Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.110675
Revised: July 18, 2025
Accepted: October 9, 2025
Published online: November 15, 2025
Processing time: 155 Days and 11.1 Hours
Colorectal cancer (CRC) is a prevalent gastrointestinal malignancy with a typi
To develop and validate a new clinical prediction model to accurately forecast overall survival (OS) in CRC patients following surgical treatment for liver metastasis.
This study included 1059 patients diagnosed with CRC liver metastases (CRLM) at the Xijing Hospital between 2010 and 2022. The patients were randomly divided into training and validation cohorts at a 7:3 ratio. Key clinical predictors were identified using least absolute shrinkage and selection operator (LASSO) regression combined with a Cox proportional hazards model, leading to the establishment of a prediction model and preparation of a nomogram to enhance its clinical utility. Decision curve analysis (DCA) and Kaplan-Meier survival analysis were employed to evaluate the precision and predictive performance of the model.
The LASSO-Cox regression analysis revealed multiple pivotal clinical biomarkers significantly linked to CRLM, including gamma-glutamyl transferase levels, blood chloride concentration, activated partial thromboplastin time, N stage, and vascular invasion. The model's receiver operating characteristic curve area under the curve exceeded 0.7 for both the training and validation groups with moderate-to-good predictive accuracy. Furthermore, DCA validated the nomogram's effectiveness for OS prediction. Kaplan-Meier risk stratification demonstrated markedly improved OS among patients classified as low-risk compared to those categorized as high-risk (P < 0.001), highlighting its clinical utility for risk assessment and treatment guidance.
The nomogram prediction model constructed in this study has good predictive value and can effectively assess the survival rate of patients with CRLM.
Core Tip: This study presents a novel nomogram for predicting overall survival in patients with colorectal cancer (CRC) liver metastasis post-surgery. In a retrospective cohort analysis of 1059 patients, we identified key prognostic factors including gamma-glutamyl transferase, blood chloride concentration, activated partial thromboplastin time, N stage, and vascular invasion. The nomogram demonstrated robust predictive accuracy with area under the curve values exceeding 0.7 in both training and validation cohorts. These findings offer a clinically relevant tool for personalized risk assessment and treatment planning in CRC liver metastasis, potentially improving patient outcomes.
- Citation: Xie MJ, Li JJ, Guo YJ, Wang Q, Tan ZB, Li YL, Li JP. Construction of a prognostic model for colorectal cancer liver metastasis: A retrospective study based on population data. World J Gastrointest Oncol 2025; 17(11): 110675
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/110675.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.110675
Colorectal cancer (CRC) exerts a significant global health burden and ranks among the top three cancers in terms of both incidence and mortality. Over the past three decades, there has been a consistent global increase in the incidence of CRC, with some regions experiencing a doubling or greater rate of growth[1]. In particular, in developing countries with disparate medical resources and varying levels of development, a significant number of patients present at an advanced stage of cancer, often with distant metastases already established at the time of their initial visit[2]. Consequently, these patients face a grim prognosis and diminished survival rates, presenting a considerable challenge for clinical treatment and compounding the societal and familial burdens associated with this disease.
The liver is the principal target organ for hematogenous metastasis in CRC, approximately 15%-25% of patients are diagnosed with CRC liver metastasis (CRLM), exerting a profound influence on patient prognosis and recurrence rates[3-5]. Despite the utility of preoperative chest and abdominal computed tomography (CT) scans in the early detection of CRC and liver metastases, which facilitates surgical intervention, neoadjuvant chemoradiotherapy, or adjuvant chemoradiotherapy, many CRC patients (approximately 15%-25%) develop liver metastases after colorectal tumor resection. In these instances, a majority of hepatic metastases, approximately 80%-90% in the initial phase, are found to be unre
The prognosis for patients with untreated CRLM is poor, characterized by a median overall survival (OS) of only 6.9 months. The 5-year OS for CRLM is dismally low, at below 5%[3,11]. In contrast, patients who undergo successful complete resection of the liver metastases or who achieve a "no evidence of disease" state exhibit a markedly improved median OS duration of 35 months[12-16]. These findings underscore the critical nature of CRLM as a significant challenge in the management of CRC[5,17].
The growing focus on CRC has led to the development of numerous prognostic models, which have been widely adopted in clinical practice. These models have demonstrated significant predictive accuracy, thereby enhancing the precision of clinical decision-making. Consequently, the identification of critical clinical characteristics in patients with CRLM to construct prognostic models is crucial for the prediction of OS and the determination of optimal therapeutic strategies aimed at extending patient lifespan. Nomograms, as effective and convenient predictive tools, integrate different prognostic and decisive risk factors to calculate the prognosis of many diseases. They have been used as prognostic devices in oncology and have shown good practical value in personalized medicine.
This study conducted a comprehensive analysis of the CRLM dataset and established a predictive model using retrospective research methods, which provides a new method for clinicians to evaluate the individualized prognosis of patients with CRLM. This model is helpful for personalized prognostic assessment of CRLM 1-year, 3-year and 5-year OS, and improves the accuracy of treatment regimens.
This retrospective analysis was performed to construct and internally validate a predictive model using data from patients who underwent CRC resection at Xijing Hospital, Shaanxi Air Force Medical University, China, between 2010 and 2022. The model was then externally validated using a separate cohort of patients from Chengdu Medical College Second Affiliated Hospital, China. All patients included in both the model development and validation cohorts underwent standard resection surgery coupled with regional lymph node dissection, ensuring a uniform surgical approach across the study groups. The following patients were enrolled in the study: Age range 18 to 75 years; confirmed histological and cytological diagnosis of CRC; evidence of liver metastases by liver histopathology, CT, or magnetic resonance imaging; and an Eastern Cooperative Oncology Group performance status of 0-1. If patients had additional primary malignancies; severe underlying conditions of the heart, kidneys, bone marrow, lungs, or central nervous system; active infection with hepatitis B or C; acquired or congenital immune deficiency; renal dysfunction; coagulation disorders or blood clotting disorders these patients were excluded from the study.
The study included 1059 patients treated at Xijing Hospital and 97 patients from the Second Affiliated Hospital of Chengdu Medical College. Following CRC diagnosis, these patients were regularly monitored by their attending physicians. The patients were followed up every three months for the first year post-discharge, and then every six months until the patient died. The follow-up study was concluded in June 2024. Follow-up data were collected after meticulous examination of medical records and supplemented by telephonic interviews. These data included comprehensive information on the timing and site of recurrence or metastasis, the emergence of additional primary malignancies, and the timing and cause of death. The primary endpoint of the investigation was OS, which was calculated as the interval from the initial diagnosis of CRLM to death from any cause.
Postoperative pathological specimens were subjected to hematoxylin and eosin staining analysis, with diagnoses provided by a panel of expert pathologists. The collected clinical and pathological data included an array of factors such as serological markers, electrolyte concentrations, complete blood counts, coagulation profiles, tumor biomarkers, histological subtypes, tumor node metastasis (TNM) staging, evidence of perineural invasion, and the presence of vascular invasion. The TNM staging was conducted in accordance with the American Joint Committee on Cancer guidelines issued in 2017.
Data from all eligible patients with CRLM were analyzed. Initially, descriptive statistical methods were employed to summarize the basic information, with categorical data presented as frequencies and percentages, and continuous data as the median of the quartile range or the mean of standard deviations. For the comparison of discrete variables, the χ2 test was used, and for continuous variables, either the Student's t-test or the Mann-Whitney U test was applied.
To enhance the robustness of the analysis in this study, we employed the Isolated Forest algorithm for outlier detection, an ensemble method specifically designed for anomaly detection. This approach constructs isolation trees by randomly selecting features and split values, effectively isolating outliers that require fewer splits. Additionally, to address missing values, we utilized the K-Nearest Neighbors imputation method, setting K to 10, which ensured the preservation of the data's local structure and distribution characteristics.
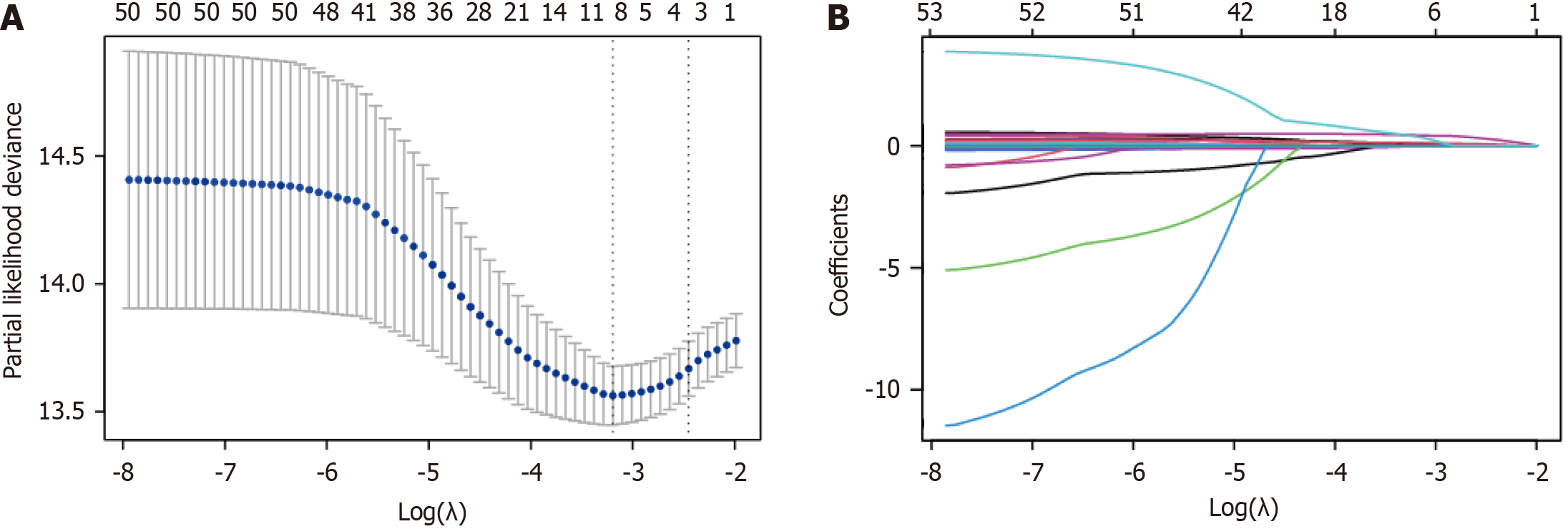
The dataset was divided into a training set and a validation set at a 7:3 ratio using the Light Gradient Boosting Machine algorithm, maintaining a consistent proportion of positive to negative samples across both subsets. Within the training set, least absolute shrinkage and selection operator (LASSO) Cox regression was employed for multivariate analysis. Compared to the traditional stepwise Cox regression approach, the LASSO-Cox regression offers a potentially more precise and interpretable risk prediction model by reducing the variance of the estimates.
A mortality prediction tool was developed for clinicians based on a nomogram created through Cox regression analysis of the training set. The accuracy of this nomogram was assessed using receiver operating characteristic (ROC) curves, and a predicted net benefit threshold was established through decision curve analysis (DCA). In this study, P < 0.05 was considered statistically significant. All statistical assessments were conducted using R software (version 4.2.2).
In this investigation, data were extracted from an electronic medical record database between 2010 and 2022, resulting in a dataset consisting of 1059 patients. Subsequently, 741 patients were assigned to the training cohort and 318 to the validation cohort. Clinical characteristics of the two groups were comprehensively compared, including variables such as sex, age, tumor type, histological grade, tumor size, microsatellite instability (MSI) status, and a range of laboratory parameters. Analysis showed no statistical difference between the two groups in terms of these clinical features (P > 0.05) (Table 1). This provided a solid foundation for subsequent analysis and modeling.
| Characteristic | Cohort | P value | |
| Training cohort, n = 741 | Test cohort, n = 318 | ||
| Sex | 0.710 | ||
| Female | 298 (40.2) | 124 (39.0) | |
| Male | 443 (59.8) | 194 (61.0) | |
| Age | 58 ± 13 | 59 ± 12 | 0.444 |
| Blood type | 0.599 | ||
| A | 215 (29.0) | 81 (25.5) | |
| AB | 66 (8.9) | 34 (10.7) | |
| B | 247 (33.3) | 108 (34.0) | |
| O | 213 (28.7) | 95 (29.9) | |
| Albumin level | 39 ± 6 | 40 ± 6 | 0.220 |
| Globulin level | 27.0 ± 4.6 | 26.8 ± 4.4 | 0.452 |
| Direct bilirubin | 6.44 ± 16.57 | 5.36 ± 4.65 | 0.101 |
| GGT | 51 ± 105 | 46 ± 88 | 0.403 |
| AST | 29 ± 32 | 27 ± 21 | 0.226 |
| Chlorine | 103.2 ± 4.0 | 103.5 ± 4.0 | 0.374 |
| Cystatin C | 0.92 ± 0.23 | 0.92 ± 0.23 | 0.952 |
| AFP | 2.72 (1.85, 3.78) | 2.53 (1.71, 3.57) | 0.136 |
| CEA | 7 (3, 33) | 7 (3, 28) | 0.718 |
| CA19-9 | 23 (10, 82) | 20 (8, 59) | 0.979 |
| CA125 | 14 (10, 23) | 14 (10, 26) | 0.455 |
| White blood cell | 7.6 ± 3.5 | 7.7 ± 3.6 | 0.753 |
| Neutral lymphoid ratio | 0.73 ± 0.14 | 0.73 ± 0.13 | 0.782 |
| Lymphocyte ratio | 0.19 ± 0.11 | 0.19 ± 0.10 | 0.639 |
| Eosinophils ratio | 0.009 (0.001, 0.019) | 0.009 (0.002, 0.021) | 0.973 |
| Platelet level | 230 ± 93 | 233 ± 101 | 0.661 |
| D-Dimer | 773 ± 1832 | 907 ± 2238 | 0.349 |
| APTT | 32 ± 8 | 32 ± 7 | 0.235 |
| Tumor type | |||
| Common adenocarcinoma | 18 (2.4) | 10 (3.1) | 0.226 |
| Adenosquamous carcinoma | 6 (0.8) | 0 (0.0) | |
| Mucinous adenocarcinoma | 702 (94.7) | 303 (95.3) | |
| Squamous cell carcinoma | 4 (0.5) | 0 (0.0) | |
| Neuroendocrine carcinoma | 7 (0.9) | 1 (0.3) | |
| Signet ring cell carcinoma | 4 (0.5) | 4 (1.3) | |
| T stage | 0.652 | ||
| T1 | 218 (29.4) | 90 (28.3) | |
| T2 | 98 (13.2) | 37 (11.6) | |
| T3 | 298 (40.2) | 139 (43.7) | |
| T4 | 127 (17.1) | 52 (16.4) | |
| N stage | 0.115 | ||
| N0 | 298 (40.2) | 122 (38.4) | |
| N1 | 271 (36.6) | 136 (42.8) | |
| N2 | 172 (23.2) | 60 (18.9) | |
| Microsatellite instability status | 0.372 | ||
| pMMR | 559 (75.4) | 248 (78.0) | |
| dMMR | 182 (24.6) | 70 (22.0) | |
| Perineural invasion | 0.654 | ||
| Negative | 86 (11.6) | 40 (12.6) | |
| Positive | 655 (88.4) | 278 (87.4) | |
| Vascular invasion | 0.873 | ||
| Negative | 225 (30.4) | 95 (29.9) | |
| Positive | 516 (69.6) | 223 (70.1) | |
Logistic regression analysis was used to investigate the association between clinical or pathological features and OS in patients with CRLM. The results of univariate analysis showed that a total of 18 variables were identified as significant predictors of OS (Figure 1). These variables included a range of clinical and biochemical parameters, including albumin, direct bilirubin, indirect bilirubin, alkaline phosphatase, gamma-glutamyl transferase (GGT), aspartate aminotransferase, triglycerides, sodium, chloride, cystatin C, carcinoembryonic antigen, carbohydrate antigen 19-9 and other factors such as D-dimer, activated partial thromboplastin time (APTT), prothrombin time, N-stage, MSI status and vascular invasion (P < 0.05) (Supplementary Table 1). These results provide a critical foundation for subsequent multivariate analysis and may have implications for clinical prognostication and therapeutic decision-making.
Subsequent multivariate Cox regression analysis showed that GGT, chloride, APTT, N stage and vascular invasion were independent prognostic factors for OS (Supplementary Table 2). The variables were centralized and standardized using 10-fold cross-validation before being incorporated into the survival nomogram construction of the training cohort. To facilitate the prediction of 1-year, 3-year and 5-year OS in patients with CRLM, the nomogram was then constructed using a weighted combination of key clinical and biochemical variables to provide a visualization tool to promote a more personalized and informed approach to patient management and treatment planning (Figure 2).
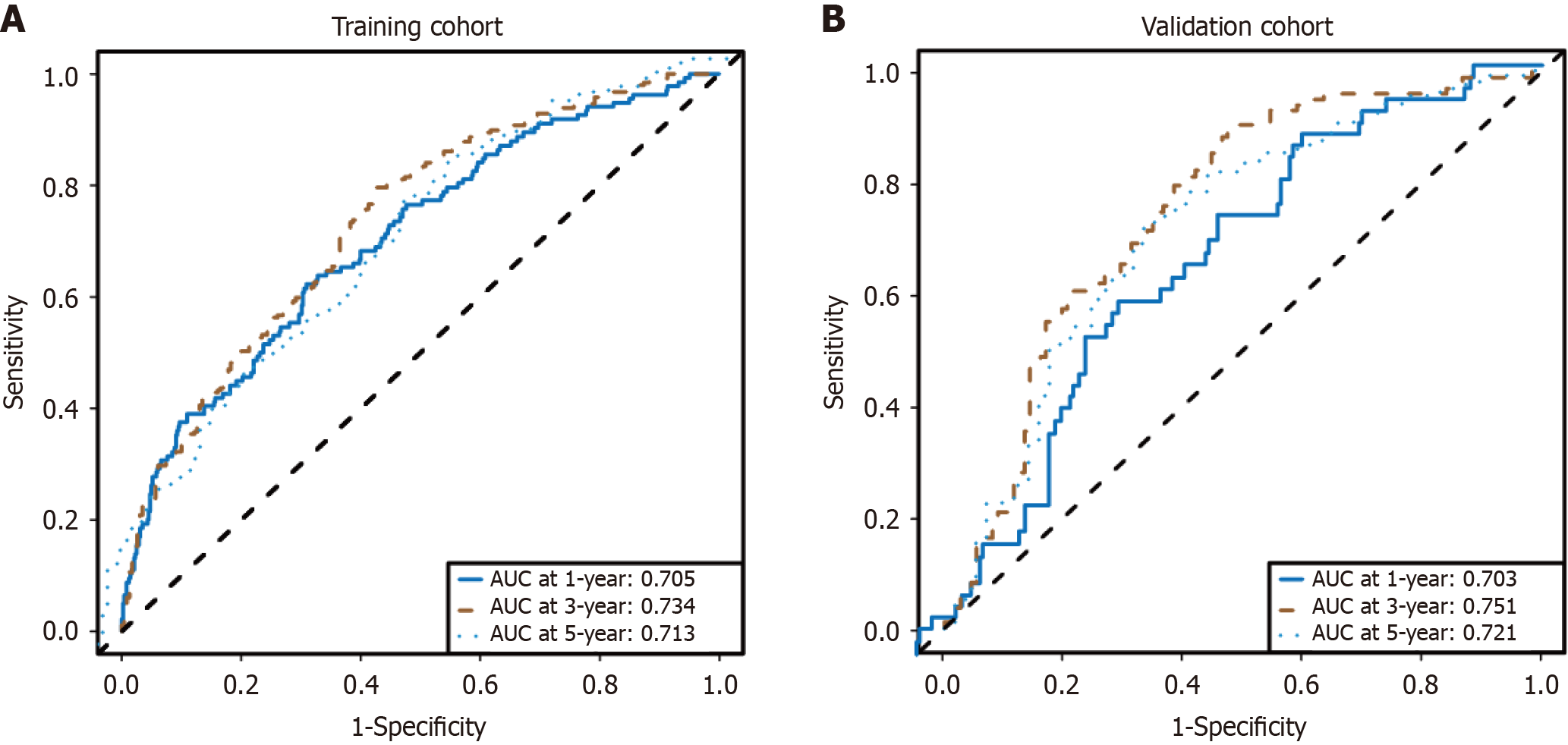
As expected, the model exhibited strong predictive performance in the training cohort for 1-year [area under the curve (AUC) = 0.705], 3-year (AUC = 0.734), and 5-year (AUC = 0.713) survival rates (Figure 3A). The ROC curves from the internal validation cohort also revealed that the survival nomogram possessed a reliable predictive ability for 1-year and 3-year survival, with AUC values of 0.703 and 0.751, respectively, and an AUC of 0.721 for 5-year survival (Figure 3B).
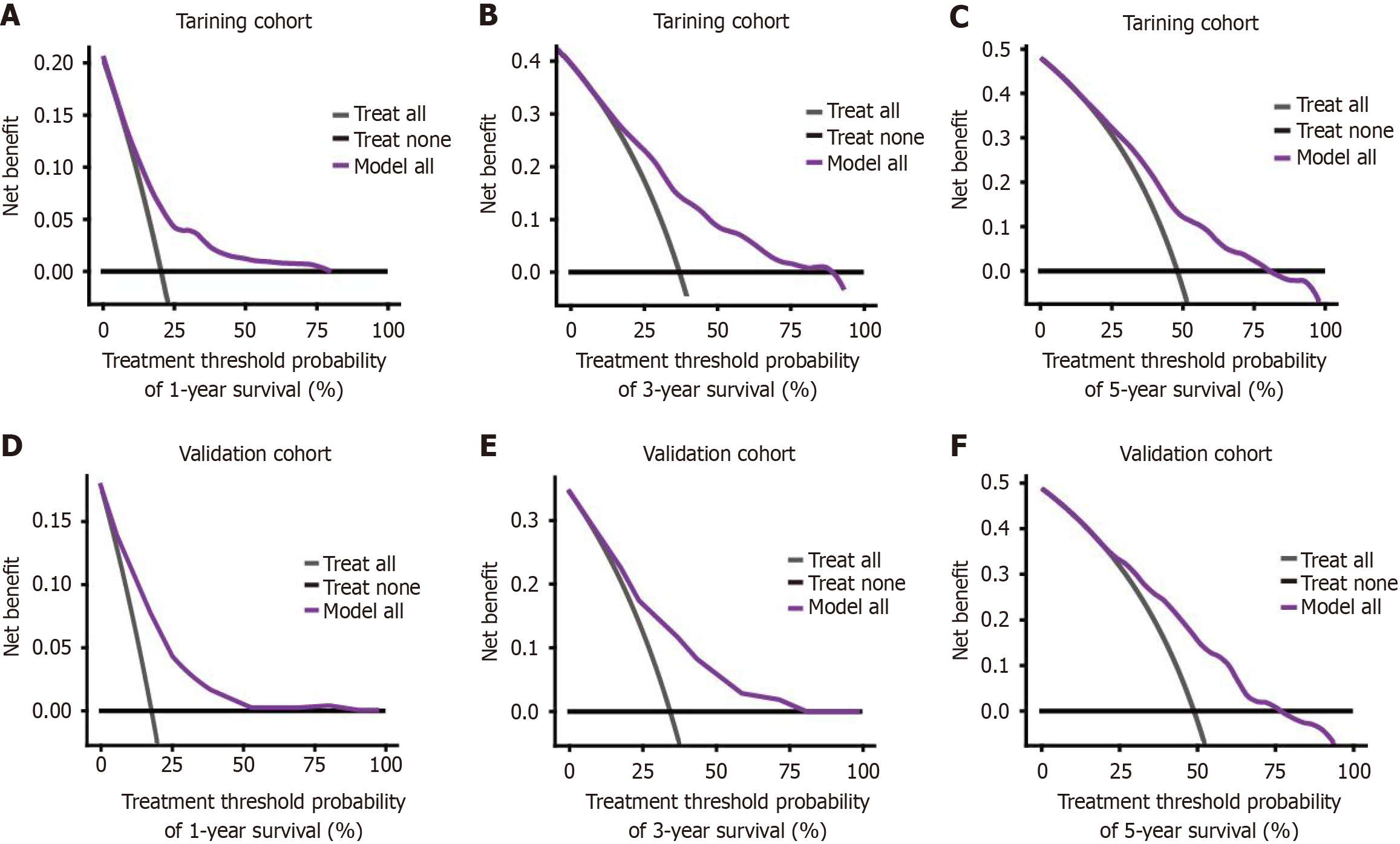
Logistic regression analysis in conjunction with DCA was conducted to assess the clinical utility of the proposed nomogram. The DCA results indicated that the nomogram exhibited a broader threshold probability range while enhancing net benefits, thereby offering robust support for the prediction of OS in CRLM within the training cohort. A comparison of the predicted 1-year, 3-year, and 5-year survival rates with the actual survival outcomes demonstrated the effectiveness of the nomogram in clinical practice, a validation further corroborated by the DCA curves for both the training and validation groups (Figure 4). These findings suggest that the nomogram is capable of providing accurate OS predictions, indicating its potential value for clinical application.
In addition to the training and internal validation cohorts, an external validation using a cohort of 97 patients from Chengdu Medical College Second Affiliated Hospital was conducted. The baseline characteristics of these patients were statistically comparable to those of the combined internal cohorts (training and internal validation), ensuring the generalizability of our findings (Supplementary Table 3). An analysis that included the construction of ROC curves and DCA to evaluate the predictive accuracy and clinical utility of the nomogram was performed.
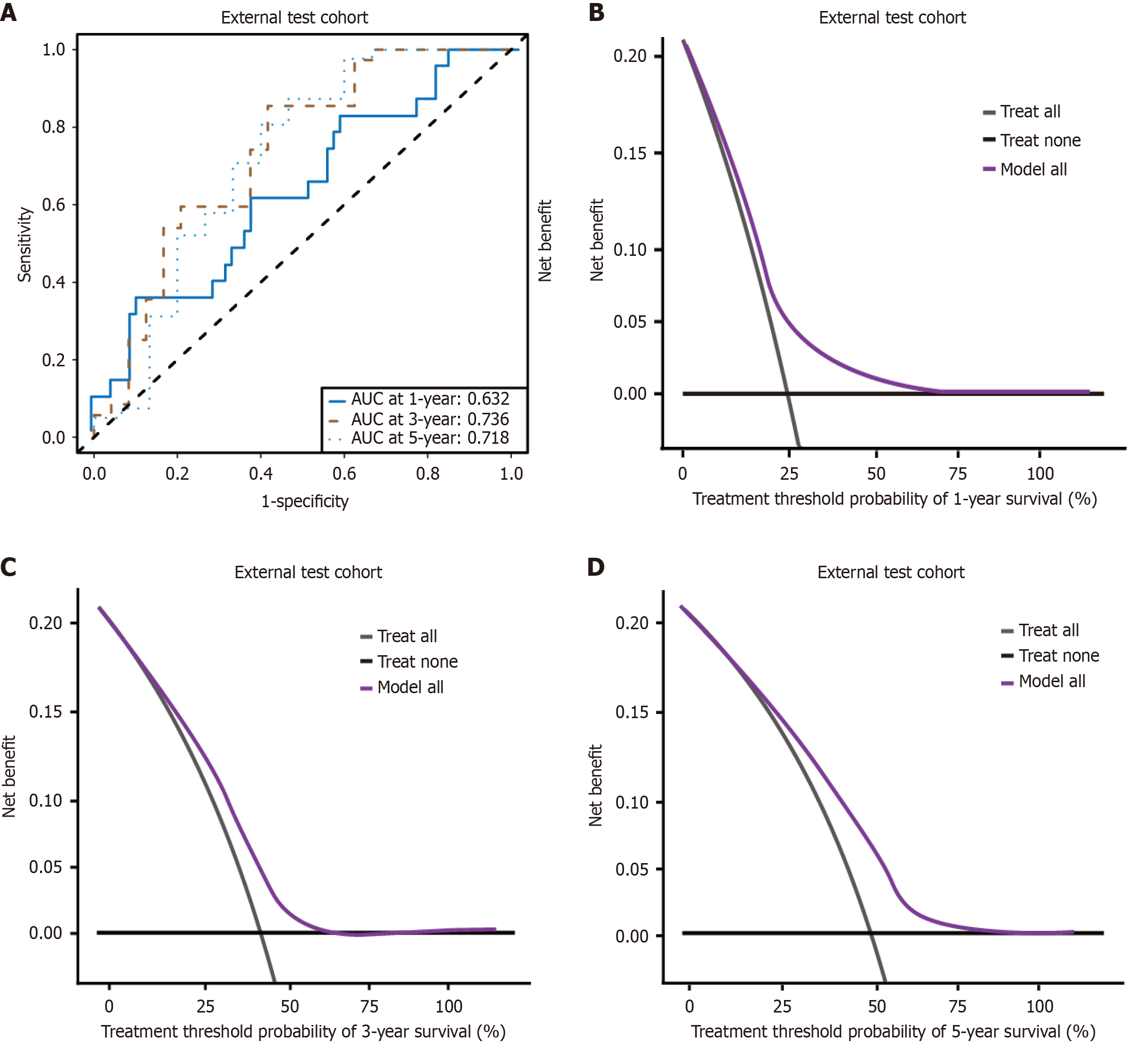
The ROC curves for the external test cohort demonstrated robust predictive performance, with AUC values of 0.632, 0.738, and 0.718 for 1-year, 3-year, and 5-year survival rates, respectively (Figure 5A). These AUC values indicate satisfactory discriminative ability of the model in an independent patient population. Furthermore, the DCA results for the external test cohort provided evidence of the clinical utility of the established nomogram (Figure 5B-D).
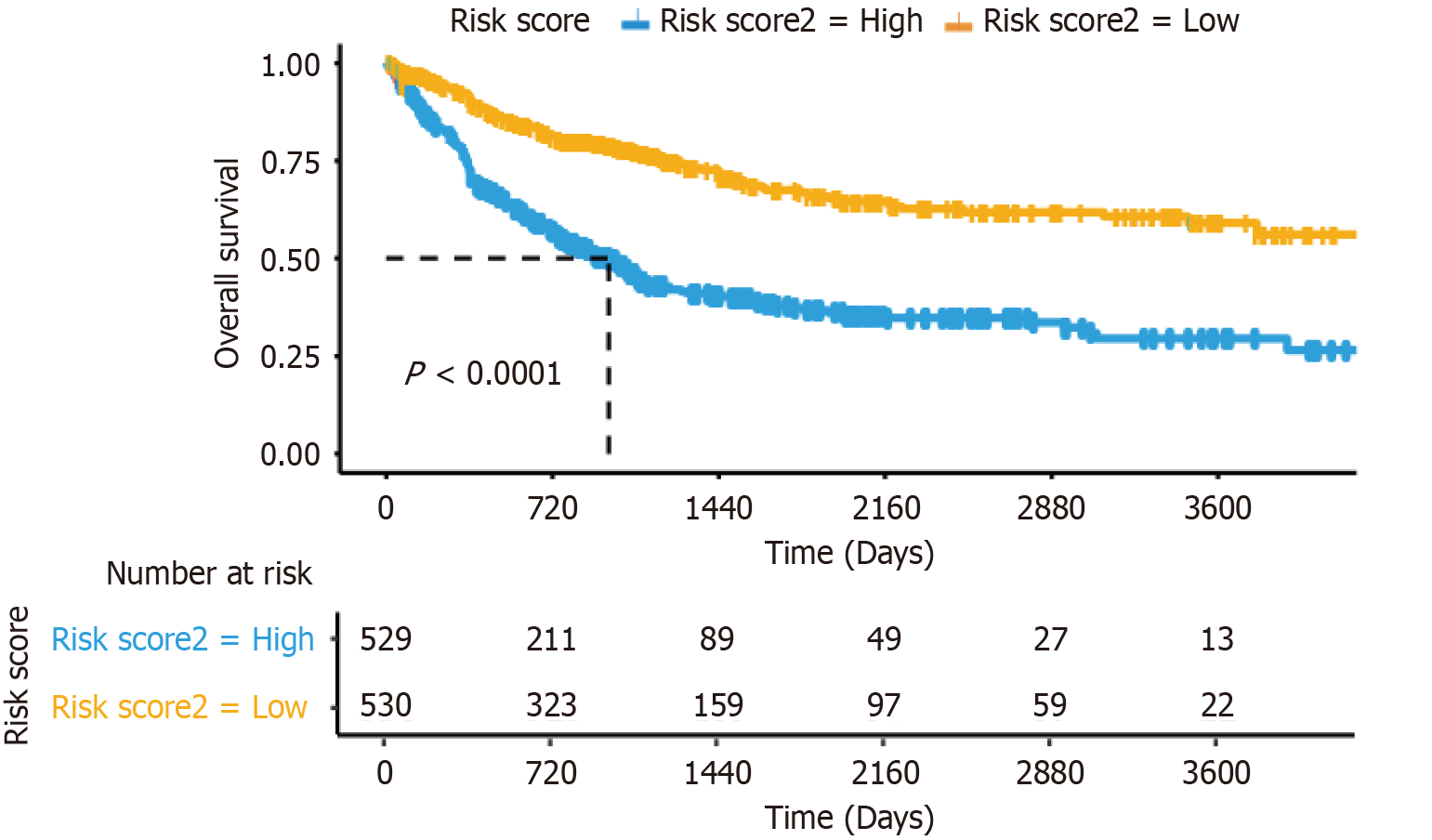
The survival prediction nomogram exhibited a high degree of accuracy in predicting outcomes. Utilizing the five parameters incorporated into the model, patients were categorized into low-risk and high-risk groups based on a median risk score, which facilitated their prognostic stratification. Kaplan-Meier survival analysis demonstrated a notable disparity in survival rates between the low-risk group, consisting of 530 patients, and the high-risk group, comprising 529 patients (P < 0.001; Figure 6). These findings underscore the utility of a risk stratification system based on liver metastasis for accurately predicting patient outcomes.
Despite the implementation of global CRC screening programs, mortality rates due to CRC continue to be unacceptably high. The development of metastases is a critical factor influencing patient survival. The liver, particularly due to its anatomical relationship with the portal venous system, is the most vulnerable site for metastasis in CRC, with approximately 25% to 30% of patients experiencing liver metastases[18,19]. The late stage at which patients often present for treatment, coupled with the unique characteristics of liver recurrence, frequently precludes the possibility of complete resection in CRLM[17]. CRLM significantly detract from patient quality of life and prognosis, necessitating an urgent exploration of novel therapeutic strategies and the enhancement of prognostic evaluation methods.
Nomogram models are now widely used in clinical medicine, particularly for CRC[20,21]. In an effort to combat the high incidence and fatality rates of CRC, numerous prognostic models have been developed to forecast outcomes post-diagnosis[22-24], several of which pertain to the risk of liver metastases[25-27]. Ding et al[28] used a nomogram to identify risk factors for liver metastasis in colorectal neuroendocrine tumors. Mo et al[29] employed a nomogram to pinpoint risk factors for liver metastasis in patients with colorectal neuroendocrine tumors. Tang et al[30] used data from the SEER database to create a nomogram predicting synchronous liver metastases in CRC, and identified several associated risk factors.
The present study aimed to identify prognostic risk factors in patients with CRLM and construct predictive models for different subsets with a view to providing valuable insights for clinical prognostic assessment. LASSO-Cox regression analysis was employed to examine the following five variables: GGT, chloride, APTT, N-stage, and vascular invasion, which exhibited a significant correlation with OS and emerged as independent prognostic factors for forecasting 1-year, 3-year, and 5-year survival outcomes.
It is worth noting that the hazard ratio for chloride suggested that lower chloride levels predict worse survival outcomes in patients with metastatic CRC. This finding initially appears to contradict the expected electrolyte imbalances typically associated with metastatic disease. However, recent studies have highlighted the potential role of chloride transport proteins in tumor progression. Specifically, the downregulation of SLC26A3, a chloride transporter, has been reported in metastatic CRC[31,32]. This down-regulation is associated with tumor progression and may contribute to altered chloride homeostasis, which could in turn affect cellular functions and survival outcomes. Our findings may reflect the impact of such molecular changes on patient outcomes, suggesting that chloride levels could serve as a biomarker for disease progression in this context. Further research is needed to elucidate the precise mechanisms underlying these associations and to explore the potential therapeutic implications.
Model validation revealed that the AUC for all variables was approximately 0.7 or higher, suggesting high predictability and accuracy. The ROC curves for individual prognostic factors were inferior to that of the composite model, indicating strong predictive capability. Moreover, DCA has been widely recognized, demonstrating its usefulness and value in clinical decision-making[33]. Consequently, DCA was performed, and the findings indicated that the predictive model offered superior clinical utility compared to isolated risk factors. Utilizing the risk score derived from the model, the patient cohort was divided into low-risk and high-risk categories. Kaplan-Meier survival analysis revealed a superior prognosis for the low-risk group relative to the high-risk group, corroborating the clinical relevance of the model.
The prognostic factors identified are already commonly accessible in clinical settings, and can promote the straightforward implementation of our model. Nonetheless, several limitations in this study should be acknowledged. Firstly, the results of this study have not yet been validated in patients from other countries. In subsequent research, further validation will be conducted in other countries. Moreover, in an era characterized by personalized medicine, a more holistic approach that incorporates comprehensive clinical attributes and multiple biomarkers may yield improved prognostic insights. The advent of innovative treatment modalities, such as immunotherapy and novel chemotherapy agents, has altered patient outcomes, emphasizing the necessity for periodic updating of predictive models in ongoing research.
We constructed and validated a prediction model for 1-year, 3-year, and 5-year OS in patients with CRLM. The model exhibits robust discriminative ability and high precision, assisting in the evaluation of hepatic metastasis risk in CRC patients. It offers critical insights for clinical treatment planning.
We are thankful to Air Force Military Medical University First Affiliation Xijing Digestive Hospital (Shaanxi Province, China) for supporting this research.
| 1. | GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:627-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 449] [Article Influence: 112.3] [Reference Citation Analysis (1)] |
| 2. | Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109:djw322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 876] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 3. | Stewart CL, Warner S, Ito K, Raoof M, Wu GX, Kessler J, Kim JY, Fong Y. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr Probl Surg. 2018;55:330-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 4. | Wu L, Fu J, Chen Y, Wang L, Zheng S. Early T Stage Is Associated With Poor Prognosis in Patients With Metastatic Liver Colorectal Cancer. Front Oncol. 2020;10:716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3360] [Article Influence: 560.0] [Reference Citation Analysis (2)] |
| 6. | Giannis D, Sideris G, Kakos CD, Katsaros I, Ziogas IA. The role of liver transplantation for colorectal liver metastases: A systematic review and pooled analysis. Transplant Rev (Orlando). 2020;34:100570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Taniai N, Akimaru K, Yoshida H, Tajiri T. Surgical treatment for better prognosis of patients with liver metastases from colorectal cancer. Hepatogastroenterology. 2007;54:1805-1809. [PubMed] |
| 8. | Qin S, Liu GJ, Huang M, Huang J, Luo Y, Wen Y, Wang Y, Chen L. The local efficacy and influencing factors of ultrasound-guided percutaneous microwave ablation in colorectal liver metastases: a review of a 4-year experience at a single center. Int J Hyperthermia. 2019;36:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, Pulitanò C, Gavazzi F, Ferla G, Di Carlo V, Staudacher C. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Vibert E, Canedo L, Adam R. Strategies to treat primary unresectable colorectal liver metastases. Semin Oncol. 2005;32:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, Park JN, Han JY, Kang JH, Lee KS, Cho JY. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15:1344-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 617] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 13. | Norén A, Sandström P, Gunnarsdottir K, Ardnor B, Isaksson B, Lindell G, Rizell M. Identification of Inequalities in the Selection of Liver Surgery for Colorectal Liver Metastases in Sweden. Scand J Surg. 2018;107:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K, Psaltopoulou T, Wang J, Buettner S, Papalois ΑE, He J, Wolfgang CL, Pawlik TM, Weiss MJ. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases: A Systematic Review and Meta-analysis. Ann Surg. 2018;267:1047-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Giuliante F, Ardito F, Vellone M, Ranucci G, Federico B, Giovannini I, Nuzzo G. Role of the surgeon as a variable in long-term survival after liver resection for colorectal metastases. J Surg Oncol. 2009;100:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Yang AD, Brouquet A, Vauthey JN. Extending limits of resection for metastatic colorectal cancer: risk benefit ratio. J Surg Oncol. 2010;102:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Adam R, Vinet E. Regional treatment of metastasis: surgery of colorectal liver metastases. Ann Oncol. 2004;15 Suppl 4:iv103-iv106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 1049] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 19. | Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 20. | Jiang T, Liu S, Wu X, Liu X, Li W, Yang S, Cai P, Xi S, Zeng Z, Gao Y, Chen G, Xiao W. Nomogram to Predict Distant Metastasis Probability for Pathological Complete Response Rectal Cancer Patients After Neoadjuvant Chemoradiotherapy. Cancer Manag Res. 2021;13:4751-4761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Zhou C, Liu HS, Liu XH, Zheng XB, Hu T, Liang ZX, He XW, He XS, Hu JC, Wu XJ, Wu XR, Lan P. Preoperative assessment of lymph node metastasis in clinically node-negative rectal cancer patients based on a nomogram consisting of five clinical factors. Ann Transl Med. 2019;7:543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Liu J, Huang X, Yang W, Li C, Li Z, Zhang C, Chen S, Wu G, Xie W, Wei C, Tian C, Huang L, Jeen F, Mo X, Tang W. Nomogram for predicting overall survival in stage II-III colorectal cancer. Cancer Med. 2020;9:2363-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Kim C, Kim WR, Kim KY, Chon HJ, Beom SH, Kim H, Jung M, Shin SJ, Kim NK, Ahn JB. Predictive Nomogram for Recurrence of Stage I Colorectal Cancer After Curative Resection. Clin Colorectal Cancer. 2018;17:e513-e518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Borumandnia N, Doosti H, Jalali A, Khodakarim S, Charati JY, Pourhoseingholi MA, Talebi A, Agah S. Nomogram to Predict the Overall Survival of Colorectal Cancer Patients: A Multicenter National Study. Int J Environ Res Public Health. 2021;18:7734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Meng Q, Zheng N, Wen R, Sui J, Zhang W. Preoperative nomogram to predict survival following colorectal cancer liver metastasis simultaneous resection. J Gastrointest Oncol. 2021;12:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Liu Z, Xu Y, Xu G, Baklaushev VP, Chekhonin VP, Peltzer K, Ma W, Wang X, Wang G, Zhang C. Nomogram for predicting overall survival in colorectal cancer with distant metastasis. BMC Gastroenterol. 2021;21:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Dai S, Ye Y, Kong X, Li J, Ding K. A predictive model for early recurrence of colorectal-cancer liver metastases based on clinical parameters. Gastroenterol Rep (Oxf). 2021;9:241-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Ding X, Tian S, Hu J, Wang G, Yu X, Fu D, Yuan Y, Wang L, Wang Z. Risk and prognostic nomograms for colorectal neuroendocrine neoplasm with liver metastasis: a population-based study. Int J Colorectal Dis. 2021;36:1915-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Mo S, Cai X, Zhou Z, Li Y, Hu X, Ma X, Zhang L, Cai S, Peng J. Nomograms for predicting specific distant metastatic sites and overall survival of colorectal cancer patients: A large population-based real-world study. Clin Transl Med. 2020;10:169-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Tang M, Wang H, Cao Y, Zeng Z, Shan X, Wang L. Nomogram for predicting occurrence and prognosis of liver metastasis in colorectal cancer: a population-based study. Int J Colorectal Dis. 2021;36:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 31. | Hosseini ST, Nemati F. Identification of GUCA2A and COL3A1 as prognostic biomarkers in colorectal cancer by integrating analysis of RNA-Seq data and qRT-PCR validation. Sci Rep. 2023;13:17086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Lin C, Lin P, Lin H, Yao H, Liu S, He R, Chen H, Teng Z, Hoffman RM, Ye J, Zhu G. SLC26A3/NHERF2-IκB/NFκB/p65 feedback loop suppresses tumorigenesis and metastasis in colorectal cancer. Oncogenesis. 2023;12:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Van Calster B, Wynants L, Verbeek JFM, Verbakel JY, Christodoulou E, Vickers AJ, Roobol MJ, Steyerberg EW. Reporting and Interpreting Decision Curve Analysis: A Guide for Investigators. Eur Urol. 2018;74:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 873] [Article Influence: 109.1] [Reference Citation Analysis (2)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/