by the release of mature IL-18 and IL-1β as well as cell pyroptosis due to a pore formation in the cellular plasma membrane caused by GSDMD-N[23,24].
A recently published study revealed that the NLRP3 variant “R779C” plays a critical role in the early onset of inflammatory bowel disease, which is tested in a dextran sulfate sodium-induced acute colitis model and biopsies derived from human patients with the R779C variant[25]. The R779C variant has been shown in patients with gastrointestinal symptoms, accompanying significantly enhanced levels of IL-1β and IL-18 production and pyroptosis. NLPR3 ubiquitination plays a key role in this process. The R779C variant not only binds with BRCA1/BRCA2-containing complex subunit 3 with a high binding affinity but gains the function of binding with Josephin Domain Containing 2, which is not shown in the non-variant wildtype. Both BRCA1/BRCA2-containing complex subunit 3 and Josephin Domain Containing can promote the ubiquitination of NLRP3 to promote its activation. This finding was further verified using a colitis model, which demonstrated that the R779C variant increased the susceptibility and severity of intestinal inflammation[25].
Another recent study demonstrated that the NLRP3 inflammasome has an important role in neuronal disease. PD is a neurodegenerative disease characterized by the aggregation of misfolded α-synuclein followed by the death of dopamine neurons. The accumulation of α-synuclein can mediate NLRP3 activation by regulating the ROS signaling pathway and cathepsin B production. The amplified assembling and activation of the NLRP3 inflammasome in cells can aggravate the death of dopamine neurons. Inhibition of NLRP3 assembling and activation can prevent parkin-associated neurodegeneration of dopamine neurons in familial and sporadic PD models[26].
Inflammatory liver disease, such as metabolic dysfunction-associated steatotic liver disease (MASLD), is often accompanied by chronic liver inflammation[27]. A recent study found that the antioxidant agent lycopene had a preventative function against MASLD via the inhibition of the hepatic NF-κB/NLRP3 signaling pathway in a high-fat and high-fructose diet-induced mouse model[28]. This study also demonstrated that dietary intervention-mediated suppression of liver inflammation is associated with the alteration of gut microbiota[28], indicating the association between the gut microbiota and NLRP3 inflammasome activation[29].
Autoinflammatory disease cryopyrin-associated periodic syndrome (CAPS) is caused by the mutation of NLRP3 and subsequent production of IL-1β. The gain-of-function CAPS-associated NLRP3 mutant, NLRP3 p.D303N variant, can continually activate the NLRP3 inflammasome. The NLRP3 p.D303N variant also has the ability of continuous expression in a mouse model of CAPS without any triggers. Triggers such as PAMPs and DAMPS initiate the activation of NF-κB to increase IL-1β production in macrophages expressing NLRP3 p.D303N. The upregulated NF-κB signaling pathway further promotes the activation of the NLRP3 inflammasome[30]. Overall, the NLRP3 inflammasome plays a pivotal role in inflammatory diseases, autoimmune diseases, and neuronal diseases.
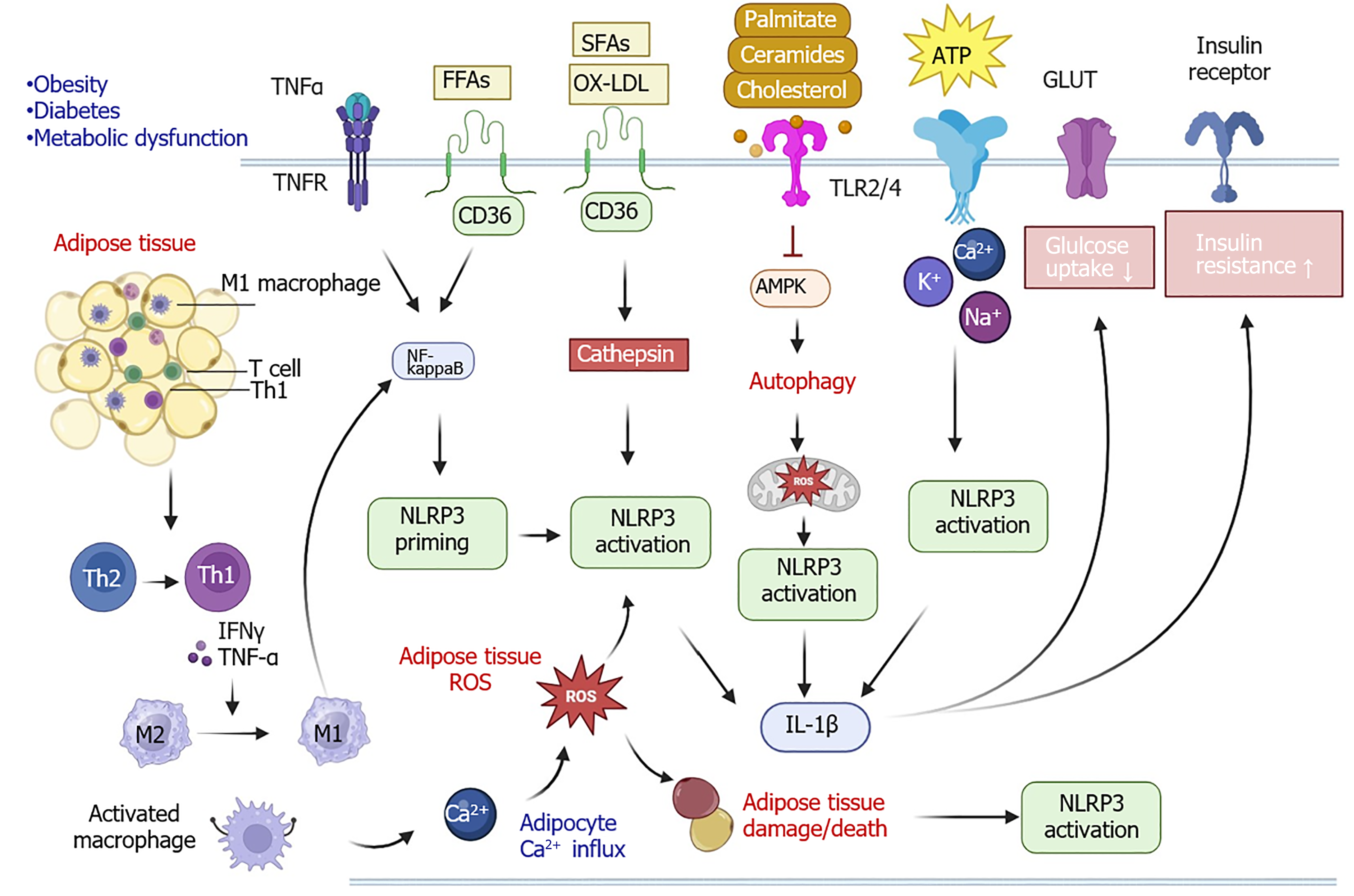
NLRP3 plays a substantial role in elevating insulin resistance by impacting the phosphoinositide 3-kinase/protein kinase B signaling pathway, which leads to a decreased level of glucose uptake[31]. Adipose tissue inflammation and immune cell infiltration are highly accompanied by the activation of the NLRP3 inflammasome in metabolic diseases such as diabetes and obesity (Figure 2). One study revealed that the expression level of NLRP3 was increased in visceral adipose tissues in comparison with subcutaneous tissues in obese individuals. The elevated level of tumor necrosis factor-α and the excessive free fatty acids in the adipose tissue of individuals with obesity and type 2 diabetes mellitus (T2DM) can trigger NF-κB-associated NLRP3 priming[32,33].
Oxidized low-density lipoproteins and short-chain fatty acids can also trigger NLRP3 activation through mitochondria and endoplasmic reticulum stress as well as lysosomes[34]. Similarly, the lipids palmitate, ceramide, and cholesterol can bind with TLR2/4 to suppress the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway, leading to autophagy and mitochondrial ROS stress, NLRP3 inflammasome activation, IL-1 production, and eventually increasing insulin resistance and decreasing glucose uptake[35,36]. Furthermore, adipocyte death, infiltration of M1 macrophages, and excessive secretion of inflammatory cytokines and adenosine triphosphates during adipose tissue damage are the major triggers of NLRP3 priming and activation[37,38].
Inflammation accelerates immune cell infiltration in adipose tissues including T cells, which can secrete interferon gamma to trigger the initiation of NF-κB signaling and NLRP3 priming. Simultaneously, T cells contribute to the activation of macrophages, regulating calcium influx. Calcium influx and ROS production can further promote the production of cytokines, chemokines, and adipokines to recruit more macrophages and induce their activation. Moreover, interferon gamma and tumor necrosis factor-α secreted from T helper 1 cells can promote macrophage differentiation from the M2 phenotype to the M1 phenotype. These activated and infiltrated M1 macrophages in adipose tissues increase insulin resistance.
Additionally, mitochondrial dysfunction contributes to the activation of the NLRP3 inflammasome[39-42]. A study using a high-fat diet mouse model demonstrated that inhibition of NLRP3 activation reduced hepatic steatosis in MASLD via the AMPK-autophagy signaling pathway[43]. Inhibiting NLRP3 inflammasome activation showed a protective effect against dietary-induced obesity, which suggests NLRP3 plays a significant role in obesity development[44]. In patients with T2DM, an upregulation of IL-18, IL-1β, and caspase-1 expression is detected, and the anti-diabetic drug metformin can impair the activation of the NLRP3 inflammasome, resulting in a decrease of mature IL-1β level via AMPK-mediated signaling pathway[45]. Therefore, NLRP3 contributes to metabolic diseases such as diabetes and obesity.
Given the important roles of NLRP3 in inflammation, pyroptosis, and immune regulation, it is recognized as one of the attractive hallmarks in cancer development and immunotherapy[46,47]. Studies have demonstrated the association between NLRP3 activation and activation with cancer progression. For instance, it has been revealed that NLRP3 is overexpressed in colorectal cancer and plays a key role in promoting tumor proliferation and migration by modulating the epithelial-mesenchymal transition process[48]. A recently published study conducted by a team using traditional medicine Yiqi Jiedu Huayu decoction to treat chronic atrophic gastritis showed that Yiqi Jiedu Huayu decoction can suppress the precancerous lesion by inhibiting NLRP3 inflammasome-mediated cell pyroptosis via suppressing TLR 4/NF-κB and IL-6/signal transducer and activator of transcription 3 signaling pathways[49]. This finding suggests the potential role of NLRP3 inflammasome in chronic atrophic gastritis and potential gastric cancer[49]. Another recent publication also demonstrated that NLRP3 inflammasome inactivation contributes to the inhibition of tumor motility, angiogenesis, and growth in hepatocellular carcinoma[50].
Emerging studies report that the upregulation of NLRP3 may neutralize or dampen the anti-tumor effect. For example, an in vivo study using a liver cancer model revealed that upregulation of NLRP3 reversed the anti-tumor effect of eriodictyol treatment[50]. Based on an analysis conducted in Pan-Cancer, the results showed that there was a significant association between NLRP3 and immune evasion via regulating the expression of programmed death-ligand 1 and lymphocyte activating 3 immune checkpoints[51]. However, in vitro and in vivo studies are required for further evaluation.
A study indicated that there was a synergistic effect of NLRP3 inhibitor together with anti-programmed cell death protein 1 (anti-PD-1) therapy compared to the anti-PD-1 monotherapy in melanoma treatment[52]. This study also revealed that NLRP3 can increase tumor infiltration of myeloid-derived suppressor cells that enable tumor cells to escape from the activation of CD8+ T cells and natural killer cells. Inhibition of NLRP3 expression can reduce the myeloid-derived suppressor cell infiltration to improve the efficacy of immunotherapy[52]. Another study used the NLRP3 inhibitor “MCC950” and Nlrp3-deficient mice to investigate the role of NLRP3 in modulating the immunotherapeutic efficacy of anti-PD-1 and anti-cytotoxic T lymphocyte-associated protein-4 in melanoma and lung cancer. The results demonstrated that the efficacy of anti-PD-1 and anti-cytotoxic T lymphocyte-associated protein-4 immunotherapies increased in NLRP3-deficient mice compared with wild-type mice[53]. Thus, targeting NLRP3 treatment holds great promise in improving cancer therapy.
NLRP3 plays a critical role in crosslinking between metabolic diseases and the risk of cancer development and progression as well as cancer immunotherapeutic efficacy[35,54]. For example, the metabolic abnormalities induced by chronic inflammation and tissue injury impact mitochondrial functions, resulting in ROS stress, mitochondrial functional disruption, and epigenetic instability, which are important factors in driving cancer development and progression[55-57]. On a molecular level, there are numerous crosslinks between metabolic disorders and cancer progression, including alteration of molecular signaling pathways[39]. For instance, the phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway[58,59], mitogen-activated protein kinase signaling pathway[60], and AMPK signaling pathway[61] are important molecular pathways that are shared by metabolic diseases and cancers. Metabolic disease and cancer reciprocally influence each other through these molecular pathways.
The exploration of potent NLRP3 inhibitors and IL-1 inhibitors attracts wide attention in both research and pharmaceutical fields[62,63]. Currently, various NLRP3 inhibitors are investigated, mainly focusing on the anti-inflammatory signaling pathways (Table 1). The Food and Drug Administration (FDA)-approved drugs for treating Nlrp3 mutation-associated diseases include rilonacept (IL-1 antagonist), canakinumab (IL-1β blocker), and anakinra (IL-1 receptor antagonist), which block inflammation. Rilonacept is an FDA-approved drug for the treatment of familial cold auto-inflammatory syndrome (for example, CAPS) disease. Rilonacept inhibits the IL-1 signaling pathway, as it has a high binding affinity with both IL-1α and IL-1β[64]. Similarly, canakinumab (ACZ885) is a monoclonal antibody that can inhibit IL-1β, which has been approved by the FDA for the treatment of CAPS such as Muckle-Wells syndrome, one type of disease commonly associated with NLRP3 gene mutation[65]. In 2001, anakinra was first approved for the treatment of rheumatoid arthritis. It was then approved for the treatment of neonatal-onset multisystem inflammatory disease (also known as chronic infantile neurologic cutaneous and articular syndrome) in 2013, and it was also approved for the treatment of deficiency of IL-1 receptor antagonist disease in 2020[65,66].
| NCT number | Phases | Conditions | Interventions (drug) | Mechanism of action | Study status |
| NCT01045772 | 2 | MWS, Schnitzler syndrome | Rilonacept | IL-1 antagonist | Completed |
| NCT00288704 | 3 | FCAS, familial cold urticaria, MWS, genetic diseases, inborn | Rilonacept 160 mg | IL-1 antagonist | Completed |
| NCT00094900 | 2 | Inflammation, familial mediterranean fever, adult-onset still’s disease | Rilonacept | IL-1 antagonist | Completed |
| NCT01276522 | 2 | Schnitzler syndrome | Canakinumab (ACZ885) | IL-1β blocker | Completed |
| NCT04510493 | 3 | Coronavirus infection, type 2 diabetes mellitus | Canakinumab (ACZ885) | IL-1β blocker | Completed |
| NCT00991146 | 3 | Cryopyrin-associated periodic syndromes, FCAS, MWS, neonatal onset multisystem inflammatory disease | Canakinumab (ACZ885) | IL-1β blocker | Completed |
| NCT00685373 | 3 | Cryopyrin-associated periodic syndromes, FCAS, MWS, neonatal onset multisystem inflammatory disease | Canakinumab (ACZ885) | IL-1β blocker | Completed |
| NCT01105507 | 3 | Cryopyrin associated periodic syndrome | Canakinumab (ACZ885) | IL-1β blocker | Completed |
| NCT01302860 | 3 | Cryopyrin-associated periodic syndromes, FCAS, MWS, neonatal onset multisystem inflammatory disease | Canakinumab (ACZ885) | IL-1β blocker | Completed |
| NCT01576367 | 3 | Cryopyrin-associated periodic syndromes, FCAS, MWS, neonatal onset multisystem inflammatory disease | Canakinumab (ACZ885) | IL-1β blocker | Completed |
| NCT00465985 | 3 | MWS | Canakinumab (ACZ885) | IL-1β blocker | Completed |
| NCT00214851 | 1 | Familial cold urticaria | Kineret (anakinra) | IL-1Ra | Completed |
| NCT05880355 | 1 | Myocardial infarction | Dapansutrile | NLRP3 inflammasome inhibition | Not recruiting |
| NCT06047262 | 2 | Type 2 diabetes mellitus | Dapansutrile | NLRP3 inflammasome inhibition | Recruiting |
| NCT05658575 | 2 and 3 | Acute gout flare, gout attack, gout flare, gouty arthritis, gout, arthritis, joint pain | Dapansutrile | NLRP3 inflammasome inhibition | Recruiting |
| NCT06212271 | 1 | Peripheral artery disease | Colchicine | Inhibit multiple proinflammatory mechanisms | Not recruiting |
| NCT04322565 | 2 | Coronavirus infections, pneumonia, viral | Colchicine | Inhibit multiple proinflammatory mechanisms | Completed |
| NCT05734612 | 3 | Reperfusion injury, myocardial | Colchicine | Inhibit multiple proinflammatory mechanisms | Enrolling-by-invitation |
| NCT04867226 | 2 | Coronavirus infection | Colchicine 0.5 mg | Inhibit multiple proinflammatory mechanisms | Completed |
| NCT06426537 | 1 | ST-elevation myocardial infarction | Colchicine 0.5 mg oral tablet | Inhibit multiple proinflammatory mechanisms | Completed |
| NCT04756128 | 2 | COVID-19 | Colchicine 0.6 mg, naltrexone | Inhibit multiple proinflammatory mechanisms and opiate antagonist | Completed |
| NCT05855746 | 3 | Acute myocarditis | Colchicine pill | Inhibit multiple proinflammatory mechanisms | Not recruiting |
| NCT05130892 | 4 | NLRP3, hs-CRP, percutaneous coronary intervention | Colchicine, tranilast, oridonin | Inhibit multiple proinflammatory mechanisms | Completed |
| NCT06097663 | 2 | Coronary heart disease, clonal hematopoiesis of indeterminate potential | MAS825, FV890 | Bispecific monoclonal antibody anti-IL-1β/IL-18, and NLRP3 inhibitor | Recruiting |
| NCT05552469 | 1 | Myeloid diseases | DFV890 | NLRP3 inhibitor | Recruiting |
| NCT04868968 | 2 | FCAS | DFV890 | NLRP3 inhibitor | Completed |
| NCT04886258 | 2 | Symptomatic knee osteoarthritis | DFV890 | NLRP3 inhibitor | Recruiting |
| NCT04086602 | 1 | Healthy volunteers, cryopyrin associated periodic syndrome | IZD334 (MCC950 related agent) | NLRP3 inflammasome inhibitor | Completed |
| NCT04972188 | 1 | Healthy | ZYIL1 capsule | NLRP3 inflammasome inhibitor | Completed |
| NCT05186051 | 2 | Cryopyrin associated periodic syndrome | ZYIL1 capsule | NLRP3 inflammasome inhibitor | Completed |
| NCT05781698 | 2 | Inflammatory bowel diseases | Mesalamine, fenofibrate | Anti-inflammatory agent and lower “bad” cholesterol and fats (such as LDL, triglycerides) and raise “good” cholesterol (HDL) in the blood | Recruiting |
| NCT03468322 | 2 | Inherited epidermolysis bullosa | AC-203 | NLRP3 inflammasome inhibition | Completed |
| NCT05812781 | 2 | Cryopyrin associated periodic syndrome | VTX2735 | Peripheral NLRP3 inhibitor | Completed |
The emerging investigation in new drugs targeting NLRP3-associated signaling pathways has drawn great interest in both preclinical studies and clinical trials. For example, the NLRP3 inflammasome inhibitor, dapansutrile, is under a clinical trial investigation for the treatment of myocardial infarction, T2DM, and gout-related disease[67]. Colchicine was originally approved as an anti-inflammatory drug for gout back in 1961. Since it has the potent function of inhibiting multiple proinflammatory mechanisms, colchicine was then intensively studied in different conditions such as peripheral artery disease, acute myocarditis, coronavirus infections, pneumonia, reperfusion injury, and myocardial coronavirus infection. In 2023, it was approved by the FDA for reducing the risk of atherosclerotic cardiovascular disease in cardiovascular patients and high-risk populations[68,69].
Bispecific monoclonal antibody MAS825 is designed for anti-IL-1β and anti-IL-18 treatments, and the NLRP3 inhibitor DFV890 is under the investigation for treatment of coronary heart disease and clonal hematopoiesis of indeterminate potential[70]. The clinical trials for DFV890 are studied in myeloid diseases, familial cold autoinflammatory syndrome, and symptomatic knee osteoarthritis[71]. IZD334 (MCC950-related agent) and ZYIL1 capsule, NLRP3 inflammasome inhibitors, are under clinical trials for evaluating their safety, tolerability, pharmacokinetic, and pharmacodynamic in the treatment of CAPS[72,73].
Fenofibrate was previously approved by the FDA for treating hypertriglyceridemia, primary hypercholesterolemia, or mixed dyslipidemia. Currently, a clinical trial is investigating the drug for repurposing for patients with inflammatory bowel diseases, such as ulcerative colitis. The underlying rationale is that fenofibrate serves as an anti-inflammatory agent that can reduce the unfavored cholesterol and fats (such as low-density lipoprotein and triglycerides) and increase the level of beneficial cholesterol (high-density lipoprotein) in the blood. This modulates the mammalian target of rapamycin/NLRP3 inflammasome[74-76]. In addition, AC-203 has been examined in the treatment of inherited epidermolysis bullosa due to the function of the NLRP3 inflammasome inhibition. Peripheral NLRP3 inhibitor, VTX2735, has been evaluated for CAPS patients. In summary, targeting the NLRP3 inflammasome and downstream signaling is critical for the treatment of NLRP3-related diseases, while more preclinical studies and clinical trial investigations are needed[77].
The NLRP3 inflammasome plays a significant role in inflammatory-associated disease, metabolic disease, cancer development, and immune infiltration-associated conditions. More mechanistic evaluation is needed to accelerate the translation from preclinical studies to clinical trials. Therapeutic agents such as NLRP3 inhibitors and bispecific antibodies have potent pharmaceutical applications in the future. The efficacy of immunotherapies can be improved by enhancing the host immunity via mediation of metabolism[78]. Metabolism, such as glucose, lactate, and fatty acid metabolism, can regulate the regulatory T cell functions, which can be targeted for cancer immunotherapy[79]. Thus, the immunotherapy response can be boosted. In obesity, the lack of oxidative phosphorylation can destroy the proinflammatory tissue macrophages in adipose tissues during inflammation, which reduces the obesity-associated pathologies[80]. Through modulating the tissue macrophage metabolic pathway, obesity-related inflammation can be alleviated. Since immunity can be enhanced through modulating and improving the metabolism function, given that many factors and conditions are associated with metabolism, multidisciplinary research is favored. For example, nutrition intervention, lifestyle, and reduction in exposure to certain pathogens and xenobiotics can reduce the stimulators of inflammation and decrease the risk of metabolic-associated diseases, resulting in an enhancement in host systemic immunity.
| 1. | Chen Y, Ye X, Escames G, Lei W, Zhang X, Li M, Jing T, Yao Y, Qiu Z, Wang Z, Acuña-Castroviejo D, Yang Y. The NLRP3 inflammasome: contributions to inflammation-related diseases. Cell Mol Biol Lett. 2023;28:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 242] [Reference Citation Analysis (0)] |
| 2. | Alam O. A single-cell-type transcriptomics map of human tissues. Nat Genet. 2021;53:1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 2634] [Article Influence: 376.3] [Reference Citation Analysis (0)] |
| 4. | Bruchard M, Rebé C, Derangère V, Togbé D, Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, Chevriaux A, Limagne E, Apetoh L, Végran F, Ghiringhelli F. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 318] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 5. | Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol. 2021;18:1141-1160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 620] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 6. | He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 1023] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 7. | Aksentijevich I, Putnam CD, Remmers EF, Mueller JL, Le J, Kolodner RD, Moak Z, Chuang M, Austin F, Goldbach-Mansky R, Hoffman HM, Kastner DL. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56:1273-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Levy R, Gérard L, Kuemmerle-Deschner J, Lachmann HJ, Koné-Paut I, Cantarini L, Woo P, Naselli A, Bader-Meunier B, Insalaco A, Al-Mayouf SM, Ozen S, Hofer M, Frenkel J, Modesto C, Nikishina I, Schwarz T, Martino S, Meini A, Quartier P, Martini A, Ruperto N, Neven B, Gattorno M; for PRINTO and Eurofever. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever Registry. Ann Rheum Dis. 2015;74:2043-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Ruiz PA, Morón B, Becker HM, Lang S, Atrott K, Spalinger MR, Scharl M, Wojtal KA, Fischbeck-Terhalle A, Frey-Wagner I, Hausmann M, Kraemer T, Rogler G. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut. 2017;66:1216-1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 10. | Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2148] [Cited by in RCA: 2123] [Article Influence: 141.5] [Reference Citation Analysis (8)] |
| 11. | Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1463] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 12. | Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nuñez G, Yodoi J, Kahn SE, Lavelle EC, O'Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1102] [Cited by in RCA: 1061] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 13. | Karasawa T, Takahashi M. Role of NLRP3 Inflammasomes in Atherosclerosis. J Atheroscler Thromb. 2017;24:443-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 14. | Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J. 2014;55:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 15. | Milner MT, Maddugoda M, Götz J, Burgener SS, Schroder K. The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer's disease. Curr Opin Immunol. 2021;68:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 16. | Amo-Aparicio J, Daly J, Højen JF, Dinarello CA. Pharmacologic inhibition of NLRP3 reduces the levels of α-synuclein and protects dopaminergic neurons in a model of Parkinson's disease. J Neuroinflammation. 2023;20:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Hamilton C, Olona A, Leishman S, MacDonald-Ramsahai K, Cockcroft S, Larrouy-Maumus G, Anand PK. NLRP3 Inflammasome Priming and Activation Are Regulated by a Phosphatidylinositol-Dependent Mechanism. Immunohorizons. 2022;6:642-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Nabil-Adam A, Ashour ML, Shreadah MA. Modulation of MAPK/NF-κB Pathway and NLRP3 Inflammasome by Secondary Metabolites from Red Algae: A Mechanistic Study. ACS Omega. 2023;8:37971-37990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Ma Q. Pharmacological Inhibition of the NLRP3 Inflammasome: Structure, Molecular Activation, and Inhibitor-NLRP3 Interaction. Pharmacol Rev. 2023;75:487-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 143] [Article Influence: 47.7] [Reference Citation Analysis (1)] |
| 20. | Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol. 2015;194:3937-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 21. | He Y, Hara H, Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem Sci. 2016;41:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1358] [Cited by in RCA: 2235] [Article Influence: 223.5] [Reference Citation Analysis (0)] |
| 22. | Chevriaux A, Pilot T, Derangère V, Simonin H, Martine P, Chalmin F, Ghiringhelli F, Rébé C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front Cell Dev Biol. 2020;8:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 23. | Fujimura K, Karasawa T, Komada T, Yamada N, Mizushina Y, Baatarjav C, Matsumura T, Otsu K, Takeda N, Mizukami H, Kario K, Takahashi M. NLRP3 inflammasome-driven IL-1β and IL-18 contribute to lipopolysaccharide-induced septic cardiomyopathy. J Mol Cell Cardiol. 2023;180:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Hu Y, Jiang Y, Li S, Ma X, Chen M, Yang R, Wen S, Moynagh PN, Wang B, Hu G, Yang S. The Gasdermin D N-terminal fragment acts as a negative feedback system to inhibit inflammasome-mediated activation of Caspase-1/11. Proc Natl Acad Sci U S A. 2022;119:e2210809119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Zhou L, Liu T, Huang B, Luo M, Chen Z, Zhao Z, Wang J, Leung D, Yang X, Chan KW, Liu Y, Xiong L, Chen P, Wang H, Ye L, Liang H, Masters SL, Lew AM, Gong S, Bai F, Yang J, Pui-Wah Lee P, Yang W, Zhang Y, Lau YL, Geng L, Zhang Y, Cui J. Excessive deubiquitination of NLRP3-R779C variant contributes to very-early-onset inflammatory bowel disease development. J Allergy Clin Immunol. 2021;147:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Panicker N, Kam TI, Wang H, Neifert S, Chou SC, Kumar M, Brahmachari S, Jhaldiyal A, Hinkle JT, Akkentli F, Mao X, Xu E, Karuppagounder SS, Hsu ET, Kang SU, Pletnikova O, Troncoso J, Dawson VL, Dawson TM. Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson's disease. Neuron. 2022;110:2422-2437.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 27. | Yang M, Liu S, Sui Y, Zhang C. Macrophage metabolism impacts metabolic dysfunction-associated steatotic liver disease and its progression. Immunometabolism. 2024;6:e00047. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | Gao X, Zhao X, Liu M, Zhao H, Sun Y. Lycopene prevents non-alcoholic fatty liver disease through regulating hepatic NF-κB/NLRP3 inflammasome pathway and intestinal microbiota in mice fed with high-fat and high-fructose diet. Front Nutr. 2023;10:1120254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 29. | Xiao L, Zheng H, Li J, Zeng M, He D, Liang J, Sun K, Luo Y, Li F, Ping B, Yuan W, Zhou H, Wang Q, Sun H. Targeting NLRP3 inflammasome modulates gut microbiota, attenuates corticospinal tract injury and ameliorates neurobehavioral deficits after intracerebral hemorrhage in mice. Biomed Pharmacother. 2022;149:112797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Molina-López C, Hurtado-Navarro L, García CJ, Angosto-Bazarra D, Vallejo F, Tapia-Abellán A, Marques-Soares JR, Vargas C, Bujan-Rivas S, Tomás-Barberán FA, Arostegui JI, Pelegrin P. Pathogenic NLRP3 mutants form constitutively active inflammasomes resulting in immune-metabolic limitation of IL-1β production. Nat Commun. 2024;15:1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 31. | Liu Z, Li J, Lin S, Wu Y, He D, Qu P. PI3K regulates the activation of NLRP3 inflammasome in atherosclerosis through part-dependent AKT signaling pathway. Exp Anim. 2021;70:488-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Rosendo-Silva D, Gomes PB, Rodrigues T, Viana S, da Costa AN, Scherer PE, Reis F, Pereira F, Seiça R, Matafome P. Clinical and molecular profiling of human visceral adipose tissue reveals impairment of vascular architecture and remodeling as an early hallmark of dysfunction. Metabolism. 2024;153:155788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 33. | Wu KK, Cheung SW, Cheng KK. NLRP3 Inflammasome Activation in Adipose Tissues and Its Implications on Metabolic Diseases. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 34. | Legrand-Poels S, Esser N, L'homme L, Scheen A, Paquot N, Piette J. Free fatty acids as modulators of the NLRP3 inflammasome in obesity/type 2 diabetes. Biochem Pharmacol. 2014;92:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 819] [Article Influence: 163.8] [Reference Citation Analysis (0)] |
| 36. | Litwiniuk A, Bik W, Kalisz M, Baranowska-Bik A. Inflammasome NLRP3 Potentially Links Obesity-Associated Low-Grade Systemic Inflammation and Insulin Resistance with Alzheimer's Disease. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Front Immunol. 2015;6:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 38. | Ahechu P, Zozaya G, Martí P, Hernández-Lizoáin JL, Baixauli J, Unamuno X, Frühbeck G, Catalán V. NLRP3 Inflammasome: A Possible Link Between Obesity-Associated Low-Grade Chronic Inflammation and Colorectal Cancer Development. Front Immunol. 2018;9:2918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 39. | Ramachandran R, Manan A, Kim J, Choi S. NLRP3 inflammasome: a key player in the pathogenesis of life-style disorders. Exp Mol Med. 2024;56:1488-1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 40. | Thrum S, Sommer M, Raulien N, Gericke M, Massier L, Kovacs P, Krasselt M, Landgraf K, Körner A, Dietrich A, Blüher M, Rossol M, Wagner U. Macrophages in obesity are characterised by increased IL-1β response to calcium-sensing receptor signals. Int J Obes (Lond). 2022;46:1883-1891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 41. | Billingham LK, Stoolman JS, Vasan K, Rodriguez AE, Poor TA, Szibor M, Jacobs HT, Reczek CR, Rashidi A, Zhang P, Miska J, Chandel NS. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat Immunol. 2022;23:692-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 252] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 42. | Xu L, Yan X, Zhao Y, Wang J, Liu B, Yu S, Fu J, Liu Y, Su J. Macrophage Polarization Mediated by Mitochondrial Dysfunction Induces Adipose Tissue Inflammation in Obesity. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 43. | Yang G, Lee HE, Lee JY. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci Rep. 2016;6:24399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Dapueto R, Rodriguez-Duarte J, Galliussi G, Kamaid A, Bresque M, Batthyány C, López GV, Escande C. A novel nitroalkene vitamin E analogue inhibits the NLRP3 inflammasome and protects against inflammation and glucose intolerance triggered by obesity. Redox Biol. 2021;39:101833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 605] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 46. | Karki R, Man SM, Malireddi RKS, Kesavardhana S, Zhu Q, Burton AR, Sharma BR, Qi X, Pelletier S, Vogel P, Rosenstiel P, Kanneganti TD. NLRC3 is an inhibitory sensor of PI3K-mTOR pathways in cancer. Nature. 2016;540:583-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 47. | Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton V, Colpitts SL, Beauchemin N, Saleh M. The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity. 2015;43:751-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 285] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 48. | Shao X, Lei Z, Zhou C. NLRP3 Promotes Colorectal Cancer Cell Proliferation and Metastasis via Regulating Epithelial Mesenchymal Transformation. Anticancer Agents Med Chem. 2020;20:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Zhou P, Zheng ZH, Wan T, Liao CW, Wu J. Yiqi Jiedu Huayu decoction inhibits precancerous lesions of chronic atrophic gastritis by inhibiting NLRP3 inflammasome-mediated pyroptosis. World J Gastrointest Oncol. 2024;16:3158-3168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (5)] |
| 50. | Huang W, Wang C, Zhang H. Eriodictyol inhibits the motility, angiogenesis and tumor growth of hepatocellular carcinoma via NLRP3 inflammasome inactivation. Heliyon. 2024;10:e24401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 51. | Ding Y, Yan Y, Dong Y, Xu J, Su W, Shi W, Zou Q, Yang X. NLRP3 promotes immune escape by regulating immune checkpoints: A pan-cancer analysis. Int Immunopharmacol. 2022;104:108512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 52. | Tengesdal IW, Menon DR, Osborne DG, Neff CP, Powers NE, Gamboni F, Mauro AG, D'Alessandro A, Stefanoni D, Henen MA, Mills TS, De Graaf DM, Azam T, Vogeli B, Palmer BE, Pietras EM, DeGregori J, Tan AC, Joosten LAB, Fujita M, Dinarello CA, Marchetti C. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 53. | Papafragkos I, Grigoriou M, Boon L, Kloetgen A, Hatzioannou A, Verginis P. Ablation of NLRP3 inflammasome rewires MDSC function and promotes tumor regression. Front Immunol. 2022;13:889075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Scully T, Ettela A, LeRoith D, Gallagher EJ. Obesity, Type 2 Diabetes, and Cancer Risk. Front Oncol. 2020;10:615375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 55. | Dai W, Jiang L. Dysregulated Mitochondrial Dynamics and Metabolism in Obesity, Diabetes, and Cancer. Front Endocrinol (Lausanne). 2019;10:570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 56. | Wang SF, Tseng LM, Lee HC. Role of mitochondrial alterations in human cancer progression and cancer immunity. J Biomed Sci. 2023;30:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 112] [Reference Citation Analysis (0)] |
| 57. | Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 717] [Cited by in RCA: 969] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 58. | Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, Kulke MH, Baird RD, Prabhu JS, Carbone D, Pecoraro C, Teh DBL, Sethi G, Cavalieri V, Lin KH, Javidi-Sharifi NR, Toska E, Davids MS, Brown JR, Diana P, Stebbing J, Fruman DA, Kumar AP. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 1277] [Article Influence: 425.7] [Reference Citation Analysis (0)] |
| 59. | Deleyto-Seldas N, Efeyan A. The mTOR-Autophagy Axis and the Control of Metabolism. Front Cell Dev Biol. 2021;9:655731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 60. | Lee S, Rauch J, Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 576] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 61. | Entezari M, Hashemi D, Taheriazam A, Zabolian A, Mohammadi S, Fakhri F, Hashemi M, Hushmandi K, Ashrafizadeh M, Zarrabi A, Ertas YN, Mirzaei S, Samarghandian S. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed Pharmacother. 2022;146:112563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 260] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 62. | Zhang X, Wang Z, Zheng Y, Yu Q, Zeng M, Bai L, Yang L, Guo M, Jiang X, Gan J. Inhibitors of the NLRP3 inflammasome pathway as promising therapeutic candidates for inflammatory diseases (Review). Int J Mol Med. 2023;51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 63. | Zahid A, Li B, Kombe AJK, Jin T, Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol. 2019;10:2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 546] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 64. | Church LD, Savic S, McDermott MF. Long term management of patients with cryopyrin-associated periodic syndromes (CAPS): focus on rilonacept (IL-1 Trap). Biologics. 2008;2:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Kuemmerle-Deschner JB, Ramos E, Blank N, Roesler J, Felix SD, Jung T, Stricker K, Chakraborty A, Tannenbaum S, Wright AM, Rordorf C. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1β mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res Ther. 2011;13:R34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Cetin Gedik K, Arici ZS, Kul Cinar O, Garcia-Bournissen F, Romano M, Demirkaya E. Practical Approach to Diagnosis and Management of IL-1-Mediated Autoinflammatory Diseases (CAPS, TRAPS, MKD, and DIRA). Paediatr Drugs. 2024;26:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 67. | Klück V, Jansen TLTA, Janssen M, Comarniceanu A, Efdé M, Tengesdal IW, Schraa K, Cleophas MCP, Scribner CL, Skouras DB, Marchetti C, Dinarello CA, Joosten LAB. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020;2:e270-e280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 68. | Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA, Tijssen JGP, Cornel JH, Thompson PL; LoDoCo2 Trial Investigators. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 1565] [Article Influence: 260.8] [Reference Citation Analysis (0)] |
| 69. | Buckley LF, Libby P. Colchicine's Role in Cardiovascular Disease Management. Arterioscler Thromb Vasc Biol. 2024;44:1031-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 70. | Hakim AD, Awili M, O'Neal HR, Siddiqi O, Jaffrani N, Lee R, Overcash JS, Chauffe A, Hammond TC, Patel B, Waters M, Criner GJ, Pachori A, Junge G, Levitch R, Watts J, Koo P, Sengupta T, Yu L, Kiffe M, Pinck A, Stein RR, Bendrick-Peart J, Jenkins J, Rowlands M, Waldron-Lynch F, Matthews J. Efficacy and safety of MAS825 (anti-IL-1β/IL-18) in COVID-19 patients with pneumonia and impaired respiratory function. Clin Exp Immunol. 2023;213:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Madurka I, Vishnevsky A, Soriano JB, Gans SJ, Ore DJS, Rendon A, Ulrik CS, Bhatnagar S, Krishnamurthy S, Mc Harry K, Welte T, Fernandez AA, Mehes B, Meiser K, Gatlik E, Sommer U, Junge G, Rezende E; Study group. DFV890: a new oral NLRP3 inhibitor-tested in an early phase 2a randomised clinical trial in patients with COVID-19 pneumonia and impaired respiratory function. Infection. 2023;51:641-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 72. | Silvis MJM, Demkes EJ, Timmers L, Arslan F, de Jager SCA, Sluijter JPG, Mosterd A, de Kleijn DPV, Bosch L, van Hout GPJ. NLRP3-Inflammasome Inhibition with IZD334 Does Not Reduce Cardiac Damage in a Pig Model of Myocardial Infarction. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 73. | Parmar DV, Kansagra KA, Momin T, Patel HB, Jansari GA, Bhavsar J, Shah C, Patel JM, Ghoghari A, Barot A, Sharma B, Viswanathan K, Patel HV, Jain MR. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Oral NLRP3 Inflammasome Inhibitor ZYIL1: First-in-Human Phase 1 Studies (Single Ascending Dose and Multiple Ascending Dose). Clin Pharmacol Drug Dev. 2023;12:202-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 74. | Jin L, Hua H, Ji Y, Jia Z, Peng M, Huang S. Anti-inflammatory role of fenofibrate in treating diseases. Biomol Biomed. 2023;23:376-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 75. | Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, Crimet DC, O'Connell RL, Colman PG; FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 774] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 76. | Liu Q, Zhang F, Zhang X, Cheng R, Ma JX, Yi J, Li J. Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol Cell Biochem. 2018;445:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 77. | Vande Walle L, Lamkanfi M. Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets. Nat Rev Drug Discov. 2024;23:43-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 207] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 78. | Jung J, Zeng H, Horng T. Metabolism as a guiding force for immunity. Nat Cell Biol. 2019;21:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 251] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 79. | De Martino M, Rathmell JC, Galluzzi L, Vanpouille-Box C. Cancer cell metabolism and antitumour immunity. Nat Rev Immunol. 2024;24:654-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 158] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 80. | Wculek SK, Heras-Murillo I, Mastrangelo A, Mañanes D, Galán M, Miguel V, Curtabbi A, Barbas C, Chandel NS, Enríquez JA, Lamas S, Sancho D. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity. 2023;56:516-530.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 173] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/