Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.112385
Revised: August 29, 2025
Accepted: October 17, 2025
Published online: November 15, 2025
Processing time: 105 Days and 23.2 Hours
Liver metastases are very common in pancreatic neuroendocrine tumors (pNETs). When surgical resection is possible, it is typically associated with survival benefits in patients with pNET and liver metastases. Patient-derived organoids are a powerful preclinical platform that show great potential for predicting treatment response, and they have been increasingly applied in precision medicine and cancer research.
A 51-year-old man was admitted to the hospital with the chief complaint of in
This case suggests that patient-derived organoids can aid in the optimization of therapeutic decisions in pNET patients with liver metastases to guide personalized treatment and improve survival outcomes.
Core Tip: Patient-derived organoids (PDOs) show great potential for predicting treatment response in various cancer types. Although gastroenteropancreatic neuroendocrine neoplasm organoids can retain the pathohistological and functional phenotypes of parental tumors, their roles in predicting clinical outcomes are not known. Here, we report a case of pancreatic neuroendocrine tumor with liver metastases in which personalized treatment guided by PDO-based drug sensitivity testing enabled successful surgery and resulted in a favorable prognosis. Therefore, for patients with pancreatic neuroendocrine tumor patients and liver metastases, PDOs can help identify effective chemotherapy regimens, enabling personalized treatment, facilitating surgical resection, and improving survival outcomes.
- Citation: Yang XM, Sun W, He YG, Peng XH, You N, Tang YC, Zheng L, Huang XB. Patient-derived organoids for the personalized treatment of pancreatic neuroendocrine tumor with liver metastases: A case report. World J Gastrointest Oncol 2025; 17(11): 112385
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/112385.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.112385
Pancreatic neuroendocrine tumors (pNETs) are a rare and heterogeneous group of neoplasms arising from pancreatic islet cells, with variations in histology, clinical characteristics, and prognosis[1]. They may present as non-infiltrative, slow-growing tumors, locally invasive tumors, or even rapidly metastasizing tumors[2]. Most metastases localize to the liver, and approximately 28%-77% of patients with pNETs will experience liver metastases in their lifetime[3]. Patients with liver metastases may be subjected to local complications such as biliary obstruction, liver insufficiency, and carcinoid syndrome. Additionally, liver metastases are a major risk factor for the prognosis of patients with pNETs[4]. When feasible, surgical resection is significantly associated with the best long-term survival outcomes[5]. Therefore, for patients with pNET liver metastases, comprehensive assessment and multidisciplinary approaches are required to determine the feasibility of surgical resection and the optimal treatment to improve the prognosis.
Over the past decade, the advent of in vitro three-dimensional technologies including organoids has revolutionized the development of human cancer models. Patient-derived organoids (PDOs), an in vitro three-dimensional microstructure, can faithfully recapitulate the intricate spatial architecture and cell heterogeneity of the tissue, and simulate the biological behaviors and functions of parental tumors while preserving biological, genetic and molecular features[6,7]. As a po
A 51-year-old man was admitted to the hospital with the primary complaint of intermittent dull pain in the upper abdomen for over 3 years.
Intermittent dull pain in the upper abdomen for over 3 years.
The patient had no history of malignancies.
The patient denied any personal and family history.
No abnormalities were found during the patient’s general physical examination.
Laboratory examinations showed alpha fetal protein of 2.88 ng/mL, carbohydrate antigen 19-9 of 75.96 U/mL, carbohy
| Indicators | AFP (ng/mL) | CA19-9 (U/mL) | CEA (ng/mL) | CA15-3 (U/mL) |
| Before treatment | 2.88 | 75.96 | 1.93 | 35.10 |
| After second TACE | 2.99 | 5.07 | 1.56 | 9.10 |
| 2 months after surgery | 2.10 | 39.00 | 1.51 | 20.0 |
| 18 months after surgery | 2.15 | 9.19 | 1.51 | 6.61 |
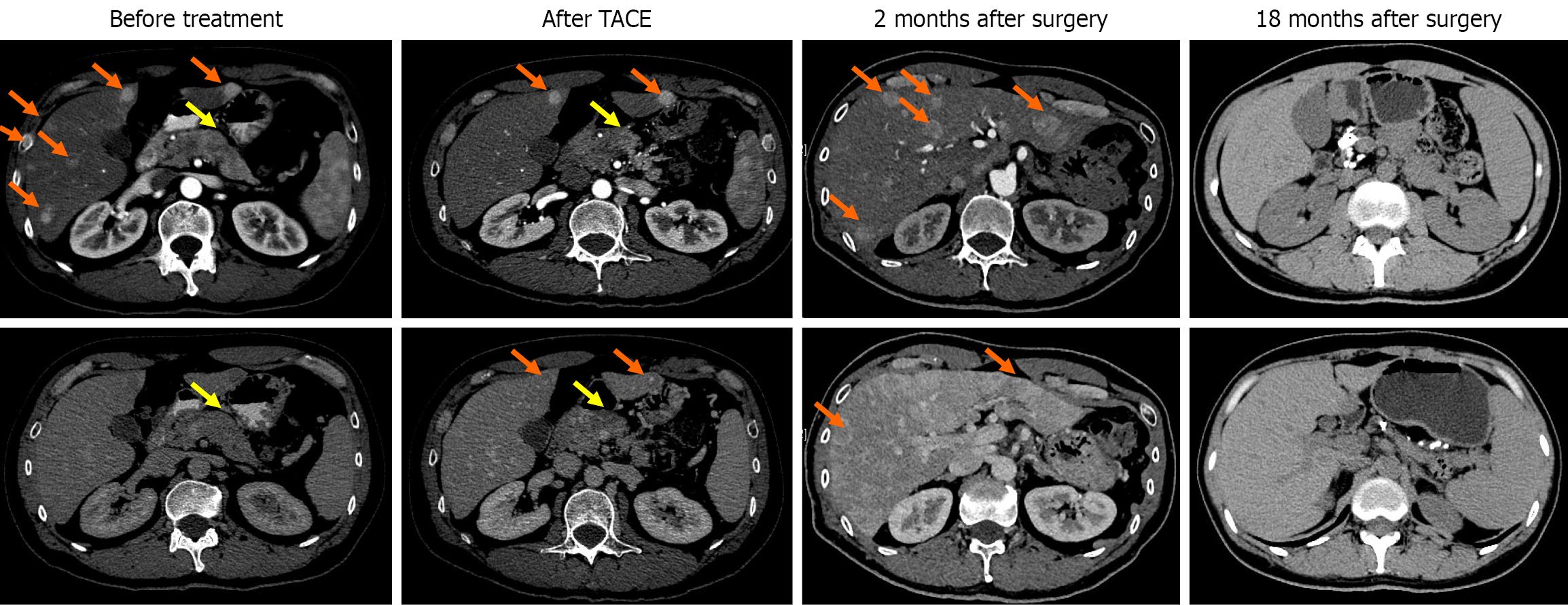
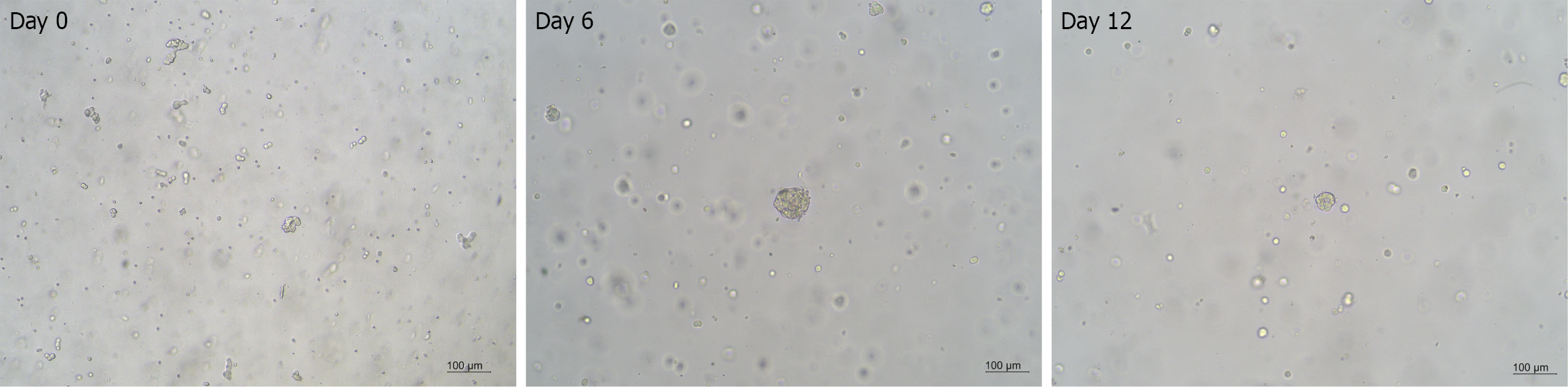
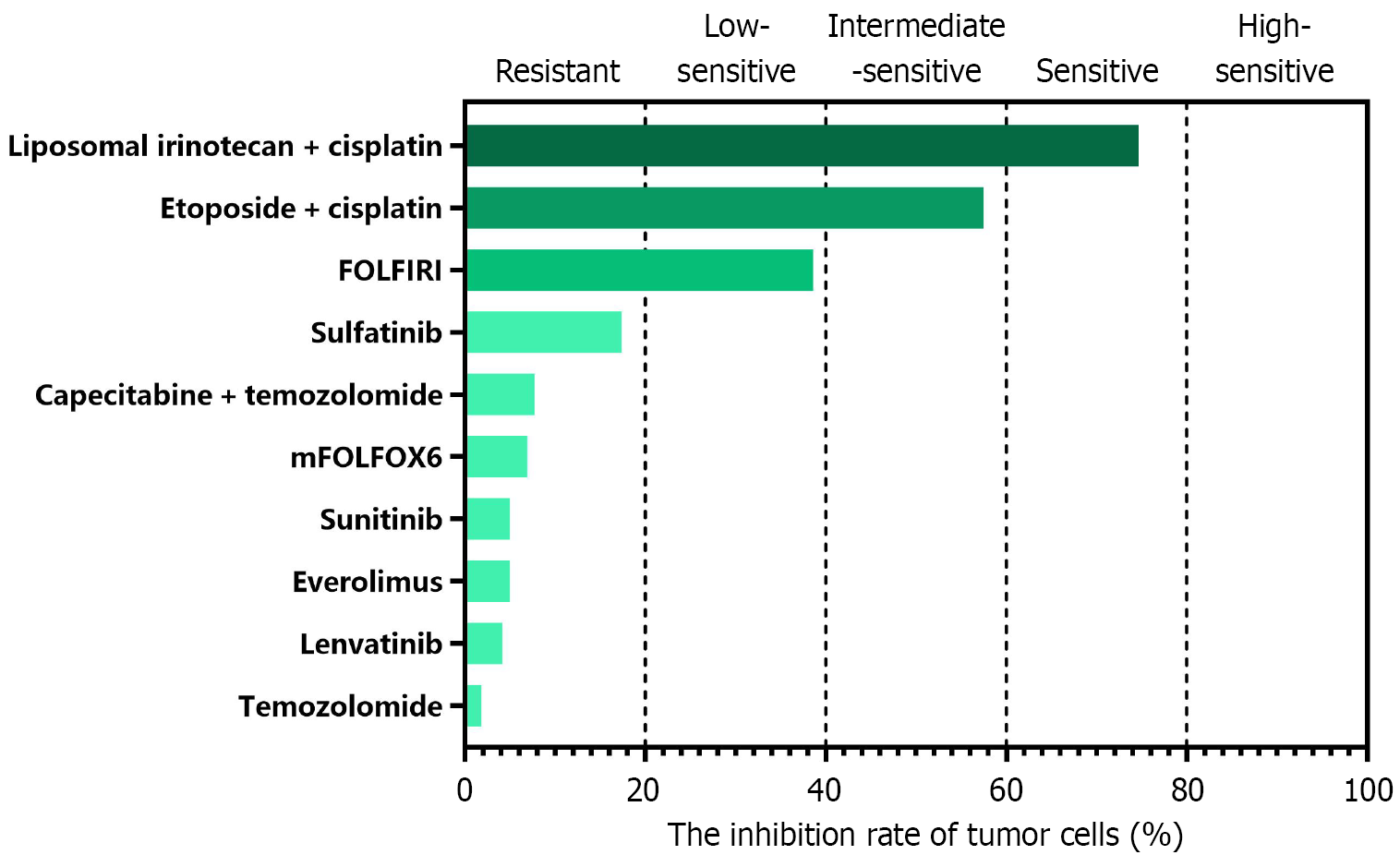
Computerized tomography (CT) scans showed multiple space-occupying lesions in the liver and a neoplasm in the pancreatic body (Figure 1). Positron emission tomography (PET)/CT examinations further revealed a pancreatic body tumor and multiple liver metastases. Meanwhile, endoscopic ultrasound-guided liver biopsy was performed, among which a portion of the biopsy sample was collected for organoid culture according to the protocols from Kingbio Medical (Chongqing) Co., Ltd. (China) after obtaining informed consent. The reagents related to organoid culture and drug sensitivity testing were all provided by Kingbio Medical (Chongqing) Co., Ltd. (China). Briefly, the biopsy tissue was minced and digested after rinsing with precooled phosphate-buffered saline, and cell pellets were collected by centrifugation. Matrigel was added, and the cells and Matrigel suspension were seeded onto a 6-well plate, with 100 cells per well. The plate was placed in an incubator at 37 °C for 15 minutes. Once the droplets were solidified, Jiabili® culture medium [Cabio Biotech (Wuhan) Co., Ltd., China] for pancreatic cancer was supplemented. At last, the plate was put in a 37 °C, 5% CO2 incubator for 13 days. Drug sensitivity testing was conducted when the organoids became solid spheroids with approximately 70 μm diameters (Figure 2). Subsequently, organoid cell masses were digested into single cells, and a total of 2 × 104 cells were obtained. Cells were resuspended in Jiabili® tumor tissue basic medium II [Cabio Biotech (Wuhan) Co., Ltd., China]. As described previously, organoid drug sensitivity testing concentrations were established based on the blood drug concentration of each drug, including the upper and lower limits of the blood drug concentration[11]. The pre-prepared drug solution and CelltiterGlo 2.0 (Promega Corporation, WI, United States) were supplemented successively, and a microplate reader was used to read the value. Notably, a binary classification method was utilized to evaluate the potential of drugs according to the quantification of the drug’s inhibitory effects on tumor organoid growth. The drug was considered resistant when the inhibitory rate < 50% or sensitive for an inhibitory rate ≥ 50%.
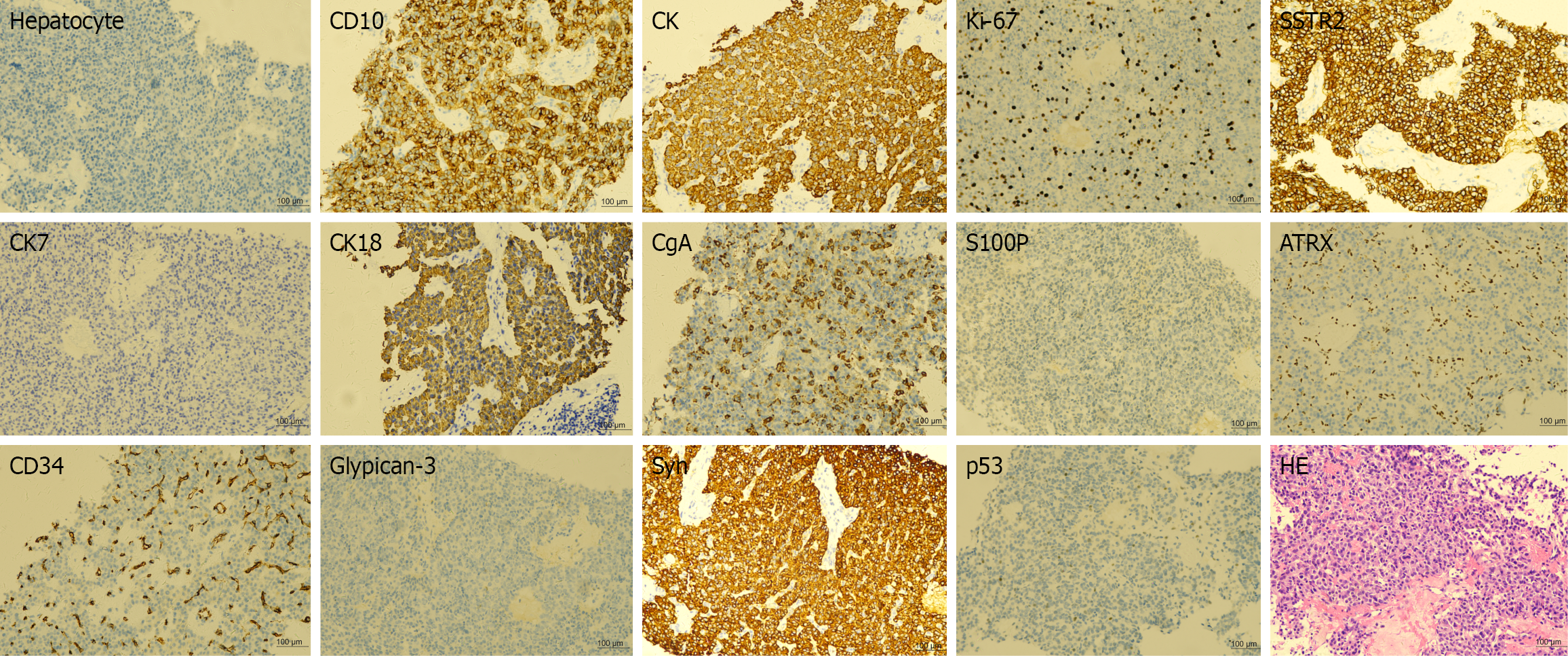
Pathological results of the patient suggested a hepatic neuroendocrine tumor derived from the pancreas, with a pro
After discussion, the patient was advised to first receive anti-tumor treatment, followed by evaluation for later treatment. After communication with the patient and his family, transarterial chemoembolization (TACE) plus oxaliplatin (85 mg/m2), 5-fluorouracil (400 mg/m2) and 5-fluorouracil (2400 mg) was initially perfused for 23 hours. Subsequently, the chemotherapy drugs were switched to liposomal irinotecan (43 mg/m2) and cisplatin (60 mg) for the second 23 hours according to the PDO-based drug sensitivity testing results (Figure 4). CT images revealed that the largest tumor diameters in the pancreas and liver were decreased from 4.6 cm to 3.3 cm and from 3.2 cm to 2.5 cm after interventional treatment, respectively (Figure 1). Based on the Response Evaluation Criteria in Solid Tumors (version 1.1), partial response was achieved in the pancreatic lesion or in the hepatic lesion. However, there was a cancer thrombus at the confluence of the splenic vein and the superior mesenteric vein, and the patient had poor treatment compliance. Considering that the patient was willing to undergo surgical resection, pancreatic body-tail resection, partial resection and anastomosis of the portal vein, total splenectomy, and liver tumor resection were all performed after discussion. Postoperative pathological results showed pNET (grade 3) and hepatic neuroendocrine tumor (grade 3), in which tumor cell metastasis occurred in 1 of 9 lymph nodes. There were no tumor cells at the margins of the portal vein and the pancreas.
Two months after surgery, CT scans showed multiple liver metastatic lesions (Figure 1). TACE with liposomal irinotecan and cisplatin was again continuously perfused for 23 hours. After surgical resection, the patient received five total treatment cycles. He is currently alive with tumors for 18 months (Figure 1), and the tumor markers have returned to normal (Table 1).
Liver metastases are very common in pNETs. When possible, surgical resection is typically associated with better survival in pNET patients with liver metastases[5,12]. According to data from a large, prospective database, hepatic resection was a significant independent factor for overall survival in neuroendocrine liver metastases[5]. In a previous meta-analysis, a median 5-year overall survival rate of 71% was pooled in patients receiving hepatic resection, and more than 95% of them achieved symptomatic relief[13].
Multimodal therapies including systemic chemotherapy are usually used for patients with liver metastases of non-neuroendocrine origin. Neoadjuvant therapy aims to promote tumor downstaging and maintain stable tumor biology, thus ensuring the feasibility of surgical resection. To identify patients with disease stability and improve survival rates, neoadjuvant chemotherapy is commonly utilized in multiple cancer types before hepatic resection[14,15]. In our study, the patient was diagnosed with pNET with liver metastases (grade 3). Considering that the patient was willing to receive surgical resection, antitumor treatment was recommended after discussion. Therefore, TACE with oxaliplatin and 5-fluorouracil was initially used, then replaced with liposomal irinotecan and cisplatin for the second perfusion based on organoid drug sensitivity testing results. The pancreatic and hepatic lesions both significantly decreased in size after organoid-guided treatment.
Over the past decade, PDOs have been established for various cancer types, with high success rates of generation. In four patients with metastatic neuroendocrine prostate cancer, organoids were successfully generated and showed a high concordance with the original tumors at the genomic, transcriptomic, and epigenomic levels. Meanwhile, organoid-based drug screening generated hypotheses for both single-agent and combination therapies, thereby supporting the clinical use of certain drugs and facilitating the development of novel treatment strategies[16]. Consequently, organoid-based drug screening can serve as a valuable tool for identifying rational therapeutic approaches in cancer. In another study, or
Notably, the results of the PDO-based drug sensitivity testing in our study only reflected the direct-action ability of drugs on “isolated tumor cells” and could not fully represent their comprehensive therapeutic efficacy in complex microenvironment in vivo. For drugs like sorafenib and lenvatinib, whose mechanisms rely on the tumor microen
For pNET patients with liver metastases, PDOs are conducive for screening effective chemotherapy drugs for TACE to facilitate personalized treatment plans, providing opportunities for surgical resection by decreasing tumor size and a novel platform for improving survival outcomes.
| 1. | Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol. 2015;21:9512-9525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Sadot E, Reidy-Lagunes DL, Tang LH, Do RK, Gonen M, D'Angelica MI, DeMatteo RP, Kingham TP, Groot Koerkamp B, Untch BR, Brennan MF, Jarnagin WR, Allen PJ. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann Surg Oncol. 2016;23:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 606] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 4. | Cloyd JM, Wiseman JT, Pawlik TM. Surgical management of pancreatic neuroendocrine liver metastases. J Gastrointest Oncol. 2020;11:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Fairweather M, Swanson R, Wang J, Brais LK, Dutton T, Kulke MH, Clancy TE. Management of Neuroendocrine Tumor Liver Metastases: Long-Term Outcomes and Prognostic Factors from a Large Prospective Database. Ann Surg Oncol. 2017;24:2319-2325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Jacob F, Salinas RD, Zhang DY, Nguyen PTT, Schnoll JG, Wong SZH, Thokala R, Sheikh S, Saxena D, Prokop S, Liu DA, Qian X, Petrov D, Lucas T, Chen HI, Dorsey JF, Christian KM, Binder ZA, Nasrallah M, Brem S, O'Rourke DM, Ming GL, Song H. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-tumoral Heterogeneity. Cell. 2020;180:188-204.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 746] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 7. | Kawasaki K, Toshimitsu K, Matano M, Fujita M, Fujii M, Togasaki K, Ebisudani T, Shimokawa M, Takano A, Takahashi S, Ohta Y, Nanki K, Igarashi R, Ishimaru K, Ishida H, Sukawa Y, Sugimoto S, Saito Y, Maejima K, Sasagawa S, Lee H, Kim HG, Ha K, Hamamoto J, Fukunaga K, Maekawa A, Tanabe M, Ishihara S, Hamamoto Y, Yasuda H, Sekine S, Kudo A, Kitagawa Y, Kanai T, Nakagawa H, Sato T. An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping. Cell. 2020;183:1420-1435.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 8. | Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, Rata M, Koh DM, Tunariu N, Collins D, Hulkki-Wilson S, Ragulan C, Spiteri I, Moorcraft SY, Chau I, Rao S, Watkins D, Fotiadis N, Bali M, Darvish-Damavandi M, Lote H, Eltahir Z, Smyth EC, Begum R, Clarke PA, Hahne JC, Dowsett M, de Bono J, Workman P, Sadanandam A, Fassan M, Sansom OJ, Eccles S, Starling N, Braconi C, Sottoriva A, Robinson SP, Cunningham D, Valeri N. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018;359:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1443] [Article Influence: 180.4] [Reference Citation Analysis (0)] |
| 9. | Ganesh K, Wu C, O'Rourke KP, Szeglin BC, Zheng Y, Sauvé CG, Adileh M, Wasserman I, Marco MR, Kim AS, Shady M, Sanchez-Vega F, Karthaus WR, Won HH, Choi SH, Pelossof R, Barlas A, Ntiamoah P, Pappou E, Elghouayel A, Strong JS, Chen CT, Harris JW, Weiser MR, Nash GM, Guillem JG, Wei IH, Kolesnick RN, Veeraraghavan H, Ortiz EJ, Petkovska I, Cercek A, Manova-Todorova KO, Saltz LB, Lavery JA, DeMatteo RP, Massagué J, Paty PB, Yaeger R, Chen X, Patil S, Clevers H, Berger MF, Lowe SW, Shia J, Romesser PB, Dow LE, Garcia-Aguilar J, Sawyers CL, Smith JJ. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med. 2019;25:1607-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 10. | Kim J, Yu D, Kwon Y, Lee KS, Sim SH, Kong SY, Lee ES, Park IH, Park C. Genomic Characteristics of Triple-Negative Breast Cancer Nominate Molecular Subtypes That Predict Chemotherapy Response. Mol Cancer Res. 2020;18:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | He Y, Zhu Y, Wang W, Yi Y, Wang Z, Zhao C, Li J, Huang X, Zheng L. Clinical efficacy and chemoresistance analysis of precision neoadjuvant chemotherapy for borderline resectable pancreatic cancer: a prospective, single-arm pilot study. Int J Surg. 2025;111:3269-3280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Mayo SC, de Jong MC, Bloomston M, Pulitano C, Clary BM, Reddy SK, Clark Gamblin T, Celinski SA, Kooby DA, Staley CA, Stokes JB, Chu CK, Arrese D, Ferrero A, Schulick RD, Choti MA, Geschwind JF, Strub J, Bauer TW, Adams RB, Aldrighetti L, Mentha G, Capussotti L, Pawlik TM. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol. 2011;18:3657-3665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Saxena A, Chua TC, Perera M, Chu F, Morris DL. Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol. 2012;21:e131-e141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Brouquet A, Abdalla EK, Kopetz S, Garrett CR, Overman MJ, Eng C, Andreou A, Loyer EM, Madoff DC, Curley SA, Vauthey JN. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol. 2011;29:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 15. | Martel G, Hawel J, Rekman J, Croome KP, Bertens K, Balaa FK, Hernandez-Alejandro R. Liver resection for non-colorectal, non-carcinoid, non-sarcoma metastases: a multicenter study. PLoS One. 2015;10:e0120569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Puca L, Bareja R, Prandi D, Shaw R, Benelli M, Karthaus WR, Hess J, Sigouros M, Donoghue A, Kossai M, Gao D, Cyrta J, Sailer V, Vosoughi A, Pauli C, Churakova Y, Cheung C, Deonarine LD, McNary TJ, Rosati R, Tagawa ST, Nanus DM, Mosquera JM, Sawyers CL, Chen Y, Inghirami G, Rao RA, Grandori C, Elemento O, Sboner A, Demichelis F, Rubin MA, Beltran H. Patient derived organoids to model rare prostate cancer phenotypes. Nat Commun. 2018;9:2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 17. | Saito Y, Muramatsu T, Kanai Y, Ojima H, Sukeda A, Hiraoka N, Arai E, Sugiyama Y, Matsuzaki J, Uchida R, Yoshikawa N, Furukawa R, Saito H. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Rep. 2019;27:1265-1276.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 18. | Grebenyuk S, Ranga A. Engineering Organoid Vascularization. Front Bioeng Biotechnol. 2019;7:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |