Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.110570
Revised: July 17, 2025
Accepted: August 18, 2025
Published online: October 15, 2025
Processing time: 127 Days and 6 Hours
This editorial comments on Yang et al’s article that reported a correlation between dynamic contrast-enhanced ultrasound (CEUS) quantitative parameters and Ki67/tumor differentiation. The validation of CEUS as a diagnostic modality in this study deserves merit. However, it raises interesting points of discussion: (1) Since pancreatic cancer is an overarching term that includes conventional pan
Core Tip: Correlations have been reported between contrast-enhanced ultrasound (CEUS) quantitative parameters and Ki67/tumor differentiation. However, the validation process is still a work in progress. Meticulous attention should be paid to subtypes of pancreatic ductal adenocarcinoma selected for testing, the suitability of Ki67 as the gold standard, the counting methodology and grade cut-offs. Overall, CEUS appears valuable in assessing pancreatic ductal adenocarcinoma. However, it should be regarded one more additional tool along with other imaging modalities such as computed tomography, magnetic resonance imaging and positron emission tomography scan. CEUS can also be augmented by endoscopic ultrasound-guided fine needle biopsy for tissue procurement. In certain situations, detective flow imaging endoscopic ultrasonography could be an alternative to CEUS.
- Citation: Moyana TN. Contrast-enhanced ultrasound as a non-invasive diagnostic modality for pancreatic ductal adenocarcinoma: The question of Ki67 for study validation. World J Gastrointest Oncol 2025; 17(10): 110570
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/110570.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.110570
Dynamic contrast-enhanced ultrasound (CEUS) offers quantitative perfusion data for the diagnosis and monitoring of various diseases[1,2]. It has found clinical application for both neoplastic and non-neoplastic conditions in virtually every body system[1-3]. From an oncologic perspective, it can provide insights into tumor vascularization and thus help in assessing mass characteristics. This editorial comments on the article by Yang et al[4] who investigated the correlation between quantitative parameters of CEUS and Ki67/tumor differentiation in an attempt to validate CEUS as a non-invasive diagnostic modality for pancreatic ductal adenocarcinoma (PDAC). The goal was to not only use CEUS for circumventing the risks associated with invasive biopsy procedures but also provide a more accurate basis for treatment decisions and prognosis assessment, thereby improving outcomes and quality of life.
To better define its place in the diagnostic armamentarium, CEUS has been compared to other imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)[1,2,5-8]. From a historical viewpoint, the first reported medical use of echo contrast dates back to the 1960s when injection of saline was utilized to study heart valves[1,5]. Since then, various solutions have been utilized ranging from hand-agitated bubble suspensions to the current commercial ultrasound contrast agents that use bubbles of standardized size. Over time, the applications of the technique extended beyond cardiovascular disease to other organs including both neoplastic and non-neoplastic diseases[1,2,5-8]. However, with increasing utilization comes the requirement for ratification. It is therefore pertinent to conduct validation assessments for the expanding role of CEUS in medical imaging, which in this context is with particular reference to PDAC.
Ultrasound imaging is a common imaging technology in the world rivaling CT and MRI due largely to its favorable characteristics e.g., easy performance, real-time scanning, non-radiating energy, and cost-effectiveness[9,10]. With respect to the pancreas, endoscopic ultrasound (EUS) may have an advantage over transabdominal ultrasound since it can overcome problems caused by intervening gas, mural bone and fat. It can therefore produce better spatial resolution, a very useful feature for small lesions. However, most lesions appear hypoechoic on EUS which limits the differential diagnosis. EUS is further limited by the scope of conventional color-Doppler and power-Doppler imaging. These conventional EUS Doppler modes are effective at assessing large vessels with fast-flowing blood but are not good for visualizing fine vessels and slow flow at the tissue perfusion level[11]. The advent of CEUS and its subsequent technical improvements over the years have been quite beneficial in this regard. The modality involves the use of micro- and nano-bubble contrast agents and specialized imaging techniques. It permits the visualization of not only the macro- and micro-vasculature but also the measurement of perfusion parameters, which in turn leads to improved diagnostic performance[10]. Unlike CT and MRI, the rapid elimination of the active compounds of CEUS by exhalation allows repeated examinations at short intervals[1]. The fast clearance through the lungs is also particularly helpful for patients with renal insufficiency.
Radiomics is an emerging field within medical imaging that aims to extract mineable quantitative data from routine medical images, such as those obtained from CT, MRI, PET, and in Yang et al’s study[4] CEUS[12,13]. The process typically involves identifying and segmenting a region of interest (ROI) such as a tumor, tumor subregion, or peritumoral zone. The pancreas was ROI for Yang et al’s study[4]. Extracted features typically describe the distribution of signal intensities and spatial relationship of pixels within the ROI. Quantitative CEUS parameters such as maximum intensity, rise time, rise slope 50%, rise slope 10%-90% are then collected and compared with morphologic parameters for pancreatic cancer[3]. All in all, based on the ease of deployment of CEUS (e.g., can be performed at the bedside or other point of care), it is anticipated that there is going to be increased utilization of this imaging modality going forward. It is therefore important to validate its diagnostic capabilities, and this was one of the key goals of Yang et al’s study[4].
Pancreatic neuroendocrine neoplasms: Yang et al[4] uses the terms pancreatic cancer, pancreatic adenocarcinoma and PDAC almost interchangeably throughout the text. This can be problematic since pancreatic cancer includes other entities such as pancreatic neuroendocrine tumors and pancreatic neuroendocrine carcinomas (panNECs), collectively referred to as pancreatic neuroendocrine neoplasms (panNENs)[14-16]. Coincidentally, it is with panNENs that Ki67 (together with mitotic activity) is officially recognized for grading purposes by both the North American Neuroendocrine Tumor Society and European Neuroendocrine Tumor Society as well as the World Health Organization (WHO) and other oncology organizations[17-21]. Indeed, the advent of Ki67 immunostaining was an important milestone for the classification of panNENs because, unlike conventional carcinomas, architectural patterns and cytologic features such as nuclear anisocytosis and atypia (e.g., pleomorphic neuroendocrine tumors) are unreliable for predicting tumor behavior[22]. Ki67 also correlated well with mitotic activity to the extent that both were incorporated into the WHO classification. To this point, the validation of Ki67/mitoses was based on survival outcomes from multiple fairly large studies in different jurisdictions[23-27]. In effect, overall survival (OS) was the gold standard. Viewed from this perspective, Ki67 is a mere parameter, one amongst many, that can be used to assess the biologic aggressiveness of neuroendocrine neoplasms (NENs). Thus, using Ki67 instead of survival makes it a surrogate, not the true gold standard. Moreover, it should also be recognized that Ki67 has its own shortcomings, not least of which are the grading cut-offs and predictive values. This has led a number of studies to question the utility of Ki67 for panNENs[28-30]. It would therefore appear more objective for radiomic studies to use survival as the real gold standard.
Of the panNENs, panNECs are the ones that draw comparisons with PDAC. Molecular profiling shows that genetically and phenotypically, they resemble PDAC in that they carry similar mutations such as TP53, RB1 and KRAS[14,31]. The prognosis for both is very poor with a 5-year OS of < 20%[14]. In contrast, pancreatic neuroendocrine tumours are characterized by MEN1, DAXX/ATRX and mammalian target of rapamycin pathway genes, are slow-growing and have 5- and 15-year OS rates of 85% and 55% respectively[14,25]. Furthermore, it should also be noted that the Ki67 proliferation index for panNECs is defined by a cutoff of > 20%, which is much lower than the 50% threshold that was used in Yang et al’s study[4].
Pancreatic carcinoma subtypes: It is well-accepted that PDAC not otherwise specified (NOS) is the dominant pancreatic cancer. Nonetheless, there are also other variants that may have different disease biology e.g., PDAC arising from intraductal papillary mucinous neoplasm or from mucinous cystic neoplasm, and medullary carcinoma[14]. For example, even though medullary carcinoma is poorly/undifferentiated, it still has a relatively better prognosis compared to PDAC (NOS). Likewise, PDAC ex- intraductal papillary mucinous neoplasm or ex- mucinous cystic neoplasm have better outcomes compared to PDAC (NOS)[14]. There are also mixed carcinomas which can include portions of any of various subtypes. From the perspective of validating a diagnostic modality, it is important to clarify the selection criteria by indicating which of these tumors were included/excluded e.g., colloid carcinoma or acinar carcinoma.
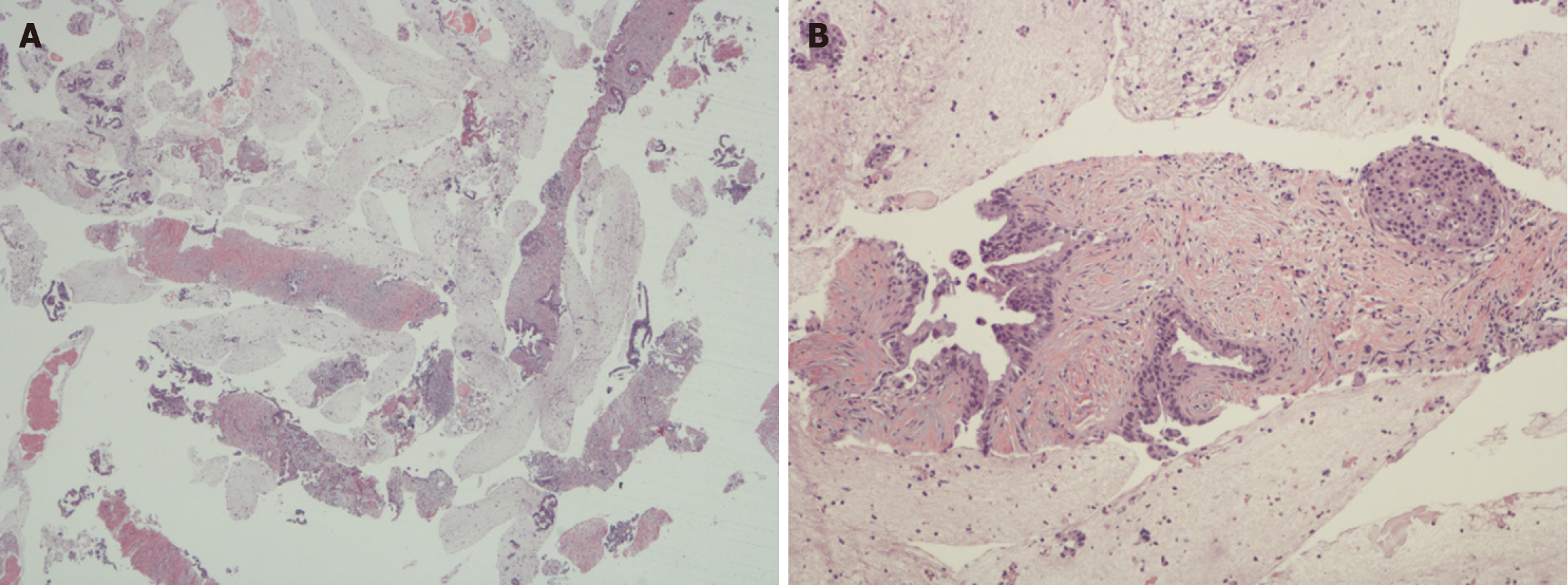
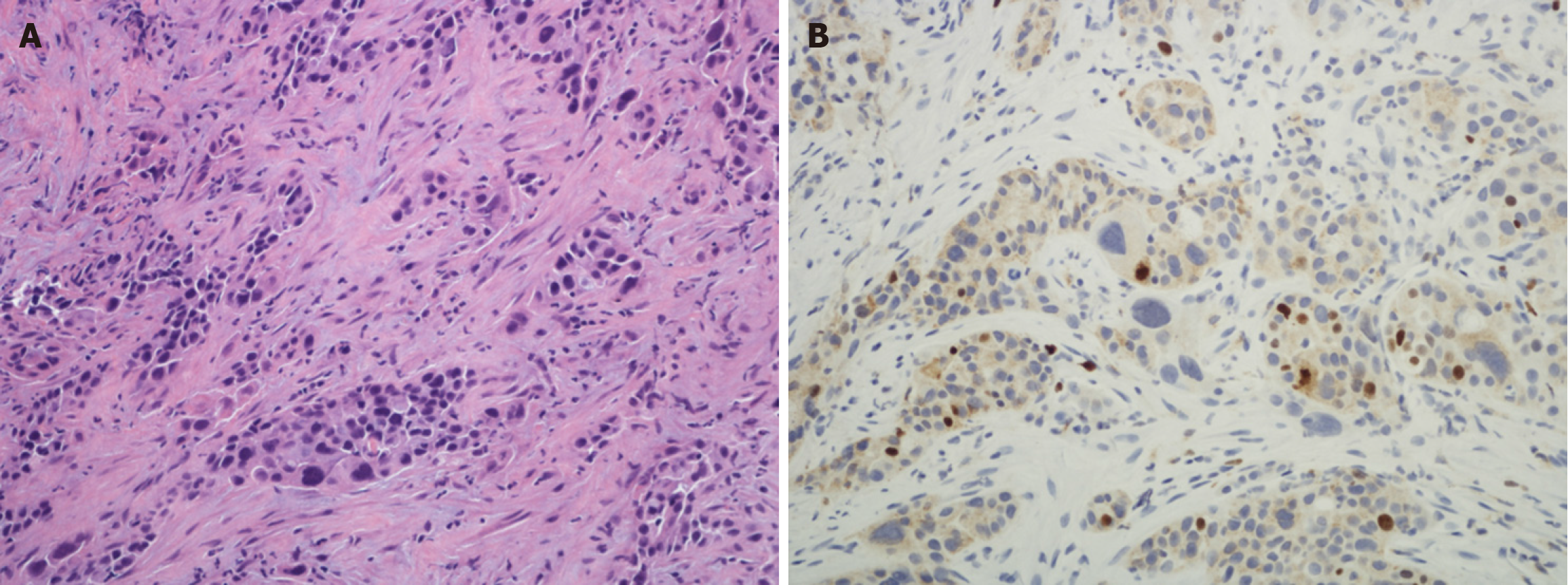
Histologic grading of PDAC: The histologic grading of PDAC is quite different from that of PanNENs. It is based on the extent of glandular differentiation (Figures 1 and 2A) along similar lines to other digestive system adenocarcinomas e.g., in the esophagus, stomach and colorectum[32]. Another grading system uses a combination of degree of glandular differentiation, mucin elaboration, mitoses and nuclear features but overall the predictive value of both these systems is comparable[33]. These systems have been endorsed by official organizations such as the WHO and College of American Pathologists[14,15]. Using this system, most PDACs are well to moderately differentiated, which contrasts with Yang
Ki67 and radiomics: Ki67 is a nuclear protein antigen marker that reflects cellular proliferation. Yang et al[4] posits that this mostly occurs in tumors but rarely in normal cells. However, it should be noted that cellular proliferation is part of normal physiologic processes especially in labile cells that continuously regenerate throughout life, e.g., the epidermis, hematopoietic stem cells, and the crypt cell compartment of the mucosal lining as in the gastrointestinal tract[36,37]. In this regard, normal tonsil is commonly used as a positive control in immunohistochemistry protocols for Ki67 not only for staining the germinal centres but also the overlying epithelium[38-40].
From an oncologic perspective, higher Ki-67 expression is often seen as connoting poorer outcomes, suggesting its potential to predict disease aggressiveness and guide treatment approaches[13,41]. As such, it has been used in many radiomics studies as a prognostic marker. However, tumor proliferation is not always the most important indicator of biologic aggressiveness. With some tumors, there may well be other important considerations. For example, tumor budding in PDAC[42], perineural invasion e.g., head and neck carcinomas[43], extramural vascular invasion in jejunoileal neuroendocrine tumors[44], cuproptosis in kidney renal clear cell carcinoma[45], exhausted CD8+ T cells in breast cancer[46], overexpression of the mitogen-activated protein kinase signaling pathway in gliomas[47], or epithelial mesenchymal transition with sarcomatoid carcinomas[14]. All this is to simply say that cell proliferation kinetics should not necessarily be regarded as the prime driver of biologic aggressiveness.
There are also issues relating to the definition of Ki67 threshold values for low- and high-risk carcinoma groups in such sites as the breast, lung and prostate. For example, the cutoffs established by various laboratories and expert committees range from 10% to 50%[34,41,48-50]. These differing Ki67 cut-off values indicate the difficulty in establishing a specific Ki67 cut-off for routine use as a standard-of-care biomarker[51]. What may even be more important is the method that was used for assessing Ki67. For NENs, hotspots are selected for evaluation[14,21-25]. However, in other organs e.g., the breast, lung and prostate, some studies use the average count whereas others use hotspots[35,41,48-50]. Parenthetically, if average counts were used for the WHO classification for NENs, the cut-off would be even lower than 20%.
One of the Yang et al’s main stated objectives in using CEUS was to establish a definitive diagnosis of PDAC while at the same time avoiding the risk of biopsy procedures[4], e.g., damage to major blood vessels in the peripancreatic region. The commonly used biopsy procedures nowadays are image-guided and they are generally considered safe[52,53]. The most widely accepted are percutaneous ultrasound and endoscopic ultrasound biopsy, offering real-time multiplanar scanning capabilities, minimal invasiveness, and a lack of radiation exposure[52-54]. Whereas there may be some form of risk associated with any biopsy procedure, the incidence of complications such as hemorrhage and pancreatitis as well as the occurrence of false-negative results have been considerably lessened over the years due to technical improvements. Another consideration in these comparisons relates to procurement of tissue, either as core biopsies or aspirates[54]. In this respect, CEUS has a major downside since it provides no tissue for diagnostic purposes. As is well known, there are many mimics of PDAC such as chronic pancreatitis, autoimmune pancreatitis, paraduodenal pancreatitis, lymphomas and metastases[55-57]. For a major operation such as Whipple procedure which has considerable morbidity and mortality, it is very important to establish the diagnosis based on firm grounds (Figure 1). Even in inoperable cases, tissue diagnosis is desirable since systemic therapy has significant side-effects. In this day and age of personalized medicine, biopsy also provides material for molecular studies which greatly helps in the selection of the appropriate treatment. At the same time, it is worth noting that there is ongoing research with liquid biopsies and molecular markers which, in the future, could lessen the reliance on traditional tissue biopsies[58].
Ultrasound contrast agents as used in CEUS are among the safest of all contrast media and numerous studies over the years are a testament to their safety profile[59]. Nonetheless, on very rare occasions, they have resulted in hypersensitivity reactions (estimated at 1 in 15000) or even death[11,59]. These issues may possibly explain the initial tardiness of the Food and Drug Administration in approving CEUS, but it was subsequently ratified based on strong multi-societal expert endorsements[59-61]. Partly in response to concerns about hypersensitivity reactions to CEUS agents, detective flow imaging-EUS was developed as a way for detecting fine vessels and low-velocity blood flow without contrast agents. Studies have shown that it can visualize microvascularity much more clearly than color-Doppler EUS, power Doppler EUS or e-FLOW EUS[11]. Therefore, it may be a useful alternative to CEUS for this purpose[11].
CEUS is a helpful adjunct to other imaging modalities such as CT, MRI and PET scan, and should be viewed as augmenting rather than replacing routine EUS-guided fine-needle biopsy[62]. As such, both can be performed concurrently or as CEUS-guided EUS-guided fine-needle biopsy[63-65]. This would not only provide more confidence in the diagnosis but also supply material for molecular testing. Future work should use survival outcomes and clear criteria for validation.
| 1. | Dietrich CF, Albrecht T, Becher H, Harvey CJ, Jenssen C, Lim AK, Möller K, Greis C. History of contrast enhanced ultrasound (CEUS). Med Ultrason. 2024;26:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Nolsøe CP, Lorentzen T. International guidelines for contrast-enhanced ultrasonography: ultrasound imaging in the new millennium. Ultrasonography. 2016;35:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Lassau N, Bonastre J, Kind M, Vilgrain V, Lacroix J, Cuinet M, Taieb S, Aziza R, Sarran A, Labbe-Devilliers C, Gallix B, Lucidarme O, Ptak Y, Rocher L, Caquot LM, Chagnon S, Marion D, Luciani A, Feutray S, Uzan-Augui J, Coiffier B, Benastou B, Koscielny S. Validation of dynamic contrast-enhanced ultrasound in predicting outcomes of antiangiogenic therapy for solid tumors: the French multicenter support for innovative and expensive techniques study. Invest Radiol. 2014;49:794-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Yang ZY, Wan WN, Zhao L, Li SN, Liu Z, Sang L. Noninvasive prediction of Ki-67 expression in pancreatic cancer via contrast-enhanced ultrasound quantitative parameters: A diagnostic model study. World J Gastrointest Oncol. 2025;17:107919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (4)] |
| 5. | Kremkau FW, Gramiak R, Carstensen EL, Shah PM, Kramer DH. Ultrasonic detection of cavitation at catheter tips. Am J Roentgenol Radium Ther Nucl Med. 1970;110:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 69] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Gaiani S, Celli N, Piscaglia F, Cecilioni L, Losinno F, Giangregorio F, Mancini M, Pini P, Fornari F, Bolondi L. Usefulness of contrast-enhanced perfusional sonography in the assessment of hepatocellular carcinoma hypervascular at spiral computed tomography. J Hepatol. 2004;41:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Giorgio A, Ferraioli G, Tarantino L, de Stefano G, Scala V, Scarano F, Coppola C, Del Viscovo L. Contrast-enhanced sonographic appearance of hepatocellular carcinoma in patients with cirrhosis: comparison with contrast-enhanced helical CT appearance. AJR Am J Roentgenol. 2004;183:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Del Prete M, Di Sarno A, Modica R, Lassandro F, Giorgio A, Bianco A, Muto M, Gasperi M, Del Prete F, Colao A, Montesarchio V, Faggiano A; ENETS Centre of Excellence Multidisciplinary Group for Neuroendocrine Tumors in Naples (Italy). Role of contrast-enhanced ultrasound to define prognosis and predict response to biotherapy in pancreatic neuroendocrine tumors. J Endocrinol Invest. 2017;40:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Hunt D, Romero J. Contrast-Enhanced Ultrasound. Magn Reson Imaging Clin N Am. 2017;25:725-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Xu HX. Contrast-enhanced ultrasound: The evolving applications. World J Radiol. 2009;1:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 11. | Yamashita Y, Yoshikawa T, Yamazaki H, Kawaji Y, Tamura T, Hatamaru K, Itonaga M, Ashida R, Ida Y, Maekita T, Iguchi M, Kitano M. A Novel Endoscopic Ultrasonography Imaging Technique for Depicting Microcirculation in Pancreatobiliary Lesions without the Need for Contrast-Enhancement: A Prospective Exploratory Study. Diagnostics (Basel). 2021;11:2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Shur JD, Doran SJ, Kumar S, Ap Dafydd D, Downey K, O'Connor JPB, Papanikolaou N, Messiou C, Koh DM, Orton MR. Radiomics in Oncology: A Practical Guide. Radiographics. 2021;41:1717-1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 13. | Luo X, Zheng R, Zhang J, He J, Luo W, Jiang Z, Li Q. CT-based radiomics for predicting Ki-67 expression in lung cancer: a systematic review and meta-analysis. Front Oncol. 2024;14:1329801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2716] [Article Influence: 452.7] [Reference Citation Analysis (3)] |
| 15. | Reid MD, Lewis MM, Willingham FF, Adsay NV. The Evolving Role of Pathology in New Developments, Classification, Terminology, and Diagnosis of Pancreatobiliary Neoplasms. Arch Pathol Lab Med. 2017;141:366-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Strosberg JR, Halfdanarson TR, Bellizzi AM, Chan JA, Dillon JS, Heaney AP, Kunz PL, O'Dorisio TM, Salem R, Segelov E, Howe JR, Pommier RF, Brendtro K, Bashir MA, Singh S, Soulen MC, Tang L, Zacks JS, Yao JC, Bergsland EK. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas. 2017;46:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 17. | Kos-Kudła B, Castaño JP, Denecke T, Grande E, Kjaer A, Koumarianou A, de Mestier L, Partelli S, Perren A, Stättner S, Valle JW, Fazio N. European Neuroendocrine Tumour Society (ENETS) 2023 guidance paper for nonfunctioning pancreatic neuroendocrine tumours. J Neuroendocrinol. 2023;35:e13343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 154] [Reference Citation Analysis (0)] |
| 18. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 781] [Article Influence: 130.2] [Reference Citation Analysis (0)] |
| 19. | Chauhan A, Chan K, Halfdanarson TR, Bellizzi AM, Rindi G, O'Toole D, Ge PS, Jain D, Dasari A, Anaya DA, Bergsland E, Mittra E, Wei AC, Hope TA, Kendi AT, Thomas SM, Flem S, Brierley J, Asare EA, Washington K, Shi C. Critical updates in neuroendocrine tumors: Version 9 American Joint Committee on Cancer staging system for gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2024;74:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Fang JM, Li J, Shi J. An update on the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. World J Gastroenterol. 2022;28:1009-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (12)] |
| 21. | Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, Gorbounova V, Gross D, Grossma A, Jense RT, Kulke M, Oeberg K, Rindi G, Sorbye H, Welin S; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology. 2017;105:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 22. | Xue Y, Reid MD. Well-Differentiated Pancreatic Neuroendocrine Tumor: Does Morphologic Variant Matter? Arch Pathol Lab Med. 2025;. [PubMed] [DOI] [Full Text] |
| 23. | Pelosi G, Bresaola E, Bogina G, Pasini F, Rodella S, Castelli P, Iacono C, Serio G, Zamboni G. Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: a comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol. 1996;27:1124-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 190] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, Panzuto F, Pederzoli P, delle Fave G, Falconi M. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 25. | Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A, Doglioni C, Delle Fave G, Fischer L, Fusai G, de Herder WW, Jann H, Komminoth P, de Krijger RR, La Rosa S, Luong TV, Pape U, Perren A, Ruszniewski P, Scarpa A, Schmitt A, Solcia E, Wiedenmann B. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 341] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 26. | Martin-Perez E, Capdevila J, Castellano D, Jimenez-Fonseca P, Salazar R, Beguiristain-Gomez A, Alonso-Orduña V, Martinez Del Prado P, Villabona-Artero C, Diaz-Perez JA, Monleon A, Marazuela M, Pachon V, Sastre-Valera J, Sevilla I, Castaño A, Garcia-Carbonero R. Prognostic factors and long-term outcome of pancreatic neuroendocrine neoplasms: Ki-67 index shows a greater impact on survival than disease stage. The large experience of the Spanish National Tumor Registry (RGETNE). Neuroendocrinology. 2013;98:156-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008;14:7798-7803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 28. | Aysal A, Agalar C, Egeli T, Unek T, Oztop I, Obuz F, Sagol O. Reconsideration of Clinicopathologic Prognostic Factors in Pancreatic Neuroendocrine Tumors for Better Determination of Adverse Prognosis. Endocr Pathol. 2021;32:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Taskin OC, Reid MD, Bagci P, Balci S, Armutlu A, Demirtas D, Pehlivanoglu B, Saka B, Memis B, Bozkurtlar E, Leblebici CB, Birceanu A, Xue Y, Erkan M, Kapran Y, Baygul A, Sokmensuer C, Scarpa A, Luchini C, Basturk O, Adsay V. Infiltration pattern predicts metastasis and progression better than the T-stage and grade in pancreatic neuroendocrine tumors: a proposal for a novel infiltration-based morphologic grading. Mod Pathol. 2022;35:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Khan MS, Luong TV, Watkins J, Toumpanakis C, Caplin ME, Meyer T. A comparison of Ki-67 and mitotic count as prognostic markers for metastatic pancreatic and midgut neuroendocrine neoplasms. Br J Cancer. 2013;108:1838-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Gao HL, Wang WQ, Yu XJ, Liu L. Molecular drivers and cells of origin in pancreatic ductal adenocarcinoma and pancreatic neuroendocrine carcinoma. Exp Hematol Oncol. 2020;9:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Adsay NV, Basturk O, Bonnett M, Kilinc N, Andea AA, Feng J, Che M, Aulicino MR, Levi E, Cheng JD. A proposal for a new and more practical grading scheme for pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2005;29:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Giulianotti PC, Boggi U, Fornaciari G, Bruno J, Rossi G, Giardino D, Di Candio G, Mosca F. Prognostic value of histological grading in ductal adenocarcinoma of the pancreas. Klöppel vs TNM grading. Int J Pancreatol. 1995;17:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Liang Y, Sheng G, Guo Y, Zou Y, Guo H, Li Z, Chang S, Man Q, Gao S, Hao J. Prognostic significance of grade of malignancy based on histopathological differentiation and Ki-67 in pancreatic ductal adenocarcinoma. Cancer Biol Med. 2024;21:416-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 35. | Striefler JK, Sinn M, Pelzer U, Jühling A, Wislocka L, Bahra M, Sinn BV, Denkert C, Dörken B, Oettle H, Riess H, Bläker H, Lohneis P. P53 overexpression and Ki67-index are associated with outcome in ductal pancreatic adenocarcinoma with adjuvant gemcitabine treatment. Pathol Res Pract. 2016;212:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Remnant L, Kochanova NY, Reid C, Cisneros-Soberanis F, Earnshaw WC. The intrinsically disorderly story of Ki-67. Open Biol. 2021;11:210120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 37. | Sobecki M, Mrouj K, Colinge J, Gerbe F, Jay P, Krasinska L, Dulic V, Fisher D. Cell-Cycle Regulation Accounts for Variability in Ki-67 Expression Levels. Cancer Res. 2017;77:2722-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 38. | Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, McShane L, Paik S, Penault-Llorca F, Prudkin L, Regan M, Salter J, Sotiriou C, Smith IE, Viale G, Zujewski JA, Hayes DF; International Ki-67 in Breast Cancer Working Group. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1155] [Cited by in RCA: 1365] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 39. | Faragalla H, Plotkin A, Barnes P, Lu FI, Kos Z, Mulligan AM, Bane A, Nofech Mozes S. Ki67 in Breast Cancer Assay: An Ad Hoc Testing Recommendation from the Canadian Association of Pathologists Task Force. Curr Oncol. 2023;30:3079-3090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 40. | Hsu CY, Yang CF, Liao LR, Ho HL, Ho DM. Tonsil surface epithelium is ideal for monitoring Ki-67 immunohistochemical staining. Histopathology. 2013;63:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Finkelman BS, Zhang H, Hicks DG, Turner BM. The Evolution of Ki-67 and Breast Carcinoma: Past Observations, Present Directions, and Future Considerations. Cancers (Basel). 2023;15:808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 42. | Lawlor RT, Veronese N, Nottegar A, Malleo G, Smith L, Demurtas J, Cheng L, Wood LD, Silvestris N, Salvia R, Scarpa A, Luchini C. Prognostic Role of High-Grade Tumor Budding in Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis with a Focus on Epithelial to Mesenchymal Transition. Cancers (Basel). 2019;11:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Bakst RL, Glastonbury CM, Parvathaneni U, Katabi N, Hu KS, Yom SS. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int J Radiat Oncol Biol Phys. 2019;103:1109-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 44. | Ranot JM, Hamid JS, Montazeri A, Harper K, McCudden C, Moyana TN. Well-Differentiated Jejunoileal Neuroendocrine Tumors and Corresponding Liver Metastases: Mesenteric Fibrogenesis and Extramural Vascular Invasion in Tumor Progression. Cancers (Basel). 2025;17:1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Liu H. Expression and potential immune involvement of cuproptosis in kidney renal clear cell carcinoma. Cancer Genet. 2023;274-275:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 46. | Liu H, Dong A, Rasteh AM, Wang P, Weng J. Identification of the novel exhausted T cell CD8 + markers in breast cancer. Sci Rep. 2024;14:19142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 96] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 47. | Liu H, Tang T. MAPK signaling pathway-based glioma subtypes, machine-learning risk model, and key hub proteins identification. Sci Rep. 2023;13:19055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 48. | Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell'Orto P, Maiorano E, MacGrogan G, Braye SG, Ohlschlegel C, Neven P, Orosz Z, Olszewski WP, Knox F, Thürlimann B, Price KN, Castiglione-Gertsch M, Gelber RD, Gusterson BA, Goldhirsch A; Breast International Group Trial 1-98. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569-5575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 267] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 49. | Berlin A, Castro-Mesta JF, Rodriguez-Romo L, Hernandez-Barajas D, González-Guerrero JF, Rodríguez-Fernández IA, González-Conchas G, Verdines-Perez A, Vera-Badillo FE. Prognostic role of Ki-67 score in localized prostate cancer: A systematic review and meta-analysis. Urol Oncol. 2017;35:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Jakobsen JN, Sørensen JB. Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer. 2013;79:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | Aung TN, Acs B, Warrell J, Bai Y, Gaule P, Martinez-Morilla S, Vathiotis I, Shafi S, Moutafi M, Gerstein M, Freiberg B, Fulton R, Rimm DL. A new tool for technical standardization of the Ki67 immunohistochemical assay. Mod Pathol. 2021;34:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 52. | Chai WL, Kuang XF, Yu L, Cheng C, Jin XY, Zhao QY, Jiang TA. Percutaneous ultrasound and endoscopic ultrasound-guided biopsy of solid pancreatic lesions: An analysis of 1074 lesions. Hepatobiliary Pancreat Dis Int. 2023;22:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 53. | Paramythiotis D, Karlafti E, Tsavdaris D, Arvanitakis K, Protopapas AA, Germanidis G, Kougias L, Hatzidakis A, Savopoulos C, Michalopoulos A. Comparative Assessment of Endoscopic Ultrasound-Guided Biopsies vs. Percutaneous Biopsies of Pancreatic Lesions: A Systematic Review and Meta-Analysis of Diagnostic Performance. J Clin Med. 2024;13:3108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Moyana TN, Kendal WS, Chatterjee A, Jonker DJ, Maroun JA, Grimard L, Shabana W, Mimeault R, Hakim SW. Role of fine-needle aspiration in the surgical management of pancreatic neuroendocrine tumors: utility and limitations in light of the new World Health Organization classification. Arch Pathol Lab Med. 2014;138:896-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Miller FH, Lopes Vendrami C, Hammond NA, Mittal PK, Nikolaidis P, Jawahar A. Pancreatic Cancer and Its Mimics. Radiographics. 2023;43:e230054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 56. | Kim SS, Choi GC, Jou SS. Pancreas Ductal Adenocarcinoma and its Mimics: Review of Cross-sectional Imaging Findings for Differential Diagnosis. J Belg Soc Radiol. 2018;102:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Boninsegna E, Negrelli R, Zamboni GA, Tedesco G, Manfredi R, Pozzi Mucelli R. Paraduodenal pancreatitis as a mimicker of pancreatic adenocarcinoma: MRI evaluation. Eur J Radiol. 2017;95:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Moyana T, Merz V. Editorial: Molecular markers for pancreatic cancers: new technologies and applications in the clinical practice. Front Oncol. 2025;15:1651566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Strom JB, Appis A, Barr RG, Chammas MC, Clevert DA, Darge K, Feinstein L, Feinstein SB, Fowlkes JB, Gorman B, Huang P, Kono Y, Lopez-Mattei J, Lyshchik A, Main ML, Matthias W Jr, Merrill C, Mulvagh SL, Nihoyannopoulos P, Olson J, Piscaglia F, Porter T, Rabischoffsky A, Senior R, Stout JL, Stanczak M, Wilson SR; International Contrast Ultrasound Society with endorsement from the British Society of Echocardiography; Canadian Society of Echocardiography; Society of Diagnostic Medical Sonography; Society for Pediatric Radiology; World Federation of Ultrasound in Medicine and Biology; Brazilian College of Radiology; Joint Review Committee for Diagnostic Medical Sonography; Chinese Ultrasound Doctors Association; American Society of Neuroimaging and affirmation/support from the American Society of Echocardiography; Association for Medical Ultrasound, and the Society for Vascular Ultrasound. Multi-societal expert consensus statement on the safe administration of ultrasound contrast agents. Echo Res Pract. 2025;12:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 60. | Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol. 2009;193:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Hussain A, Weimer DS, Mani N. Diagnosing Pancreatic Adenocarcinoma With Contrast-Enhanced Ultrasonography: A Literature Review of Research in Europe and Asia. Cureus. 2022;14:e22080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 62. | D'Onofrio M, Biagioli E, Gerardi C, Canestrini S, Rulli E, Crosara S, De Robertis R, Floriani I. Diagnostic performance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced endoscopic ultrasound (ECEUS) for the differentiation of pancreatic lesions: a systematic review and meta-analysis. Ultraschall Med. 2014;35:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Otsuka Y, Kamata K. A review of contrast-enhanced harmonic endoscopic ultrasonography for pancreatic solid tumors. J Med Ultrason (2001). 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 64. | Itonaga M, Kitano M, Kojima F, Hatamaru K, Yamashita Y, Tamura T, Nuta J, Kawaji Y, Shimokawa T, Tanioka K, Murata SI. The usefulness of EUS-FNA with contrast-enhanced harmonic imaging of solid pancreatic lesions: A prospective study. J Gastroenterol Hepatol. 2020;35:2273-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 65. | Seicean A, Samarghitan A, Bolboacă SD, Pojoga C, Rusu I, Rusu D, Sparchez Z, Gheorghiu M, Al Hajjar N, Seicean R. Contrast-enhanced harmonic versus standard endoscopic ultrasound-guided fine-needle aspiration in solid pancreatic lesions: a single-center prospective randomized trial. Endoscopy. 2020;52:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |