Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.110667
Revised: August 7, 2025
Accepted: August 26, 2025
Published online: October 15, 2025
Processing time: 97 Days and 23.9 Hours
Implementing nursing interventions in patients undergoing endoscopic treatment for intestinal polyps and early stage cancer can serve as a reference for reducing the incidence of complications, accelerating the recovery process, and improving the quality of life.
To impact of systematic nursing intervention on recovery, complications prevention, and quality of life after endoscopic surgery for intestinal polyps.
This retrospective study included 157 patients who underwent endoscopic mucosal resection or endoscopic submucosal dissection at our hospital. The patients were divided into intervention and conventional groups, with no significant differences in age, sex, or surgical methods. The intervention group received multidimensional nursing interventions, including preoperative evaluation, intraoperative cooperation, postoperative rehabilitation, psychological support and nutritional management. The conventional group received st
On the 7th day after surgery, C-reactive protein (CRP) and white blood cell levels were lower in the intervention group than in the conventional group. Complications occurred in 9.33% of the patients in the intervention group and 23.17% in the conventional group, with significant differences in fever and abdominal distension. The intervention group had shorter first exhaust and hospitalization durations than the control group. By day 3 post-surgery, the intervention group showed lower VAS scores and reduced anxiety and depression. High-risk factors included diabetes [relative risk (RR) = 2.43, 95%CI: 1.21-4.86], laparotomy (RR = 2.86, 95%CI: 1.22-6.71), CRP > 15 mg/L (RR = 3.12, 95%CI: 1.54-6.33), and procalcitonin > 0.5 ng/mL 1 day after surgery (RR = 2.91. 95%CI: 1.31-6.44), while systematic nursing interventions (OR = 0.40, 95%CI: 0.18-0.89) reduced the complication risk by 60%.
Multidimensional nursing interventions have clinical value in endoscopic treatment of intestinal polyps and early stage cancer, reducing complications and hospital stay. This study provides a basis for establishing patient-centered guidelines.
Core Tip: This retrospective study highlights the significant role of systematic nursing interventions in improving clinical outcomes in patients undergoing endoscopic treatment for intestinal polyps and early stage cancer. By incorporating preoperative assessment, intraoperative cooperation, postoperative rehabilitation, psychological support, and nutritional guidance, the intervention group showed reduced inflammatory markers, lower complication rates, faster recovery, and less postoperative pain than the conventional care group. These findings support the establishment of patient-centered, evidence-based nursing pathways to enhance recovery quality and safety, and provide valuable reference data for future clinical nursing practices and guideline development.
- Citation: Zhuang YY, Chen HY, Zhang JR, Lai SL, Zheng YY, Huang YD. Clinical outcomes of nursing interventions after endoscopic treatment for intestinal polyps and early-stage cancer. World J Gastrointest Oncol 2025; 17(10): 110667
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/110667.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.110667
With the widespread adoption of endoscopic techniques, such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), minimally invasive treatment of intestinal polyps and early stage colorectal cancer has achieved significant clinical success, offering high resection rates and low complication risks. Intestinal polyps are recognized as precancerous lesions, and early intervention is crucial to prevent their progression to malignancy. However, current studies have primarily focused on surgical techniques, pathological features, and technical parameters, whereas systematic research on postoperative nursing and interventions remains limited[1]. Most existing nursing studies only address general postoperative complication management and lack quantitative assessments of intervention efficacy and standardized care models[2]. Additionally, many studies suffer from small sample sizes, short follow-up durations, and inconsistent nursing protocols across institutions, limiting the comparability and generalizability of their findings[3-5]. Although ERAS-based concepts, such as psychological support, dietary guidance, and physical rehabilitation, have been preliminarily introduced into digestive endoscopy, there is still no systematic framework linking specific nursing strategies with treatment modalities or assessing their impact on long-term outcomes[6-8]. Moreover, patient-centered evaluation systems are rarely established, and comprehensive multi-index efficacy evaluations are lacking[9].
This study innovatively integrated retrospective clinical data from patients who underwent endoscopic treatment for intestinal polyps and early cancer to evaluate the impact of multilevel nursing interventions across the preoperative, intraoperative, and postoperative stages. The nursing model incorporates psychological counseling, nutritional support, and functional rehabilitation to create a closed-loop intervention pathway. Both objective indicators (complication rates, length of stay, and recurrence) and subjective measures (patient satisfaction and quality of life) were used to enhance the robustness and clinical relevance of the evaluation. By standardizing the inclusion criteria and nursing documentation, this study improved data consistency and comparability, laying a foundation for future multicenter research and the development of evidence-based, standardized nursing protocols.
First, this was a retrospective study. A total of 157 patients who underwent endoscopic treatment for intestinal polyps or early cancerous changes at the Gastrointestinal Endoscopy Center of our hospital between January 2024 and February 2025 were included in this study. They were divided into intervention and conventional groups according to whether they received a systematic nursing intervention. There were 75 and 82 patients in the intervention and conventional groups, respectively. The inclusion criteria were as follows: (1) Diagnosis of gastric or colorectal adenomatous polyps or early carcinogenesis in the mucosal layer confirmed through endoscopy and pathology; (2) Patients who underwent EMR or ESD, and no distant metastasis found on preoperative imaging examination; (3) Aged 18-75 years with complete clinical data; and (4) The follow-up time after surgery should not be less than 3 months. The exclusion criteria were as follows: (1) Patients with acute and critical diseases, such as active gastrointestinal bleeding and perforation (n = 6); (2) Patients with severe cardiovascular and cerebrovascular diseases or liver and kidney dysfunction (n = 9); (3) Patients with a previous history of malignant tumors of the digestive tract or other systems (n = 4); (4) Patients who underwent conversion to laparotomy or incomplete resection during endoscopic surgery (n = 3); and (5) Patients with incomplete nursing records or follow-up data (n = 5). Finally, 157 patients were included in this study. The basic data are provided in Table 1. No significant differences were observed in age, sex, lesion type, or surgical method between the two groups (P > 0.05), indicating that they were comparable.
| Indicators | Conventional group (n = 82) | Intervention group (n = 75) | χ2/t | P value |
| Age (years) | 54.62 ± 10.71 | 53.84 ± 11.23 | 0.454 | 0.651 |
| Number of males | 46 (56.10%) | 40 (53.33%) | 0.137 | 0.711 |
| Lesion type | Polyps: 59; Early cancer: 23 | Polyps: 54; Early cancer: 21 | 0.022 | 0.883 |
| Surgical method | EMR: 50; ESD: 32 | EMR: 46; ESD: 29 | 0.043 | 0.836 |
Patients in the conventional group underwent standard endoscopic treatment without systematic nursing intervention. All patients were routinely subjected to intestinal endoscopy before surgery and were treated by senior qualified endoscopists after confirmation of the diagnosis. During surgery, either EMR or ESD was selected based on the lesion type. A CF-HQ290I colonoscope (Olympus, Tokyo, Japan) combined with an EVIS LUCERA ELITE CV-290 host system was used for the endoscopy. The preoperative local injection was a mixture of normal saline and a small amount of 1:100000 epinephrine (2-5 mL per injection); in some cases, a dye (0.4% indigo carmine) was added according to the submucosal development of the lesion. A thermal trap (Olympus SD-230U-25) was used to remove the lesions. During ESD, an IT Knife 2 mucosal dissection knife (KD-611 L, Olympus) was used for complete dissection, and a disposable, rotatable, and repetitive soft tissue clamp was used for closure. After surgery, 10 mL of lactulose oral solution (Dankang) was administered orally twice a day, 10 mL of Kangfuxin solution (Jingxin) was given orally three times a day, and three tablets of Bifidobacterium quadrivalent active bacteria tablets (Siliankang) were administered orally three times a day for a treatment period of 597 days. Patients at high risk for co-infection may be prophylactically treated with cefuroxime sodium (Unacon Sinopharm, 1.5 g intravenous infusion, twice a day) for 3 days postoperatively. Further surgical treatment or follow-up review was performed based on the pathological results. The patients were observed in the general ward for 24-48 hours after surgery without special nursing intervention, except for routine vital sign monitoring and dietary guidance.
The intervention group received the same endoscopic treatment, equipment, and postoperative drug regimen as the conventional group, and a nursing intervention scheme was implemented before and after surgery. The treatment scheme is described in Section Therapeutic methods of this study, and the nursing intervention details are shown in Section Nursing intervention methods.
All patients in the intervention group underwent a detailed evaluation before surgery and signed an informed consent form. The endoscopic nurse and attending physician developed individualized surgical strategies. During surgery, the device configuration, injection method, resection technique, and hemostatic method were consistent with those used in the conventional group. All surgeries were performed in a painless state. The sedative drug was propofol (Guorui Pharmaceutical, Guoyao Group, 1-2 mg/kg, single intravenous injection before surgery) supplemented with fentanyl when necessary (Yichang Renfu Pharmaceutical, 50 µg static push before surgery). All patients in the intervention group were admitted to a dedicated digestive endoscopy ward after surgery and followed the standard nursing path. They underwent daily endoscopic ward rounds within 48 hours after surgery, were closely monitored for complications such as hemorrhage and perforation, and underwent phased dietary progression according to patient tolerance.
Conventional group: Patients in the conventional group received standard perioperative care and basic health education before surgery. This included general instructions, such as fasting for 8 hours and refraining from water intake for 6 hours before the surgery. Preoperative bowel preparation was performed with assistance, involving a cleansing enema using 2-3 bottles of Kesselu via rectal infusion. Individualized psychological or behavioral counseling was not provided to the participants. During surgery, the nursing staff cooperated with the physicians to perform routine monitoring of vital signs, including blood pressure, pulse rate, and oxygen saturation. Postoperative dietary guidance followed the physician’s instructions, involving fasting for the first 6 hours, followed by a gradual transition to a liquid diet. While daily observations for complications were conducted, no proactive psychological support, dietary optimization, or discharge education was implemented.
Intervention group: Based on standard care, the intervention group received a comprehensive stage-specific nursing protocol that spanned the preoperative, intraoperative, and postoperative periods. This study aimed to enhance patient compliance, reduce postoperative complications and promote recovery.
Upon hospital admission, nursing staff initiated structured preoperative education. The pathogenesis, treatment approach, and postoperative precautions for intestinal polyps and early stage cancer were explained using illustrated brochures and videos. For patients with limited health literacy, one-on-one counseling in layman’s terms was provided, with particular emphasis on fasting (8 hours) and water deprivation (6 hours) requirements to improve understanding and compliance.
The patients were guided to complete preoperative bowel cleansing. Those with intestinal disease took a prepared bowel-clearing solution made from two packets of compound polyethylene glycol electrolyte powder mixed with four bottles of water per dose and were administered in two divided doses. Vital signs and coagulation function were evaluated, and nurses assisted physicians in formulating individualized anesthesia plans.
Intraoperatively, the nursing team supported the procedure by preparing instruments and injections, collecting specimens, and continuously monitoring vital signs, particularly heart rate, blood pressure, and oxygen saturation, to prevent hypoxemia and sedation-related complications. Intraoperative verbal reassurance was provided to patients with anxiety to reduce emotional stress.
Postoperatively, the vital signs were closely monitored. On the first postoperative day, blood pressure, pulse rate, and temperature were recorded every 2 hours. Nurses observed signs of complications such as melena, abdominal pain, and hematemesis. Patients were encouraged to begin moderate ambulation 24 hours after surgery to stimulate gastrointestinal motility and to alleviate flatulence.
Dietary management followed a phased approach: Patients fasted for 6 hours postoperatively, followed by the consumption of milk or rice soup for 6-12 hours to prevent venous thrombosis. After 24 hours, the patients were transitioned to a liquid or semiliquid diet tailored to the lesion site and surgical method.
Psychological care was integrated throughout the hospital stay. The nursing staff regularly assessed the patients' emotional status, encouraged the expression of anxiety or fear, and referred patients to the psychological department when needed. A follow-up instruction file was created after the discharge of the patients. Patients were advised to review wound healing and pathology results on postoperative day 7 and to undergo follow-up endoscopy within 1-3 months after surgery. Standardized guidelines for medication adherence, dietary regulations, and recurrence prevention were provided.
Detection of inflammation and infection indices: Four milliliters of fasting venous blood was drawn within 24 hours before surgery and on the 1st, 3rd, and 7th days after surgery and placed in EDTA anticoagulation tubes for timely submission. The test items included C-reactive protein (CRP) level, white blood cell (WBC) count, neutrophil percentage (NEU%), and procalcitonin (PCT). CRP levels were detected using immunoturbidimetry with a BS-800M automatic biochemical analyzer (Shenzhen Meirui Biological Medical Electronics Co., Ltd.). Blood cell analysis was performed using a Hessenmeikang XN-1000 All-automated hematology analyzer (Sysmex Co., Ltd., Japan). PCT levels were measured using a Roche Cobas e411 Electrochemiluminescence Analyzer (Roche Diagnostics, Germany). All blood samples were tested within 2 hours of their collection. The data were entered into the database by a professional test technician, and abnormal indicators were checked and confirmed by the Clinical Laboratory Department for the second time.
Complication monitoring: Within 7 days after surgery, the responsible nurse will make a visit twice a day in the morning and evening to closely observe the patient’s body temperature, abdominal pain, abdominal distension, bloody stools, and other symptoms. If the patient has a suspected perforation or persistent abdominal pain, an abdominal computed tomography (CT) examination (NeuViz ACE 64-slice spiral CT scanner) will be performed immediately, and the presence of complications (such as hemorrhage, perforation, and infection) will be comprehensively assessed based on the clinical manifestations. All complications were recorded in the postoperative complication register and reviewed by two clinicians.
Detection of rehabilitation process indicators: Every patient after surgery was observed and recorded by a dedicated nurse, including first exhaust time: The nurse evaluated every 6 hours and asked the patient or confirmed by auscultation beside the bed; first time to get out of bed; the patient's ability to move independently was assessed every day, and patients had to be accompanied by a nurse and registered for the first time when getting out of bed; hospitalization time: The number of days from admission to the meeting of discharge criteria, which was automatically generated and exported by the medical record system.
Pain assessment: Pain assessments were performed by the nursing staff using a visual analog scale (VAS) before 8:00 a.m. on the 1st and 3rd days postoperatively. During the evaluation, the patient was presented with a 10 cm ruler scale and asked to subjectively mark the current degree of pain. The assessment results were recorded in the nursing record system and reviewed by another caregiver.
Inflammation and infection indices: The levels of CRP, WBC count, NEU%, and PCT were measured preoperatively and on days 1, 3, and 7 postoperatively. The reference values were as follows: CRP: < 8 mg/L; WBC: 4.0-10.0 × 109/L; NEU%: 40%-75%; PCT: < 0.05 ng/mL was normal, > 0.5 ng/mL indicated infection.
Complication incidence: Complications such as hemorrhage, perforation, fever, abdominal distension, and infection were observed within 7 days postoperatively. The incidence of various complications was noted.
Rehabilitation indicators: The first exhaust time, first time to get out of bed, and hospitalization duration were recorded. The first exhaust time was normal within 24 hours after surgery; the first time to get out of bed within 12 hours after surgery was optimal; hospitalization time less than 5 days after surgery was considered optimal, and > 7 days was considered as an extension.
Pain score: The VAS was used to assess pain on the 1st and 3rd days after surgery. The VAS scores were as follows: 0 as painless, 1-3 as mild, 4-6 as moderate and 7-10 as severe.
Psychological status: The Hospital Anxiety and Depression Scale (HADS) was used to evaluate the patients’ psychological condition preoperatively and on postoperative days 3 and 7. The HADS consists of 14 items, including 7 for anxiety (HADS-A) and 7 for depression (HADS-D), with each item scored from 0 to 3. The total score for each subscale ranged from 0 to 21. Scores of 0-7 indicate no anxiety or depression, 8-10 suggest borderline abnormality, and ≥ 11 indicate clinically significant anxiety or depression. This scale helps assess the emotional impact of surgery and the effectiveness of perioperative psychological interventions.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0. Measurement data were expressed as mean ± SD, enumeration data were expressed as frequency and percentage, and intra-group comparisons were performed using t-test and χ2 test. An independent sample t-test or analysis of variance was used to compare the groups. Non-normally distributed data were expressed as medians (quartiles) [M (P25, P75)], and intergroup comparisons were made using the Mann-Whitney U test or Kruskal-Wallis H test. Postoperative efficacy was mainly determined by the patient's postoperative inflammation and infection control status (CRP, WBC, NEU%, and PCT levels), incidence of complications, rehabilitation process (first exhaust time, ambulation, and length of hospital stay), and pain score (VAS). The relationship between inflammatory markers (CRP, WBC, NEU%, and PCT), postoperative clinical efficacy, and complication rate was analyzed using Spearman’s rank correlation analysis, and the correlation coefficient (R) and P value were calculated to evaluate the correlation between non-parametric variables. For risk factor analysis, the relative risk (RR), OR, and 95%CI were calculated using the contingency table method. RR is mainly used to compare the outcomes of the exposed and non-exposed groups in prospective studies, and OR is used to determine the degree of influence of the variables on outcome events in case–control studies or regression models. The relationship between variables, such as basic disease, surgical method, and inflammation index, and the occurrence of postoperative complications was analyzed by grouping to assess whether they were high-risk factors. Statistical significance was evaluated based on P < 0.05, values of RR or OR were kept to two decimal places, and the 95%CI was accurate to two decimal places. All statistical tests were performed bilaterally, and P < 0.05 indicated statistical significance.
No statistically significant differences were observed in the levels of CRP, WBC count, NEU%, and PCT between the two groups before surgery and on the 1st day after surgery (P > 0.05). On the 3rd and 7th day after surgery, the above indicators in the intervention group were significantly lower than those in the control group, and the difference was statistically significant (P ≤ 0.001), indicating that systematic nursing intervention can help alleviate postoperative inflammatory reactions (Table 2).
| Time point | Group | CRP (mg/L) | t | P value | WBC (× 109/L) | t | P value | NEU% (%) | t | P value | PCT (ng/mL) | t | P value |
| Preoperative | Intervention (n = 75) | 6.91 ± 1.58 | 0.731 | 0.466 | 7.56 ± 1.08 | 0.947 | 0.345 | 65.27 ± 6.83 | 1.182 | 0.239 | 0.046 ± 0.012 | 0.531 | 0.596 |
| Conventional (n = 82) | 7.04 ± 1.66 | 7.41 ± 1.21 | 64.12 ± 7.04 | 0.048 ± 0.011 | |||||||||
| 1 day after surgery | Intervention | 16.72 ± 3.14 | 1.265 | 0.208 | 11.42 ± 1.84 | 1.312 | 0.192 | 81.03 ± 5.32 | 1.354 | 0.178 | 0.189 ± 0.041 | 1.261 | 0.209 |
| Conventional | 17.21 ± 3.64 | 12.14 ± 2.04 | 83.16 ± 5.83 | 0.216 ± 0.052 | |||||||||
| 3 days after surgery | Intervention | 10.43 ± 2.01 | 3.263 | 0.001 | 8.62 ± 1.32 | 3.785 | < 0.001 | 71.54 ± 6.03 | 3.254 | 0.001 | 0.089 ± 0.024 | 4.192 | < 0.001 |
| Conventional | 12.17 ± 2.28 | 9.56 ± 1.57 | 75.21 ± 6.76 | 0.126 ± 0.035 | |||||||||
| 7 days after surgery | Intervention | 6.94 ± 1.34 | 4.675 | < 0.001 | 6.85 ± 1.11 | 3.914 | < 0.001 | 63.12 ± 5.26 | 4.003 | < 0.001 | 0.047 ± 0.014 | 5.012 | < 0.001 |
| Conventional | 8.32 ± 1.75 | 7.84 ± 1.29 | 67.49 ± 5.87 | 0.063 ± 0.016 |
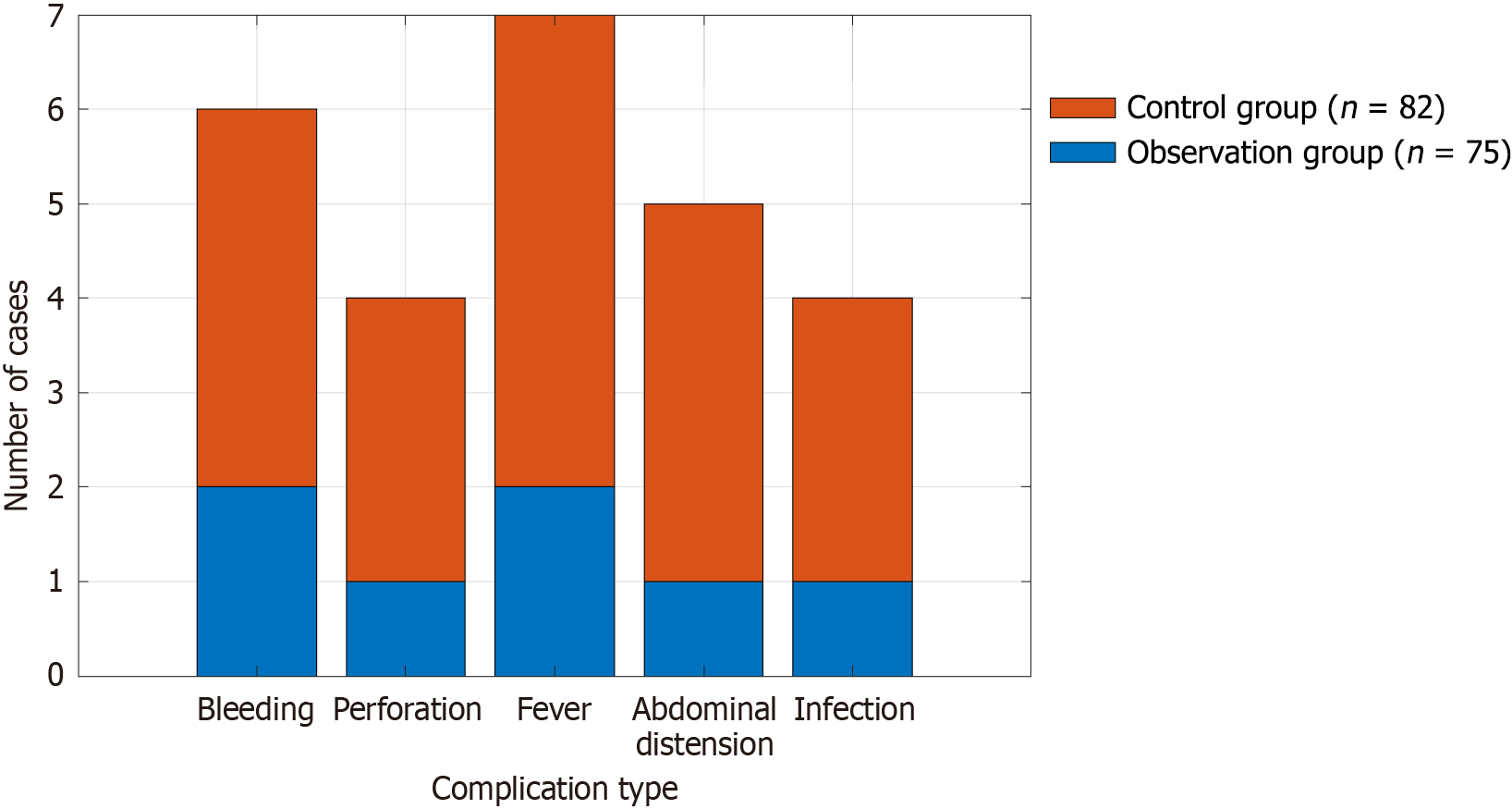
Within 7 days after surgery, the total complication incidence rate in the intervention group was 9.33%, which was significantly lower than that in the conventional group (23.17%) (P < 0.05). The difference between fever and abdominal distension was statistically significant (P < 0.05) (Table 3; Figure 1).
| Types | Time point | Intervention group (n = 75) | Conventional group (n = 82) | χ2/t | P value |
| Hemorrhage | 7 days after surgery | 2 (2.67) | 4 (4.88) | 0.545 | 0.46 |
| Perforation | 7 days after surgery | 1 (1.33) | 3 (3.66) | 3.065 | 0.08 |
| Fever | 7 days after surgery | 2 (2.67) | 5 (6.10) | 4.187 | 0.041 |
| Abdominal distention | 7 days after surgery | 1 (1.33) | 4 (4.88) | 3.982 | 0.046 |
| Infection | 7 days after surgery | 1 (1.33) | 3 (3.66) | 1.209 | 0.271 |
| Total | 7 (9.33) | 19 (23.17) | 5.598 | 0.018 |
The first exhaust time of the intervention group was 21.45 ± 2.56 hours, which was better than that of the control group (24.18 ± 2.91 hours). The excellent/normal rate was 76.00%, which was significantly higher than that in the control group (57.32%; P < 0.001). The first time of getting out of bed in the intervention group was 10.62 ± 1.74 hours, while that in the control group was 13.07 ± 2.10 hours. The excellence rate in the intervention group was 81.33%, which was significantly higher than that in the control group (61.00%; P < 0.001). The intervention group had a hospital stay of 4.72 ± 1.21 days, which was better than that of the control group (6.13 ± 1.44). The excellence rate was 69.33%, which was higher than that of the control group (46.34%; P < 0.001). These differences were significant (Table 4).
| Indicators | Group | Time value | Excellent/normal | Poor | χ2/t | P value |
| First exhaust time (hour) | Intervention group | 21.45 ± 2.56 | 57 (76.00) | 18 (24.00) | 6.089 | < 0.001 |
| Conventional group | 24.18 ± 2.91 | 47 (57.32) | 35 (42.68) | 6.095 | 0.014 | |
| First time to get out of bed (hour) | Intervention group | 10.62 ± 1.74 | 61 (81.33) | 14 (18.67) | 7.683 | < 0.001 |
| Conventional group | 13.07 ± 2.10 | 50 (61.00) | 32 (39.00) | 7.628 | 0.006 | |
| Hospitalization time (day) | Intervention group | 4.72 ± 1.21 | 52 (69.33) | 23 (30.67) | 6.522 | < 0.001 |
| Conventional group | 6.13 ± 1.44 | 38 (46.34) | 44 (53.66) | 7.933 | 0.005 |
The VAS scores of the intervention group were lower than those of the conventional group on the 1st and 3rd day. The proportion of patients with mild pain was higher, and that of patients with severe pain was significantly lower. The difference between the two groups was statistically significant (P < 0.05) (Table 5), suggesting that the analgesic effect of the intervention group was better than that of the conventional group.
| Time point | Group | VAS score | Mild | Moderate | Severe | χ2/t | P value |
| 1 day after surgery | Intervention group (n = 75) | 4.15 ± 0.94 | 29 (38.67) | 35 (46.67) | 11 (14.67) | 10.802 | 0.005 |
| Conventional (n = 82) | 5.03 ± 1.08 | 12 (14.63) | 46 (56.10) | 24 (29.27) | |||
| 3 days after surgery | Intervention group | 2.37 ± 0.73 | 57 (76.00) | 15 (20.00) | 3 (4.00) | 11.704 | 0.003 |
| Conventional group | 3.18 ± 0.92 | 43 (52.44) | 30 (36.59) | 9 (10.98) |
No statistically significant differences in HADS-A and HADS-D scores were observed between the two groups before surgery (P > 0.05). On the 3rd and 7th day after surgery, the anxiety and depression scores of the intervention group were significantly lower than those of the control group (both P < 0.001), and the difference was statistically significant, indicating that systematic nursing intervention can effectively improve postoperative psychological status (Table 6).
| Time Point | Group | HADS-A (mean ± SD) | χ2/t | P value | HADS-D (mean ± SD) | χ2/t | P value |
| Preoperative | Intervention | 8.01 ± 2.42 | -0.682 | 0.496 | 7.89 ± 2.30 | -0.863 | 0.39 |
| Conventional | 8.21 ± 2.51 | 8.17 ± 2.38 | |||||
| Postoperative day 3 | Intervention | 6.12 ± 2.15 | -5.879 | < 0.001 | 6.08 ± 2.02 | -5.29 | < 0.001 |
| Conventional | 8.45 ± 2.58 | 8.07 ± 2.36 | |||||
| Postoperative day 7 | Intervention | 4.89 ± 1.76 | -7.125 | < 0.001 | 4.76 ± 1.69 | -6.662 | < 0.001 |
| Conventional | 7.42 ± 2.31 | 7.06 ± 2.14 |
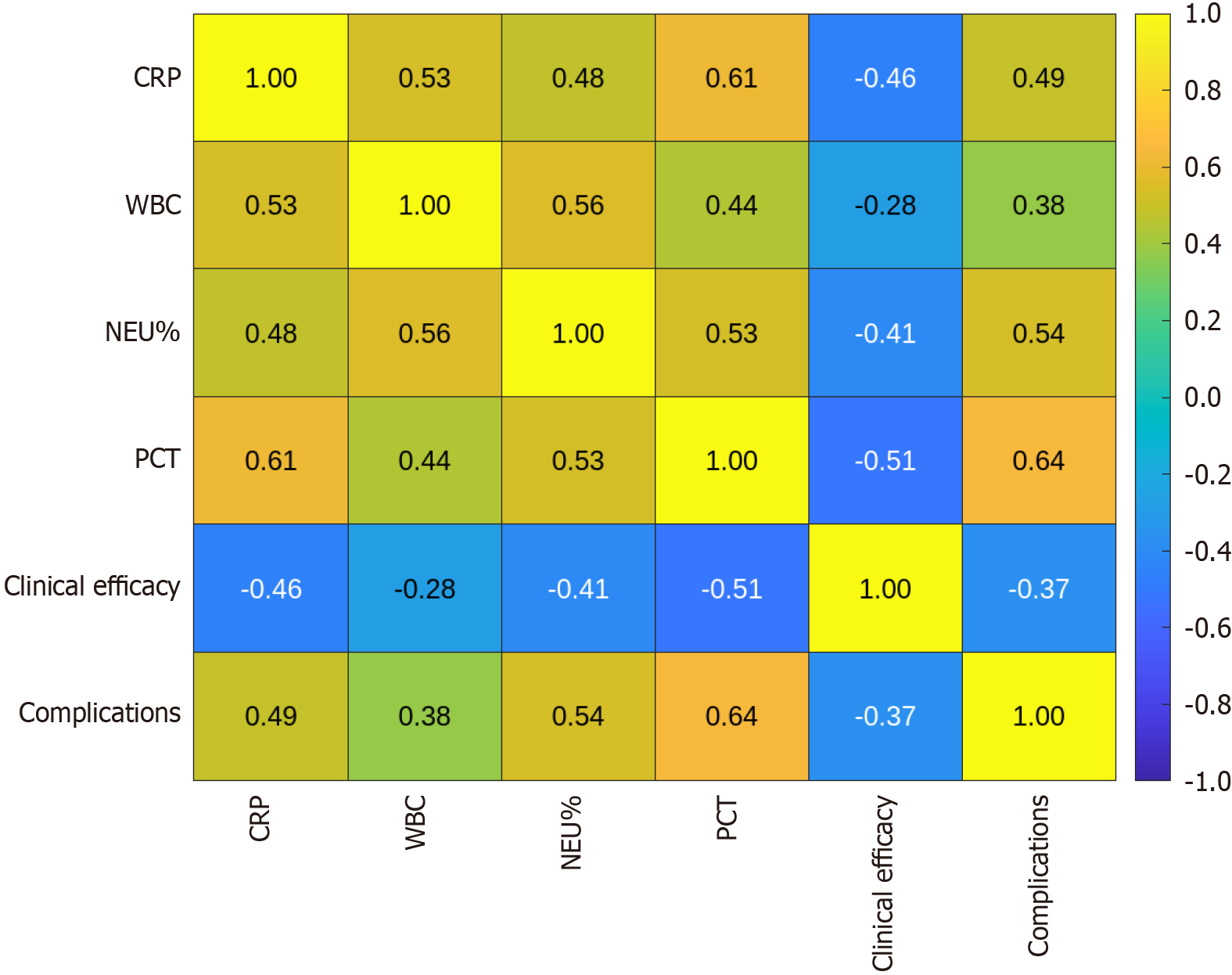
Inflammatory markers (CRP, WBC, NEU%, and PCT) were significantly correlated with clinical efficacy and complications after surgery. CRP levels were negatively correlated with clinical efficacy (r = -0.459, P < 0.01) and positively correlated with complications (r = 0.491, P < 0.01) (Table 7; Figure 2). Other indicators showed a similar trend, suggesting that increased inflammation is closely related to adverse postoperative outcomes.
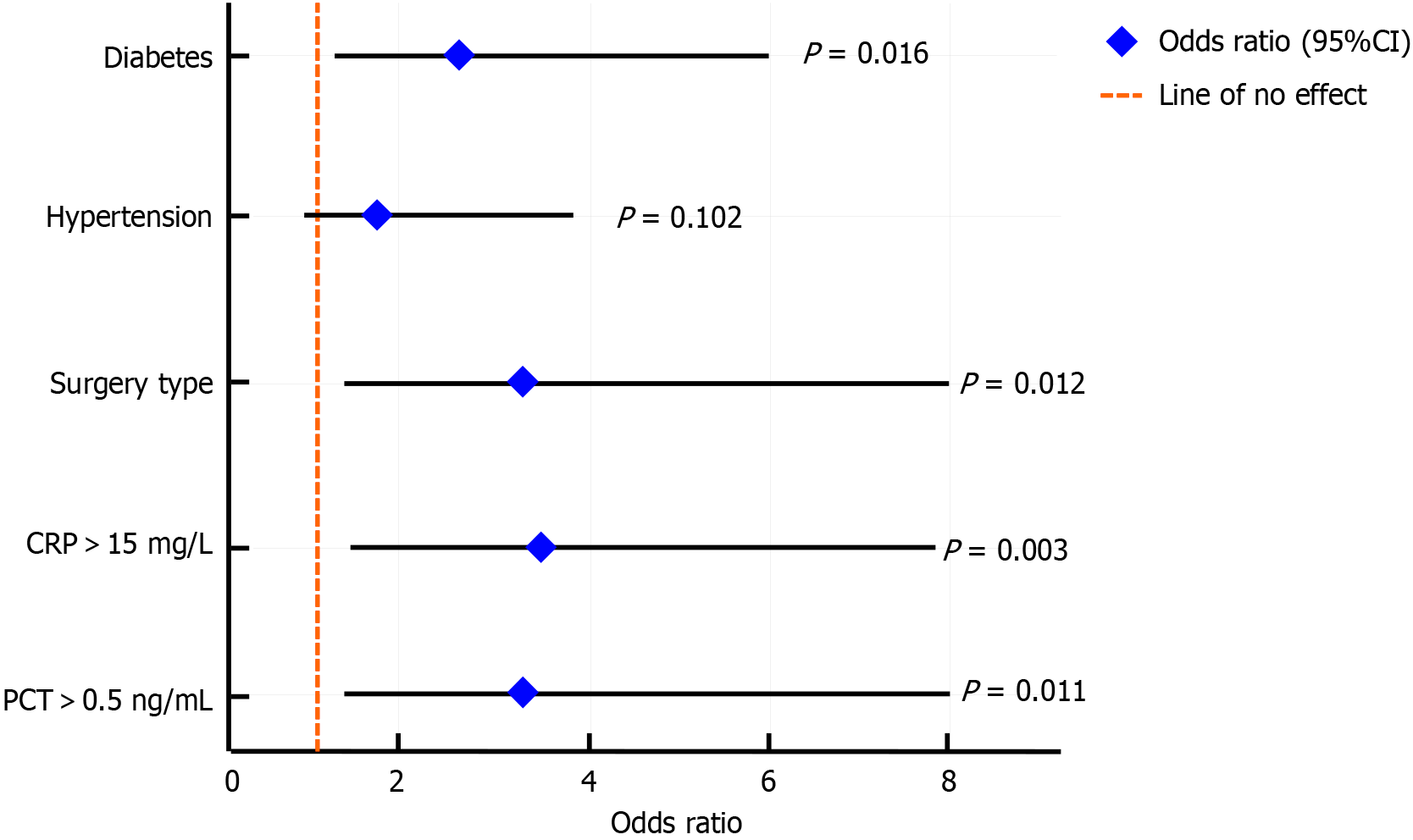
The results showed that the incidence of postoperative complications in patients with diabetes was significantly higher than that in patients without diabetes (RR = 2.43, P = 0.012), indicating that diabetes was an independent risk factor. The incidence of complications was higher than that in the laparoscopic group (RR = 2.86, P = 0.015). The influence of the surgical method was significant. Patients with CRP > 15 mg/L and PCT > 0.5 ng/mL on the first day after surgery had a significantly increased risk of complications, with RR values of 3.12 and 2.91, respectively (all P < 0.01) (Table 8; Figure 3), suggesting that elevated inflammatory markers were an important risk factor. Although hypertension showed a trend of increasing risk, this was not statistically significant.
| Variable | Group | Complication incidence, n (%) | RR | 95%CI | P value | OR | 95%CI | P value |
| Diabetes | Yes (n = 46) | 14 (30.43) | 2.43 | 1.21-4.86 | 0.012 | 2.67 | 1.19-5.98 | 0.016 |
| No (n = 111) | 14 (12.50) | |||||||
| Hypertension | Yes (n = 44) | 11 (25.00) | 1.77 | 0.90-3.47 | 0.097 | 1.85 | 0.89-3.83 | 0.102 |
| No (n = 113) | 17 (14.13) | |||||||
| Surgical method | Laparotomy (n = 49) | 14 (28.57) | 2.86 | 1.22-6.71 | 0.015 | 3.23 | 1.30-7.99 | 0.012 |
| Laparoscopy (n = 108) | 14 (10.00) | |||||||
| CRP (1 day after surgery) | > 15 mg/L (n = 52) | 18 (34.62) | 3.12 | 1.54-6.33 | 0.002 | 3.46 | 1.56-7.67 | 0.003 |
| ≤ 15 mg/L (n = 105) | 14 (11.11) | |||||||
| PCT (1 day after surgery) | > 0.5 ng/mL (n = 36) | 14 (38.89) | 2.91 | 1.31-6.44 | 0.006 | 3.2 | 1.28-7.98 | 0.011 |
| ≤ 0.5 ng/mL (n = 121) | 18 (13.33) |
Intestinal polyps and early tumors commonly occur in the mucosal layer of the gastrointestinal tract and are common lesions of the digestive system. If left untreated, some polyps may progress to the early stages of cancer, increasing patient mortality[10]. Currently, EMR and ESD are important minimally invasive treatments for intestinal polyps and early stage cancer. They are widely applied because of minimal trauma, rapid recovery, and low recurrence rates[11,12]. However, existing studies have primarily focused on surgical techniques and pathological features, neglecting the impact of postoperative nursing and patient psychological status on therapeutic outcomes. Moreover, nursing care often focuses on complication management and lacks a systematic and quantitative evaluation of nursing models[13,14]. Preoperative anxiety and depression have been shown to correlate negatively with postoperative recovery quality, and psychological interventions play a crucial role in improving compliance and optimizing overall treatment efficacy[15]. This study retrospectively analyzed data and incorporated psychological assessments using the HADS to systematically explore how nursing interventions synergize with endoscopic treatment to promote recovery and enhance overall efficacy[16].
Our results showed that the intervention group receiving systematic nursing care had significantly better outcomes in objective measures, such as postoperative complication incidence, hospital stay, and recurrence rate, than the conventional care group, supporting the positive role of nursing interventions in postoperative rehabilitation and prognostic improvement[17,18]. Enhanced monitoring and early warning management within 48 hours after surgery allowed for the timely detection and management of complications such as delayed bleeding and abdominal pain, overcoming the limitations of previous reliance solely on physician directives. The shortened hospitalization time further demonstrated the value of systematic nursing in promoting functional recovery, consistent with the “accelerated rehabilitation” concept of the ERAS program.
This study is one of the first to incorporate psychological assessments into nursing evaluations. The results indicated significantly lower anxiety and depression scores postoperatively in the intervention group than in the control group (P < 0.001), suggesting that psychological interventions effectively alleviated pre- and postoperative emotional stress and improved treatment adherence. This aligns with prior research emphasizing the critical role of psychological care in recovery after gastrointestinal endoscopic procedures[19]. Additionally, phased nutritional guidance and functional exercise facilitate early recovery of gastrointestinal function, reflecting a patient-centered nursing model[20].
The analysis of postoperative complications identified several risk factors affecting recovery, including lesion type and size, surgical method, operation time, operator experience, patient comorbidities, and preoperative psychological state[21]. ESD poses a higher risk of complications than EMR because of its technical complexity and larger resection area. Individualized nursing interventions, especially psychological counseling and postoperative compliance management for high-risk patients, are vital for optimizing overall treatment outcomes.
Theoretically, systematic nursing promotes postoperative recovery through multiple pathways: Preoperative education enhances patient understanding and compliance, intraoperative monitoring reduces the risk of acute complications, postoperative comprehensive management accelerates the restoration of digestive function, and standardized documentation with feedback ensures closed-loop quality control. This constitutes an effective coupling between clinical nursing and minimally invasive endoscopic treatment[22,23]. Unlike previous studies that focused mainly on complication management, this study used a multidimensional indicator system to evaluate the comprehensive effects of holistic nursing interventions, thus providing strong empirical and theoretical guidance[24]. Based on the ERAS concept, the nursing pathway of the intervention group differed from that of traditional intestinal surgery care by emphasizing the coordinated regulation of psychology, behavior, and nutrition, highlighting the proactive role of digestive endoscopy in rehabilitation medicine.
The limitations of this study include its single-center retrospective design, limited sample size, and reliance on medical records, which may have introduced selection bias and incomplete data. Future research should expand the sample size, adopt multicenter prospective designs, incorporate long-term follow-ups, and use multifactor models to explore the key factors influencing postoperative recovery, thereby improving the precision and clinical effectiveness of the nursing interventions.
The viewpoint that "systematic nursing intervention can effectively and synergistically improve the effect of endoscopic treatment" was proposed and verified in this study, which added to the current gap in research on postoperative endoscopic nursing and supported the key position of nursing in postoperative rehabilitation management with data. Through the systematic derivation of the evidence chain and the theoretical sublimation of the principle mechanism, the collaborative value concept of "technology + nursing" was further strengthened, and a solid basis was provided for promoting the establishment of a standardized path for digestive endoscopy nursing in the future.
| 1. | Dong Z, Li G, Wang M, Wang T, Jiang C. Effect of systematic nursing intervention on rehabilitation after colorectal polyps of endoscopic removal in children: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e25109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Zhu L, Zhu L, Yu W. Analysis of pathological characteristics and nursing intervention of patients with gastric polyps based on image stitching algorithm and endoscopy. Pak J Med Sci. 2021;37:1620-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Liu A, Wang H, Lin Y, Fu L, Liu Y, Yan S, Chen H. Gastrointestinal endoscopy nurse assistance during colonoscopy and polyp detection: A PRISMA-compliant meta-analysis of randomized control trials. Medicine (Baltimore). 2020;99:e21278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Morrow L, Greenwald B. The American Society for Gastrointestinal Endoscopy Quality Assurance in Endoscopy Committee's Three Priority Quality Indicators for Screening Colonoscopy Services. Gastroenterol Nurs. 2022;45:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Dave H, Vithalani H, Singh H, Yadav I, Jain A, Kumar S, Bhatia Z, Seshadri S, Hassan S, Dhanka M. Easily injectable gelatin-nonanal hydrogel for endoscopic resectioning of gastrointestinal polyps. Int J Biol Macromol. 2024;279:135405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Messmann H, Bisschops R, Antonelli G, Libânio D, Sinonquel P, Abdelrahim M, Ahmad OF, Areia M, Bergman JJGHM, Bhandari P, Boskoski I, Dekker E, Domagk D, Ebigbo A, Eelbode T, Eliakim R, Häfner M, Haidry RJ, Jover R, Kaminski MF, Kuvaev R, Mori Y, Palazzo M, Repici A, Rondonotti E, Rutter MD, Saito Y, Sharma P, Spada C, Spadaccini M, Veitch A, Gralnek IM, Hassan C, Dinis-Ribeiro M. Expected value of artificial intelligence in gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2022;54:1211-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 7. | Xiong J, Jiang J, Chen Y, Chen Y, Xie C, Xu S. Application of Endoscopic Ultrasound Combined with Multislice Spiral CT in Diagnosis and Treatment of Patients with Gastrointestinal Eminence Lesions. Dis Markers. 2022;2022:1417104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Erdoğan Ç, Arı D, Yeşil B, Koşar K, Coşkun O, Tenlik İ, Köseoğlu HT, Yüksel M. Evaluation of non-gastric upper gastrointestinal system polyps: an epidemiological assessment. Sci Rep. 2023;13:6168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Jiang Q, Yan X, Wang D, Zhang S, Zhang Y, Feng Y, Yang A, Wu D. Endoscopic mucosal resection using cold snare versus hot snare in treatment for 10-19 mm non-pedunculated colorectal polyps: protocol of a non-inferiority randomised controlled study. BMJ Open. 2023;13:e070321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ferlitsch M, Hassan C, Bisschops R, Bhandari P, Dinis-Ribeiro M, Risio M, Paspatis GA, Moss A, Libânio D, Lorenzo-Zúñiga V, Voiosu AM, Rutter MD, Pellisé M, Moons LMG, Probst A, Awadie H, Amato A, Takeuchi Y, Repici A, Rahmi G, Koecklin HU, Albéniz E, Rockenbauer LM, Waldmann E, Messmann H, Triantafyllou K, Jover R, Gralnek IM, Dekker E, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2024. Endoscopy. 2024;56:516-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 140] [Article Influence: 70.0] [Reference Citation Analysis (1)] |
| 11. | Meier B, Caca K. [Endoscopic diagnosis, treatment, and follow-up of polyps of the upper gastrointestinal tract]. Internist (Berl). 2021;62:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Vanbiervliet G, Moss A, Arvanitakis M, Arnelo U, Beyna T, Busch O, Deprez PH, Kunovsky L, Larghi A, Manes G, Napoleon B, Nalankilli K, Nayar M, Pérez-Cuadrado-Robles E, Seewald S, Strijker M, Barthet M, van Hooft JE. Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:522-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Tripathi PR, Sen Sarma M, Yachha SK, Lal R, Srivastava A, Poddar U. Gastrointestinal Polyps and Polyposis in Children: Experience of Endoscopic and Surgical Outcomes. Dig Dis. 2021;39:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Taghiakbari M, Kim DHD, Djinbachian R, von Renteln D. Endoscopic resection of large non-pedunculated colorectal polyps: current standards of treatment. eGastroenterology. 2024;2:e100025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Cai MY, Zhu L, Xu XY, Xu JX, Zhang DF, Zhang Z, Li QL, Qin WZ, Feng L, Xu JG, Li P, Zhou PH. Endoscopic mucosal resection of gastrointestinal polyps with a novel low-temperature plasma radio frequency generator: a non-inferiority multi-center randomized control study. Surg Endosc. 2023;37:3272-3279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Lu X, Ma J. Risk factors of delayed post-polypectomy bleeding after treatment of gastrointestinal polyps with snare-assisted endoscopic sub-mucosal dissection. J Minim Access Surg. 2023;19:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Mandarino FV, Danese S, Uraoka T, Parra-Blanco A, Maeda Y, Saito Y, Kudo SE, Bourke MJ, Iacucci M. Precision endoscopy in colorectal polyps' characterization and planning of endoscopic therapy. Dig Endosc. 2024;36:761-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Tate DJ, Argenziano ME, Anderson J, Bhandari P, Boškoski I, Bugajski M, Desomer L, Heitman SJ, Kashida H, Kriazhov V, Lee RRT, Lyutakov I, Pimentel-Nunes P, Rivero-Sánchez L, Thomas-Gibson S, Thorlacius H, Bourke MJ, Tham TC, Bisschops R. Curriculum for training in endoscopic mucosal resection in the colon: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2023;55:645-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Song EM, Park B, Ha CA, Hwang SW, Park SH, Yang DH, Ye BD, Myung SJ, Yang SK, Kim N, Byeon JS. Endoscopic diagnosis and treatment planning for colorectal polyps using a deep-learning model. Sci Rep. 2020;10:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 20. | Yan T, Qin YY, Wong PK, Ren H, Wong CH, Yao L, Hu Y, Chan CI, Gao S, Chan PP. Semantic Segmentation of Gastric Polyps in Endoscopic Images Based on Convolutional Neural Networks and an Integrated Evaluation Approach. Bioengineering (Basel). 2023;10:806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Mehdi AH. Prevalence of neoplasia in solitary and multiple esophago-gastrointestinal polyps: 5 years retrospective histopathological study. Cell Mol Biol (Noisy-le-grand). 2021;67:44-51. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Spadaccini M, Albéniz E, Pohl H, Maselli R, Thoguluva Chandrasekar V, Correale L, Anderloni A, Carrara S, Fugazza A, Badalamenti M, Iwatate M, Antonelli G, Enguita-Germán M, Álvarez MA, Sharma P, Rex DK, Hassan C, Repici A. Prophylactic Clipping After Colorectal Endoscopic Resection Prevents Bleeding of Large, Proximal Polyps: Meta-analysis of Randomized Trials. Gastroenterology. 2020;159:148-158.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 23. | Colak Y, Hasan B, Hassaballa W, Ur Rashid M, Strassmann V, DaSilva G, Wexner SD, Erim T. Risk factors for local recurrence of large gastrointestinal lesions after endoscopic mucosal resection. Tech Coloproctol. 2022;26:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Dias E, Marques M, Santos-Antunes J, Baldaque-Silva F, Moutinho-Ribeiro P, Macedo G. The role of endoscopic submucosal dissection in the management of gastric inflammatory fibroid polyps: a single-center experience. Rev Esp Enferm Dig. 2022;114:592-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |