Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.110503
Revised: July 2, 2025
Accepted: September 16, 2025
Published online: October 15, 2025
Processing time: 128 Days and 22.8 Hours
As one of the most prevalent malignant tumors, hepatocellular carcinoma (HCC) represents a major global public health burden. Traditionally, HCC pathogenesis has been attributed to chronic liver diseases (viral hepatitis, cirrhosis) and af
Core Tip: Hepatocellular carcinoma (HCC) remains a globally prevalent malignancy, traditionally attributed to etiological factors such as viral hepatitis, alcohol-related liver disease, and metabolic dysfunction-associated fatty liver disease. Alarmingly, the global incidence of HCC continues to rise, with more than 900000 new cases reported in 2020, a trend not fully explained by these established risk factors. Emerging evidence highlights sleep disorders as a novel contributor to hepatocarcinogenesis. This review systematically explores the association between sleep disturbances and HCC pathogenesis, focusing on the underlying pathological mechanisms through which disrupted sleep patterns may drive HCC progression.
- Citation: Qian J, Ruan MH, Wang ZJ, Dong W, Jia JY, Feng XC, Liu H. Sleep disorders in hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(10): 110503
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/110503.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.110503
Hepatocellular carcinoma (HCC) is one of the most prevalent malignant tumors globally and represents a major health burden worldwide. According to the 2020 global cancer statistics, HCC accounts for over 900000 new cases and approximately 830000 deaths annually. Both its incidence and mortality rates have shown a significant upward trend over the past decade, particularly in East Asia and Africa[1].
Common etiological factors for HCC include viral hepatitis, alcohol-associated liver disease, and metabolic dysfunction-associated fatty liver disease (MAFLD). The global prevalence of MAFLD, closely linked to factors such as obesity and insulin resistance, continues to rise alongside changing lifestyles[2]. Concurrently, sleep disorders, as another unhealthy lifestyle factor, have been implicated in liver cancer development. Yang et al[3] investigated the relationship between various sleep traits and primary liver cancer (PLC), finding that adequate and regular sleep plays a significant protective role. Specifically, longer nocturnal sleep duration was significantly inversely associated with reduced PLC risk (β = -0.002, P = 0.026). Conversely, daytime napping (β = 0.001, P = 0.043) and insomnia (inverse-variance weighted P = 0.022) both demonstrated significant positive associations with increased HCC risk. Wu et al[4] found that obstructive sleep apnea (OSA) significantly increases liver cancer incidence (RR: 1.19, 95%CI: 1.10-1.29).
Furthermore, animal studies indicate that sleep disorders can exacerbate liver fibrosis progression, induce pre-neoplastic lesions, and indirectly elevate HCC risk through multiple pathways, such as promoting hepatic steatosis and insulin resistance, and aggravating hepatocellular DNA damage[5-7]. These findings suggest that sleep disorders may constitute a novel risk factor, warranting systematic investigation into their pathophysiological interactions with HCC development.
Currently, chronic insomnia symptoms affect approximately 25% of adults[8], while OSA impacts nearly 1 billion individuals globally[9]. Without intervention, these sleep disorder patients face a substantially elevated risk of developing liver cancer, posing a serious public health challenge. This review primarily examines the risk of HCC development in individuals with sleep disorders and the associated pathobiological mechanisms. It also explores the potential value of targeted sleep interventions for HCC primary prevention, along with current clinical challenges and future research directions.
According to the International Classification of Sleep Disorders, Third Edition, sleep disorders are categorized into seven major types[10]. Among these, insomnia, OSA, and circadian rhythm disruption are currently considered the most common sleep disorders associated with liver cancer. However, the epidemiological associations between other sleep disorders and the pathogenesis of HCC require further investigation, and scientifically robust evidence is needed to substantiate the potential relationships between different sleep disorders and liver cancer occurrence.
Insomnia is one of the most common sleep disorders contributing to liver cancer development. It is characterized by difficulty initiating sleep, frequent nocturnal awakenings, early morning awakening with an inability to return to sleep; and overall manifests as reduced sleep duration and poor sleep quality. Clinically, insomnia affects up to 80% of patients with chronic liver disease (e.g., cirrhosis)[11], and the severity of their disease shows a significant correlation with worsening sleep disorders[12]. A prospective cohort study involving 356894 United Kingdom Biobank participants demonstrated that unhealthy sleep patterns, including insomnia, significantly increased the risk of HCC [hazard ratio (HR): 1.46, 95%CI: 1.15-1.85][13]. The NIH-AARP cohort study further revealed a U-shaped association between sleep duration and HCC risk, with both short (< 5 hours; HR: 2.00, 95%CI: 1.22-3.26) and long (≥ 9 hours; HR: 1.63, 95%CI: 1.04-2.65) sleep durations increasing risk[14]. Globally, insomnia symptoms affect 25%-30% of adults, with approximately 50% progressing to chronic insomnia[15]. Among Asian populations, the prevalence of insomnia in Chinese adults has reached 15%, with higher rates observed in younger individuals[16], potentially attributable to lifestyle factors. Therefore, improving insomnia is crucial for preventing liver cancer development.
OSA is characterized by recurrent episodes of apnea and hypopnea during sleep, with a higher prevalence in middle-aged men (approximately 4%) compared to women (2%)[17]. Evidence suggests that OSA is not only a risk factor for liver cancer but may also exacerbate disease progression. A meta-analysis of 18 cohort studies indicated that OSA significantly increased liver cancer incidence (RR: 1.19, 95%CI: 1.10-1.29)[4]. Lin et al[18] found significant correlations between sleep-related hypoxemia in MAFLD patients and markers of hepatocellular injury (e.g., elevated alanine aminotransferase) as well as hepatic steatosis indices. This confirms that the hypoxic state induced by OSA can directly cause liver injury and promote chronic liver disease progression. Qu et al[19] reported consistent findings, further demonstrating that hepatic hypoxia activates inflammatory pathways, leading to enhanced liver inflammation and oxidative stress, changes that may promote hepatocarcinogenesis.
Under normal conditions, the circadian rhythm system maintains homeostasis in numerous biological functions, including cell proliferation, metabolic balance, and immune regulation[20]. Desynchronization between the endogenous circadian clock and environmental light-dark cycles, caused by factors like shift work or transmeridian travel, disrupts organismal homeostasis (including sleep-wake patterns, metabolic processes, and hormone secretion), thereby increasing carcinogenic risk[21,22]. In 2007, shift work involving circadian disruption was classified as “probably carcinogenic to humans” (Group 2A)[23]. Clinical research by Wegrzyn et al[24] supported this, showing that long-term rotating night-shift work was associated with a higher risk of breast cancer (HR = 1.40, 95%CI: 1.00-1.97). Multiple clinical studies also indicate an elevated cancer risk among male and female flight attendants frequently crossing time zones, e.g., skin cancer: Melanoma [standardised incidence ratio (SIR): 2.3, 95%CI: 1.7-3.0], non-melanoma (SIR: 2.1, 95%CI: 1.7-2.8), basal cell carcinoma (SIR: 2.5, 95%CI: 1.9-3.2)[25]. VoPham et al[26] also identified circadian disruption as a potential independent risk factor for liver cancer, reporting that within the same time zone, each 5-degree increase in longitude from east to west was significantly associated with increased HCC risk (incidence rate ratio: 1.07; 95%CI: 1.01-1.14, P = 0.03).
Collectively, these findings establish sleep disorders as independent risk factors for the development and progression of HCC as shown in Table 1. In clinical practice, enhanced sleep management for high-risk populations (e.g., patients with chronic liver disease) is warranted to mitigate disease risk. Future research should further explore the impact of sleep interventions on liver cancer prognosis and elucidate the interactions between sleep disorders and other risk factors.
| Ref. | Country | Type of sleep disorders | Type of cancer | Outcomes |
| [13] | United Kingdom | Insomnia | HCC | HR: 1.46, 95%CI: 1.15-1.85 |
| [14] | United States | Insomnia | HCC | HR: 2.00, 95%CI: 1.22-3.26 |
| Long sleep durations | HCC | HR: 1.63, 95%CI: 1.04-2.65 | ||
| Daytime napping | HCC | HR: 1.46, 95%CI: 1.04-2.06 | ||
| [4] | China | Obstructive sleep apnea | Liver cancer | RR: 1.19, 95%CI: 1.10-1.29 |
| [26] | United States | Circadian rhythm disruption | HCC | IRR: 1.07; 95%CI: 1.01-1.14 |
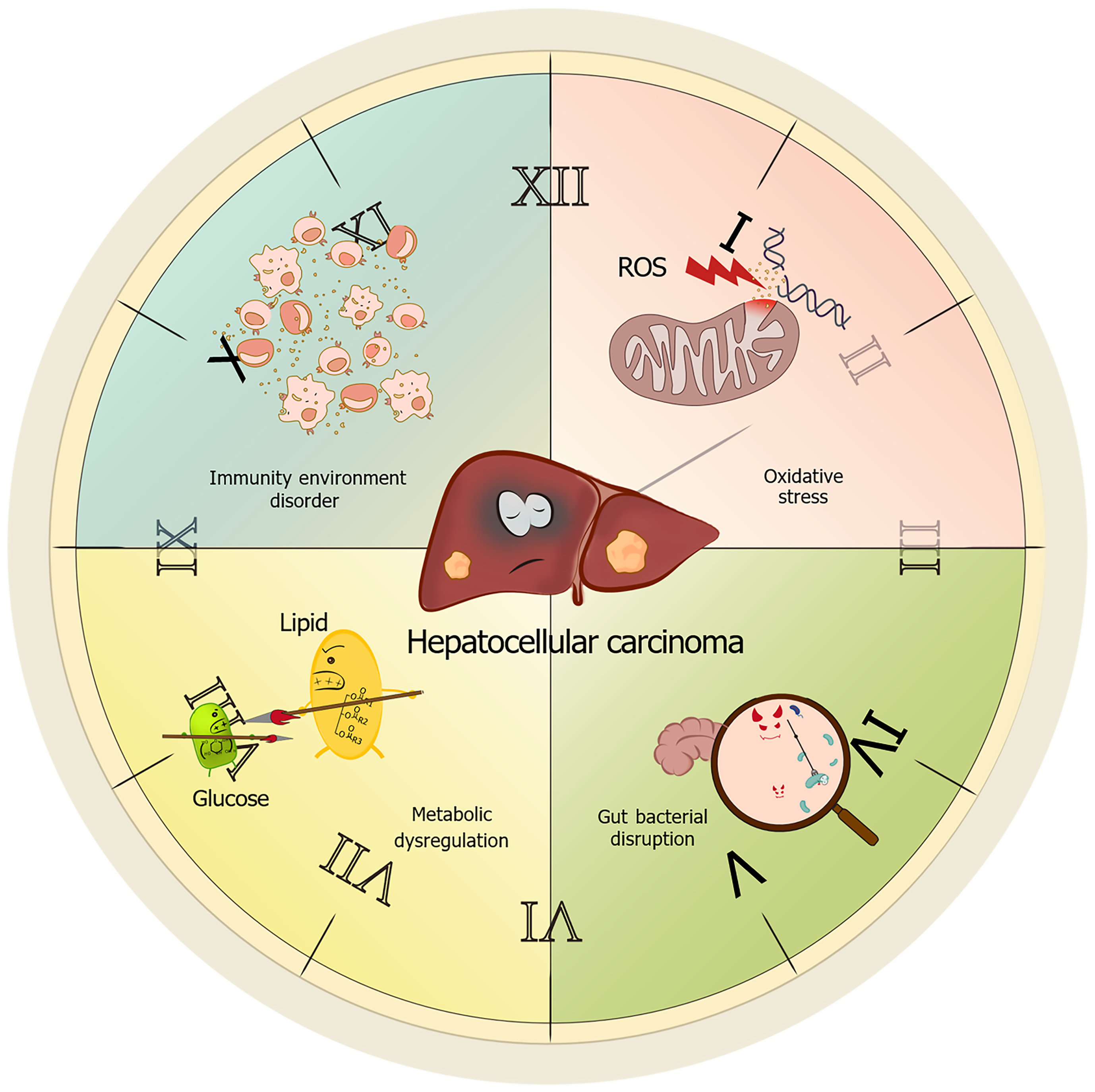
With increasing health awareness in modern society and a rising prevalence of sleep disorders, research related to sleep disorders has garnered significant attention. Substantial evidence now demonstrates that sleep disorders can promote liver cancer development through multiple mechanisms. We will delineate these mechanisms, focusing on immune dysregulation, oxidative stress, metabolic disorders, gut dysbiosis, and circadian rhythm disruption (Figure 1).
Sleep disorders disrupt immune homeostasis. On one hand, they can induce persistent activation of pro-inflammatory cytokines, fostering a chronic inflammatory microenvironment within the liver. On the other hand, sleep disorders impair immune cell activity, diminishing their capacity to recognize and eliminate nascent cancer cells. Collectively, these effects contribute to the development of HCC. Firstly, sleep disorders amplify inflammatory-immune crosstalk, accelerating HCC progression[14]. Chronic infection with hepatitis B virus (HBV) or hepatitis C virus inherently causes sustained hepatocyte damage and inflammatory responses, rendering hepatocytes more susceptible to malignant transformation[27-29]. Concomitant sleep deprivation further elevates systemic levels of pro-inflammatory cytokines [e.g., tumor necrosis factor-α (TNF-α), interleukin (IL)-6][30]. Existing research has also identified that inflammatory mediators within the tumor microenvironment (TME), such as IL-6 and β-arrestin 1, persistently activate the NF-κB and STAT3 signaling cascades. This activation directly promotes tumor cell proliferation while simultaneously inhibiting apoptosis[31,32]. Preclinical evidence indicates that sleep deprivation upregulates hepatic lipase activity, thereby promoting hepatic steatosis and insulin resistance[5]. These complications synergistically exacerbate hepatic lipid accumulation, perpetuating the inflammatory cascade[33]. Secondly, sleep disorders impair tumor immune surveillance by disrupting immune cell effector functions and cytokine networks[34]. Huang et al[35] demonstrated that sleep deprivation in mice leads to reduced numbers and impaired function of T lymphocytes and natural killer cells in peripheral circulation. Concurrently, it increases the infiltration of immunosuppressive CD11b+ cells from the spleen into the TME via peripheral blood. Notably, sleep-related immune dysregulation extends beyond peripheral circulation to lymphoid organs. Zager et al[36] found that sleep disruption causes a generalized reduction across all splenocyte subsets (primarily T and B cells). Furthermore, sleep disorders disrupt the Th1/Th2 homeostasis, which is critical for immune balance. This results in Th2 cell hyperfunction and the secretion of cytokines such as IL-4 and IL-10. These cytokines promote tumor angiogenesis while simultaneously suppressing Th1 cell function. This dual action weakens the host's anti-tumor immune response, enabling tumor cells to evade immune surveillance, proliferate unchecked, and establish an immune escape microenvironment, thereby further promoting HCC development[37].
Oxidative stress refers to a pathological state characterized by an imbalance between reactive oxygen species (ROS) production and antioxidant defense systems under endogenous or exogenous stimuli, leading to oxidative damage accumulation. Mitochondrial dysfunction constitutes the predominant mechanism underlying this process[38-40]. Excessive mitochondrial-derived ROS disrupts lipid bilayer integrity, induces DNA strand breaks, and promotes protein carbonyl modifications, ultimately triggering apoptosis or malignant transformation[41]. Oxidative stress serves as a critical pathogenic nexus in HCC, where sleep disorders exacerbate hepatic redox imbalance through mitochondrial dysfunction and impaired antioxidant defense, thereby accelerating hepatocarcinogenesis via ROS-mediated genomic instability and pro-tumorigenic signaling.
Preclinical models further confirm that sleep deprivation leads to the accumulation of ROS in the intestine, which can ultimately be fatal without antioxidant intervention[42,43]. Within the hepatic context, sustained ROS assault on hepatocytes triggers chronic inflammation, activates hepatic stellate cells (HSCs), and accelerates fibrosis[44,45]. Furthermore, oxidative stress induces cell cycle arrest by interfering with the function of checkpoint kinase 2[46-48] and increases genomic instability, thereby promoting tumorigenesis[49].
Sleep disorders profoundly disrupt redox homeostasis. Multiple studies indicate that chronic sleep disorders impair mitochondrial dynamics, leading to compromised mitochondrial function and a further exacerbation of ROS generation[50,51]. Previous research has established that the Kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2 (Nrf2) pathway is a crucial defense mechanism protecting the liver against oxidative stress damage[52]. Concurrently, Nrf2 serves as a key transcription factor in maintaining cellular redox homeostasis during the sleep-wake cycle[53]. Mechanistically, sleep disorders may suppress Nrf2 nuclear translocation, downregulate antioxidant gene expression, and impair ROS scavenging capacity, collectively promoting hepatocarcinogenesis. Relevant clinical studies also corroborate the link between oxidative stress and liver cancer. Oxidative stress-induced autophagosome formation promotes extensive HBV replication, thereby increasing the risk of HCC recurrence[54]. Additionally, elevated ROS levels in patients with chronic hepatitis B suppress the expression of suppressor of cytokine signaling 3 via Snail-mediated epigenetic silencing. This suppression results in sustained activation of the IL-6/STAT3 signaling pathway, ultimately leading to HCC development[55]. In summary, oxidative stress drives hepatocarcinogenesis through ROS-mediated DNA damage, inflammation activation, and dysfunction of protein inhibitory mechanisms[56,57]. As a key instigator, sleep disorders amplify hepatic oxidative damage through mitochondrial dysfunction, suppression of antioxidant defenses, and enhancement of pro-carcinogenic signaling, thereby establishing a pathogenic "sleep disorder-oxidative stress-HCC" axis.
Hepatic metabolic dysfunction - centered on insulin resistance - constitutes a critical pathological foundation for HCC, with sleep disorders exacerbating hepatocarcinogenesis through disruption of glucose/Lipid homeostasis, aberrant energy sensing, and circadian misalignment.
The liver, acting as the central hub for glucose and lipid metabolism, is susceptible to metabolic dysfunction, which underpins the pathogenesis of HCC. Insulin resistance drives this process by disrupting glucose and lipid homeostasis: Under physiological conditions, insulin stimulates hepatic glucose uptake and glycogen synthesis while inhibiting gluconeogenic gene expression via the PI3K/AKT pathway[58,59]. During insulin resistance, reduced hepatocyte insulin sensitivity impairs GLUT4 membrane translocation, diminishes glycogen synthesis, and compromises AKT/FOXO1-mediated suppression of gluconeogenesis, thereby increasing hepatic glucose output. This persistent hyperglycemia provides an energy substrate for tumorigenesis[60-64]. Clinically, type 2 diabetes mellitus promotes HCC development through IGF-1-mediated inhibition of apoptosis, fostering hepatocyte hyperproliferation[65]. Concurrently, insulin resistance increases pathological free fatty acid uptake and triglyceride synthesis, inducing hepatic steatosis. The generation of excess ROS, diacylglycerol, and ceramides further impairs insulin signaling through a positive feedback loop. The synergy between lipid accumulation and mitochondrial oxidative stress establishes a vicious cycle that accelerates the progression of metabolic liver disease and significantly elevates HCC risk[66,67].
Sleep disorders profoundly amplify these metabolic derangements: Animal studies demonstrate that sleep-deprived mice develop hepatic steatosis and insulin resistance, exhibiting up to a 67.9% increase in hepatic triglycerides and upregulation of lipogenic genes (e.g., Elovl3, Lipin1)[5,68]. Metabolic homeostasis in young adult mice is more vulnerable to sleep deprivation than in older mice, subsequently inducing aging-like features[69]. Furthermore, phosphatidylinositol-5-phosphate 4-kinase type 2 gamma-deficient cells exhibit heightened sensitivity to insulin-mediated PI3K/AKT signaling and exploit the insulin-rich hepatic environment for organ-specific metastasis, thereby increasing the burden of cancer liver metastases[70]. In an experimental model investigating spontaneous liver cancer formation, circadian disruption was found to activate the constitutive androstane receptor, accelerating MAFLD-associated HCC development by promoting increased lipid synthesis, disruption of peripheral clocks, and sympathetic nervous system dysfunction[71].
In summary, sleep disorders disrupt glucose and lipid metabolic homeostasis, activate oncogenic pathways, and ultimately drive carcinogenesis through tripartite mechanisms: Insulin resistance, aberrant energy sensing, and circadian disruption. Future research should further explore the long-term impact of sleep disorders on metabolic pathways to optimize clinical practice.
Sleep disorders exacerbate microbial dysregulation through circadian- neuroendocrine crosstalk, while gut-derived inflammatory/metabolic signals reciprocally drive HCC progression. The core circadian regulator, brain and muscle ARNT-like 1 (BMAL1), governs diurnal oscillations of gut microbial composition. Circadian disruption downregulates intestinal BMAL1 expression, reducing beneficial genera (Bacteroides, Lactobacillus) while enriching pathobionts (Helicobacter, Enterobacteriaceae), thereby instigating dysbiosis-driven inflammation[72,73]. Preclinical models reveal that sleep deprivation elevates serum corticotropin-releasing hormone, adrenocorticotropic hormone, and corticosterone levels, indicating hypothalamic-pituitary-adrenal (HPA) axis hyperactivation. Concurrently, sleep-deprived rodents exhibit an increased abundance of opportunistic pathogens, suggesting that sleep disorders may contribute to gut microbial dysbiosis via HPA axis activation. When the gut microbiota is imbalanced, it can lead to the breakdown of the intestinal mucosal barrier, causing lipopolysaccharide (LPS) to enter the portal vein system. Elevated hepatic LPS concentrations trigger Toll-like receptor 4 (TLR4) signaling in Kupffer cells and HSCs. This activation stimulates excessive release of inflammatory factors, driving hepatic inflammation and oxidative injury while suppressing apoptosis and inducing DNA lesions[74]. Simultaneously, LPS engages TLR4 receptors on liver endothelial cells, initiating the MyD88-dependent TLR cascade. Through extracellular protease modulation, MyD88 governs angiogenesis - a pivotal process in HCC development - and facilitates cirrhosis-to-HCC transition[75]. Furthermore, LPS provokes hepatic progenitor cells (HPCs) to differentiate into myofibroblasts. Secreted IL-6 and TNF-α from these cells activate oncogenic Ras pathways and inactivate p53 tumor suppressor signaling in HPCs, ultimately accelerating aberrant HPC proliferation, malignant transformation, and HCC initiation[76,77]. Gut microbiota dysbiosis disrupts hepatic bile acid metabolism, elevating toxicity. Such imbalance promotes liver tumor advancement via the BA-CXCL16-CXCR6 axis[78]. In addition, gut microbiota can indirectly lead to disease by stimulating inflammatory states, such as cancer inflammation induced by enterotoxigenic fragile bacteria developing through STAT3- and Th17-dependent pathways[79,80], while inflammatory states also increase the production of ROS, inducing DNA damage and promoting tumor progression[81-83].
Currently, targeted sleep interventions for high-risk HCC patients face several clinical challenges. Firstly, patients with chronic liver disease often present with multiple comorbidities and complex health conditions. Certain sedative-hypnotic drugs may adversely affect hepatic function[84], imposing an additional burden on an already compromised liver. Consequently, careful consideration of both drug safety and efficacy is essential[85]. Secondly, HCC treatments, including surgery, chemotherapy, and radiotherapy, may induce or exacerbate sleep disorders[86-88]. Concurrently, treatment-related side effects such as pain and fatigue can further disrupt sleep[89], complicating the management of sleep disorders. Additionally, medications used to manage these symptoms may potentially interact with sleep-targeting agents. Furthermore, psychological factors cannot be overlooked in sleep management. HCC patients frequently experience comorbid anxiety and depression, which can significantly worsen sleep disorders[90]. Implementing effective psychological interventions requires specialized mental health professionals; however, resource constraints, including personnel shortages in clinical practice, often hinder optimal management.
Therefore, individualized treatment represents a key future direction. Personalized management plans must be developed through comprehensive consideration of patient-specific factors, including genetic background, disease progression, sleep disorder subtype, medication adherence, and tolerance levels.
Maintaining consistent sleep-wake schedules constitutes a critical component of circadian rhythm management. Establishing fixed bedtimes and wake-up times facilitates circadian entrainment, thereby preserving normal physiological rhythms[91,92]. Chronic sleep deprivation disrupts circadian homeostasis, precipitating endocrine dysfunction, metabolic dysregulation, and elevated risks of sleep disorders and HCC[71]. For individuals engaged in transmeridian travel or shift work, proactive circadian adaptation strategies are essential to mitigate jet lag-induced chronodisruption[93]. Empirical evidence indicates that adherence to 7-8 hours of nocturnal sleep duration establish robust physiological defenses through maintenance of circadian integrity and optimal physiological functioning[14].
Ambient factors including photic exposure, thermal conditions, and acoustic stimuli significantly influence sleep architecture. Blue light, a high-energy visible spectrum emitted by electronic devices (e.g., smartphones, computers, and tablets), exerts melatonin-suppressive effects via retinal photoreceptor activation. Given melatonin's pivotal role in sleep initiation, such photic suppression correlates with prolonged sleep latency and diminished sleep efficiency[94]. Implementing digital device abstinence 1-2 hours pre-sleep or utilizing blue light filtering eyewear represents effective countermeasures[95,96]. Thermal optimization within 18-22 °C enhances sleep continuity through autonomic thermoregulation, whereas thermal deviations promote sleep fragmentation[97]. Noise is also an important factor affecting sleep. The noise level in the sleeping environment should be controlled below 30 decibels[98]. Earplugs, soundproof curtains, and other devices can be used to reduce noise interference and create a quiet sleeping environment.
Pharmacotherapy demonstrates clinical efficacy in managing sleep disorders (Table 2). The current pharmacological armamentarium primarily comprises non-benzodiazepine receptor agonists (Z-drugs), benzodiazepines (BZDs), melatonin and melatonin receptor agonists, sedating antidepressants, and orexin receptor antagonists (ORAs). BZDs (estazolam) potentiate γ-aminobutyric acid (GABA) ergic neurotransmission through GABA receptor modulation, effectively ameliorating prolonged sleep latency and sleep maintenance difficulties[99]. However, their clinical utility is constrained by dependence liability, tolerance development, and residual sedation, necessitating rigorous risk-benefit analysis[100,101]. Z-drugs (e.g., zopiclone, zolpidem) share similar GABAergic mechanisms but demonstrate rapid onset, abbreviated elimination half-lives, and reduced adverse effect profiles, rendering them preferable for short-term insomnia management[102,103]. In hepatopathy patients with anxiety or depression, antidepressants (e.g., mirtazapine, trazodone) exert dual therapeutic effects by modulating monoaminergic transmission, concurrently improving sleep architecture and affective symptoms[104,105]. Melatonin and its receptor agonists constitute effective therapeutics for circadian rhythm sleep-wake disorders[106,107]. ORAs represent a novel therapeutic class for sleep disorders, exerting their therapeutic effects by modulating the physiological sleep-wake cycle. Clinical evidence demonstrates their capacity to significantly reduce sleep onset latency and wake after sleep onset duration, thereby promoting sleep patterns that more closely resemble natural physiological sleep. Notably, these agents exhibit superior efficacy compared to placebos and traditional medications such as zolpidem, while demonstrating significantly lower risks of addiction and withdrawal reactions relative to BZDs[108]. The favorable cognitive profile, characterized by minimal impact on daytime cognitive function, renders them particularly suitable for elderly populations and patients with intolerance or dependency risks associated with conventional therapies[109]. However, current clinical research remains insufficient in addressing the heterogeneity of therapeutic responses and establishing comprehensive long-term safety profiles. Clinical implementation requires careful consideration of individual patient characteristics, necessitating a balanced evaluation of therapeutic benefits vs potential risks to achieve precision medicine objectives in sleep disorders management.
| Ref. | Category | Advantages | Limitations | Target population |
| [102,103] | Non-benzodiazepines | Rapid onset, lower risk of dependence, minimal next-day residual effects | No anxiolytic efficacy; sleepwalking risk | Short-term insomnia, elderly patients |
| [99-101] | Benzodiazepines | Potent anxiolytic effects; well-established efficacy | High dependence risk; cognitive impairment; fall risk | Anxiety-related insomnia (short-term use) |
| [106,107] | Melatonin receptor agonists | No dependence risk; favorable safety | Limited efficacy in primary insomnia | Circadian rhythm disorders; comorbid respiratory conditions |
| [104,105] | Antidepressants | Effective for mood-related insomnia | Diverse side effects (e.g., weight gain, hypotension) | Chronic insomnia with depression/anxiety |
| [108,109] | Orexin receptor antagonists | Novel mechanism; fewer adverse effects | Limited long-term efficacy data | Treatment-resistant insomnia; patients requiring prolonged therapy |
Notably, pharmacotherapy in cirrhotic populations requires meticulous evaluation of hepatic metabolism pathways and potential drug-drug interactions to optimize the therapeutic index.
Cognitive behavioral therapy for insomnia (CBT-I), endorsed as a first-line therapeutic modality in European insomnia guidelines, demonstrates robust efficacy in managing chronic insomnia[99,110]. This multimodal intervention comprises three core components: Sleep hygiene optimization, stimulus control therapy (SCT), and sleep restriction therapy (SRT)[111]. Sleep hygiene education provides structured guidance on sleep-conducive behaviors, emphasizing circadian alignment through fixed sleep-wake schedules, avoiding the use of electronic devices before bedtime, and avoiding activities unrelated to sleep in the bedroom[112]. SCT employs operant conditioning principles to reinforce bed-sleep associations, mandating: (1) Bedtime initiation only upon sleepiness onset; (2) Conditioned arousal reduction through immediate bedroom exit following 15-20 minutes of wakefulness; and (3) Re-initiation of sleep attempts exclusively in drowsy states[113]. SRT systematically curtails time-in-bed to enhance homeostatic sleep pressure, thereby improving sleep efficiency metrics[114]. Research has shown that CBT-I can effectively improve the sleep quality of insomnia patients, reduce the occurrence of insomnia symptoms, and improve their quality of life[115,116].
For patients diagnosed with OSA, continuous positive airway pressure (CPAP) remains the gold-standard in
Emerging evidence indicates that structured exercise regimens demonstrate therapeutic efficacy in sleep disorder management. Moderate-intensity aerobic modalities (e.g., ambulation, jogging, yoga) enhance cardiorespiratory fitness and promote autonomic nervous system equilibrium, thereby facilitating sleep homeostasis. A randomized controlled trial in oncological populations revealed that protocolized aerobic exercise significantly ameliorates sleep architecture parameters and improves health-related quality of life metrics[118]. Complementary and alternative medicine approaches, including acupuncture and therapeutic massage, exhibit clinical potential in sleep pathology management[119,120]. Mechanistic studies suggest massage therapy attenuates musculoskeletal hypertonicity and nociceptive signaling through somatosensory afferent modulation, consequently enhancing sleep continuity[121]. In HCC cohorts, such non-pharmacological interventions demonstrate dual benefits: Sleep optimization and psychological distress alleviation through cortisol rhythm normalization.
Deep brain stimulation (DBS), a stereotactic neurosurgical intervention utilizing high-frequency electrical modulation of target nuclei, has established therapeutic superiority in movement disorders including Parkinson's disease and essential tremor[122,123]. Recent advances in sleep-wake circuitry elucidation have propelled DBS applications into circadian dysregulation domains. DBS's unique advantages-stereotactic precision, reversible neuromodulation, and dose-titratable intervention-position it as a paradigm-shifting therapeutic strategy for refractory sleep disorders.
Overall, sleep disorders play an extremely important role in the occurrence and development of liver cancer, and there is a complex and closely related relationship between the two. Sleep disorders, such as insomnia, OSA-hypopnea syndrome, and circadian rhythm disorders significantly increase the risk of liver cancer through multiple mechanisms such as chronic inflammation, oxidative stress, metabolic dysregulation, immune suppression, disruption of gut microbiota, and clock gene dysregulation. All these lead to complex physiological and pathological changes, which include immune inflammation imbalance weakening the body's defense, DNA damage repair obstruction increasing the risk of gene mutations, biological clock gene loss leading to metabolic disorders, and liver fibrosis and cirrhosis accelerating deterioration of the liver microenvironment. While some progress has been made in evaluating the relationship between sleep disorders and liver cancer, there are still many issues that need to be addressed. Future research should be oriented to conduct large-scale, prospective cohort studies to unravel the causal factors, to identify the differential effects of different subtypes, and to explore the molecular mechanisms by which sleep disorders affect the occurrence and development of liver cancer. These would lead to identification of more potential targets, thereby providing a theoretical basis for developing new treatment strategies. Additionally, the development of precise interventions based on circadian rhythms, will become one of the key directions for future research. These studies are expected to open up new venues for early diagnosis and prevention, and individualized treatment, to reduce the incidence rate and mortality of liver cancer, improve the quality of life and survival rate of patients, and finally achieve the goal of translational medicine of "from sleep management to liver cancer prevention".
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68193] [Article Influence: 13638.6] [Reference Citation Analysis (201)] |
| 2. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4390] [Article Influence: 878.0] [Reference Citation Analysis (4)] |
| 3. | Yang X, Wang J, Wang H. Association between sleep traits and primary liver cancer: A Mendelian randomization analysis. Eur J Clin Invest. 2023;53:e14002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 4. | Wu D, Zhao Z, Chen C, Lu G, Wang C, Gao S, Shen J, Liu J, He J, Liang W. Impact of obstructive sleep apnea on cancer risk: a systematic review and meta-analysis. Sleep Breath. 2023;27:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 5. | Shigiyama F, Kumashiro N, Tsuneoka Y, Igarashi H, Yoshikawa F, Kakehi S, Funato H, Hirose T. Mechanisms of sleep deprivation-induced hepatic steatosis and insulin resistance in mice. Am J Physiol Endocrinol Metab. 2018;315:E848-E858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Trzepizur W, Boursier J, Le Vaillant M, Ducluzeau PH, Dubois S, Henni S, Abraham P, Aubé C, Calès P, Gagnadoux F; on the behalf of the METABOL group. Increased liver stiffness in patients with severe sleep apnoea and metabolic comorbidities. Eur Respir J. 2018;51:1800601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 7. | Everson CA, Henchen CJ, Szabo A, Hogg N. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep. 2014;37:1929-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Morin CM, LeBlanc M, Daley M, Gregoire JP, Mérette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 780] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 9. | Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin JL, Peppard PE, Sinha S, Tufik S, Valentine K, Malhotra A. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 2668] [Article Influence: 381.1] [Reference Citation Analysis (0)] |
| 10. | Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1305] [Cited by in RCA: 2318] [Article Influence: 210.7] [Reference Citation Analysis (1)] |
| 11. | Ghabril M, Jackson M, Gotur R, Weber R, Orman E, Vuppalanchi R, Chalasani N. Most Individuals With Advanced Cirrhosis Have Sleep Disturbances, Which Are Associated With Poor Quality of Life. Clin Gastroenterol Hepatol. 2017;15:1271-1278.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Saleh K, Javaheri S. Sleep in ambulatory patients with stable cirrhosis of the liver. Sleep Med. 2018;41:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Song J, Fan L, Shi D, Lai X, Wang H, Liu W, Yu L, Liang R, Zhang Y, Wan S, Yang Y, Wang B. Sleep and liver function biomarkers in relation to risk of incident liver cancer: a nationwide prospective cohort study. BMC Med. 2024;22:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Long L, Zhao L, Petrick JL, Liao LM, Huang T, Hakim A, Yang W, Campbell PT, Giovannucci E, McGlynn KA, Zhang X. Daytime napping, nighttime sleeping duration, and risk of hepatocellular carcinoma and liver disease-related mortality. JHEP Rep. 2023;5:100819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 15. | Morin CM, Buysse DJ. Management of Insomnia. N Engl J Med. 2024;391:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 16. | Cao XL, Wang SB, Zhong BL, Zhang L, Ungvari GS, Ng CH, Li L, Chiu HF, Lok GK, Lu JP, Jia FJ, Xiang YT. The prevalence of insomnia in the general population in China: A meta-analysis. PLoS One. 2017;12:e0170772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 17. | Quan SF. Sleep Disturbances and their Relationship to Cardiovascular Disease. Am J Lifestyle Med. 2009;3:55s-59s. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Lin QC, Chen LD, Chen GP, Zhao JM, Chen X, Huang JF, Wu LH. Association between nocturnal hypoxia and liver injury in the setting of nonalcoholic fatty liver disease. Sleep Breath. 2015;19:273-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Qu A, Taylor M, Xue X, Matsubara T, Metzger D, Chambon P, Gonzalez FJ, Shah YM. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54:472-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Yoon JA, Han DH, Noh JY, Kim MH, Son GH, Kim K, Kim CJ, Pak YK, Cho S. Meal time shift disturbs circadian rhythmicity along with metabolic and behavioral alterations in mice. PLoS One. 2012;7:e44053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Filipski E, Lévi F. Circadian disruption in experimental cancer processes. Integr Cancer Ther. 2009;8:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS, Yoo SH, Chen Z. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016;23:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 412] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 23. | IARC Monographs Vol 124 group. Carcinogenicity of night shift work. Lancet Oncol. 2019;20:1058-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 24. | Wegrzyn LR, Tamimi RM, Rosner BA, Brown SB, Stevens RG, Eliassen AH, Laden F, Willett WC, Hankinson SE, Schernhammer ES. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses' Health Studies. Am J Epidemiol. 2017;186:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 25. | Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, Hammar N, Hrafnkelsson J, Kyyrönen P, Linnersjö A, Rafnsson V, Storm H, Tveten U. Incidence of cancer among Nordic airline pilots over five decades: occupational cohort study. BMJ. 2002;325:567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | VoPham T, Weaver MD, Vetter C, Hart JE, Tamimi RM, Laden F, Bertrand KA. Circadian Misalignment and Hepatocellular Carcinoma Incidence in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 752] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 28. | McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30:1969-1983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 29. | D'souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020;26:5759-5783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (8)] |
| 30. | Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrilhon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011;56:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Nenu I, Toadere TM, Topor I, Țichindeleanu A, Bondor DA, Trella ȘE, Sparchez Z, Filip GA. Interleukin-6 in Hepatocellular Carcinoma: A Dualistic Point of View. Biomedicines. 2023;11:2623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Xu X, Lei Y, Chen L, Zhou H, Liu H, Jiang J, Yang Y, Wu B. Phosphorylation of NF-κBp65 drives inflammation-mediated hepatocellular carcinogenesis and is a novel therapeutic target. J Exp Clin Cancer Res. 2021;40:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253-E261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Liu X, Chen B, Huang Z, Duan R, Li H, Xie L, Wang R, Li Z, Gao Y, Zheng Y, Su W. Effects of poor sleep on the immune cell landscape as assessed by single-cell analysis. Commun Biol. 2021;4:1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Huang J, Song P, Hang K, Chen Z, Zhu Z, Zhang Y, Xu J, Qin J, Wang B, Qu W, Huang Z, Liang C. Sleep Deprivation Disturbs Immune Surveillance and Promotes the Progression of Hepatocellular Carcinoma. Front Immunol. 2021;12:727959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Zager A, Ruiz FS, Tufik S, Andersen ML. Immune outcomes of paradoxical sleep deprivation on cellular distribution in naive and lipopolysaccharide-stimulated mice. Neuroimmunomodulation. 2012;19:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Axelsson J, Rehman JU, Akerstedt T, Ekman R, Miller GE, Höglund CO, Lekander M. Effects of sustained sleep restriction on mitogen-stimulated cytokines, chemokines and T helper 1/ T helper 2 balance in humans. PLoS One. 2013;8:e82291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Hansford RG, Hogue BA, Mildaziene V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr. 1997;29:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 344] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 39. | Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2999] [Cited by in RCA: 3367] [Article Influence: 160.3] [Reference Citation Analysis (0)] |
| 40. | Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1002] [Article Influence: 58.9] [Reference Citation Analysis (6)] |
| 41. | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8502] [Cited by in RCA: 9055] [Article Influence: 452.8] [Reference Citation Analysis (0)] |
| 42. | Vaccaro A, Kaplan Dor Y, Nambara K, Pollina EA, Lin C, Greenberg ME, Rogulja D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell. 2020;181:1307-1328.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 350] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 43. | Wu Z, Liu L, Li L, Cao X, Jia W, Liao X, Zhao Z, Qi H, Fan G, Lu H, Shu C, Zhen M, Wang C, Bai C. Oral nano-antioxidants improve sleep by restoring intestinal barrier integrity and preventing systemic inflammation. Natl Sci Rev. 2023;10:nwad309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 44. | Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082-8091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 910] [Cited by in RCA: 840] [Article Influence: 70.0] [Reference Citation Analysis (15)] |
| 45. | Almalki WH, Almujri SS. Aging, ROS, and cellular senescence: a trilogy in the progression of liver fibrosis. Biogerontology. 2024;26:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 46. | Chen JS, Chiu SC, Huang SY, Chang SF, Liao KF. Isolinderalactone Induces Apoptosis, Autophagy, Cell Cycle Arrest and MAPK Activation through ROS-Mediated Signaling in Colorectal Cancer Cell Lines. Int J Mol Sci. 2023;24:14246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 47. | Dong M, Ding Y, Liu Y, Xu Z, Hong H, Sun H, Huang X, Yu X, Chen Q. Molecular insights of 2,6-dichlorobenzoquinone-induced cytotoxicity in zebrafish embryo: Activation of ROS-mediated cell cycle arrest and apoptosis. Environ Toxicol. 2023;38:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Li C, Deng C, Wang S, Dong X, Dai B, Guo W, Guo Q, Feng Y, Xu H, Song X, Cao L. A novel role for the ROS-ATM-Chk2 axis mediated metabolic and cell cycle reprogramming in the M1 macrophage polarization. Redox Biol. 2024;70:103059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 71] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 49. | Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1147] [Cited by in RCA: 1486] [Article Influence: 212.3] [Reference Citation Analysis (0)] |
| 50. | Song F, Lin J, Zhang H, Guo Y, Mao Y, Liu Z, Li G, Wang Y. Long-Term Sleep Deprivation-Induced Myocardial Remodeling and Mitochondrial Dysfunction in Mice Were Attenuated by Lipoic Acid and N-Acetylcysteine. Pharmaceuticals (Basel). 2022;16:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Yi ZY, Liang QX, Zhou Q, Yang L, Meng QR, Li J, Lin YH, Cao YP, Zhang CH, Schatten H, Qiao J, Sun QY. Maternal total sleep deprivation causes oxidative stress and mitochondrial dysfunction in oocytes associated with fertility decline in mice. PLoS One. 2024;19:e0306152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 52. | Xu W, Hellerbrand C, Köhler UA, Bugnon P, Kan YW, Werner S, Beyer TA. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab Invest. 2008;88:1068-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 53. | Davinelli S, Medoro A, Savino R, Scapagnini G. Sleep and Oxidative Stress: Current Perspectives on the Role of NRF2. Cell Mol Neurobiol. 2024;44:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 54. | Yang J, Zheng L, Yang Z, Wei Z, Shao J, Zhang Y, Yao J, Li M, Wang X, Zheng M. 5-FU promotes HBV replication through oxidative stress-induced autophagy dysfunction. Free Radic Biol Med. 2024;213:233-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 55. | Yuan K, Lei Y, Chen HN, Chen Y, Zhang T, Li K, Xie N, Wang K, Feng X, Pu Q, Yang W, Wu M, Xiang R, Nice EC, Wei Y, Huang C. HBV-induced ROS accumulation promotes hepatocarcinogenesis through Snail-mediated epigenetic silencing of SOCS3. Cell Death Differ. 2016;23:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 910] [Cited by in RCA: 963] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 57. | Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583-G589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 565] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 58. | Savova MS, Vasileva LV, Mladenova SG, Amirova KM, Ferrante C, Orlando G, Wabitsch M, Georgiev MI. Ziziphus jujuba Mill. leaf extract restrains adipogenesis by targeting PI3K/AKT signaling pathway. Biomed Pharmacother. 2021;141:111934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1021] [Cited by in RCA: 996] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 60. | Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 61. | Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med. 2012;18:388-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 62. | O-Sullivan I, Zhang W, Wasserman DH, Liew CW, Liu J, Paik J, DePinho RA, Stolz DB, Kahn CR, Schwartz MW, Unterman TG. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat Commun. 2015;6:7079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 63. | Titchenell PM, Chu Q, Monks BR, Birnbaum MJ. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nat Commun. 2015;6:7078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 64. | Herman R, Kravos NA, Jensterle M, Janež A, Dolžan V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int J Mol Sci. 2022;23:1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 65. | Shan Y, Lu C, Wang J, Li M, Ye S, Wu S, Huang J, Bu S, Wang F. IGF-1 contributes to liver cancer development in diabetes patients by promoting autophagy. Ann Hepatol. 2022;27:100697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 725] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 67. | Bo T, Gao L, Yao Z, Shao S, Wang X, Proud CG, Zhao J. Hepatic selective insulin resistance at the intersection of insulin signaling and metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2024;36:947-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 119] [Reference Citation Analysis (0)] |
| 68. | Zhou C, Hu Z, Liu X, Wang Y, Wei S, Liu Z. Disruption of the peripheral biological clock may play a role in sleep deprivation-induced dysregulation of lipid metabolism in both the daytime and nighttime phases. Biochim Biophys Acta Mol Cell Biol Lipids. 2024;1869:159530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 69. | Sengupta A, Tudor JC, Cusmano D, Baur JA, Abel T, Weljie AM. Sleep deprivation and aging are metabolically linked across tissues. Sleep. 2023;46:zsad246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Rogava M, Aprati TJ, Chi WY, Melms JC, Hug C, Davis SH, Earlie EM, Chung C, Deshmukh SK, Wu S, Sledge G, Tang S, Ho P, Amin AD, Caprio L, Gurjao C, Tagore S, Ngo B, Lee MJ, Zanetti G, Wang Y, Chen S, Ge W, Melo LMN, Allies G, Rösler J, Gibney GT, Schmitz OJ, Sykes M, Creusot RJ, Tüting T, Schadendorf D, Röcken M, Eigentler TK, Molotkov A, Mintz A, Bakhoum SF, Beyaz S, Cantley LC, Sorger PK, Meckelmann SW, Tasdogan A, Liu D, Laughney AM, Izar B. Loss of Pip4k2c confers liver-metastatic organotropism through insulin-dependent PI3K-AKT pathway activation. Nat Cancer. 2024;5:433-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Kettner NM, Voicu H, Finegold MJ, Coarfa C, Sreekumar A, Putluri N, Katchy CA, Lee C, Moore DD, Fu L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell. 2016;30:909-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 386] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 72. | Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 446] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 73. | Teng F, Goc J, Zhou L, Chu C, Shah MA, Eberl G, Sonnenberg GF. A circadian clock is essential for homeostasis of group 3 innate lymphoid cells in the gut. Sci Immunol. 2019;4:eaax1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 74. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1074] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 75. | Jagavelu K, Routray C, Shergill U, O'Hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 76. | Ren Z, Li A, Jiang J, Zhou L, Yu Z, Lu H, Xie H, Chen X, Shao L, Zhang R, Xu S, Zhang H, Cui G, Chen X, Sun R, Wen H, Lerut JP, Kan Q, Li L, Zheng S. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. 2019;68:1014-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 555] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 77. | Gram A, Kowalewski MP. Molecular Mechanisms of Lipopolysaccharide (LPS) Induced Inflammation in an Immortalized Ovine Luteal Endothelial Cell Line (OLENDO). Vet Sci. 2022;9:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Ponziani FR, Putignani L, Paroni Sterbini F, Petito V, Picca A, Del Chierico F, Reddel S, Calvani R, Marzetti E, Sanguinetti M, Gasbarrini A, Pompili M. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther. 2018;48:1301-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 79. | Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1113] [Cited by in RCA: 1356] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 80. | Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, Platz EA, Pardoll DM, Sears CL. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 475] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 81. | Mangerich A, Knutson CG, Parry NM, Muthupalani S, Ye W, Prestwich E, Cui L, McFaline JL, Mobley M, Ge Z, Taghizadeh K, Wishnok JS, Wogan GN, Fox JG, Tannenbaum SR, Dedon PC. Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc Natl Acad Sci U S A. 2012;109:E1820-E1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 82. | Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 2036] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 83. | Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 766] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 84. | Stöhr T, Colin PJ, Ossig J, Pesic M, Borkett K, Winkle P, Struys MMRF, Schippers F. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 85. | Kaul M, Zee PC, Sahni AS. Effects of Cannabinoids on Sleep and their Therapeutic Potential for Sleep Disorders. Neurotherapeutics. 2021;18:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 86. | Zhao FJ, Huo RR, Li FR, You XM. Associations of tumor-related psychiatric symptoms and healthy behaviors with dynamic quality of life after hepatocellular carcinoma hepatectomy. Support Care Cancer. 2024;32:589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 87. | Elamin N, Althebity N, Alkhamisi TA, Al-Foheidi M. Sleep quality and psychological disorders in breast cancer female patients receiving radiotherapy at a tertiary oncology center in West Saudi Arabia. Support Care Cancer. 2024;32:163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 88. | Li W, Kwok CC, Chan DC, Ho AW, Ho CS, Zhang J, Wing YK, Wang F, Tse LA. Disruption of sleep, sleep-wake activity rhythm, and nocturnal melatonin production in breast cancer patients undergoing adjuvant chemotherapy: prospective cohort study. Sleep Med. 2019;55:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 89. | Lai YH, Shun SC, Hsiao YL, Chiou JF, Wei LL, Tsai JT, Kao CY. Fatigue experiences in hepatocellular carcinoma patients during six weeks of stereotactic radiotherapy. Oncologist. 2007;12:221-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Huang TW, Lin CC. The mediating effects of depression on sleep disturbance and fatigue: symptom clusters in patients with hepatocellular carcinoma. Cancer Nurs. 2009;32:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, Jeffries AR, Dashti HS, Hillsdon M, Ruth KS, Tuke MA, Yaghootkar H, Sharp SA, Jie Y, Thompson WD, Harrison JW, Dawes A, Byrne EM, Tiemeier H, Allebrandt KV, Bowden J, Ray DW, Freathy RM, Murray A, Mazzotti DR, Gehrman PR, Lawlor DA, Frayling TM, Rutter MK, Hinds DA, Saxena R, Weedon MN. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 486] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 92. | Kalsbeek A, Scheer FA, Perreau-Lenz S, La Fleur SE, Yi CX, Fliers E, Buijs RM. Circadian disruption and SCN control of energy metabolism. FEBS Lett. 2011;585:1412-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 93. | Chellappa SL, Qian J, Vujovic N, Morris CJ, Nedeltcheva A, Nguyen H, Rahman N, Heng SW, Kelly L, Kerlin-Monteiro K, Srivastav S, Wang W, Aeschbach D, Czeisler CA, Shea SA, Adler GK, Garaulet M, Scheer FAJL. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci Adv. 2021;7:eabg9910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 94. | Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. The sleep and technology use of Americans: findings from the National Sleep Foundation's 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 299] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 95. | Tähkämö L, Partonen T, Pesonen AK. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int. 2019;36:151-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 96. | Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112:1232-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 767] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 97. | Okamoto-Mizuno K, Mizuno K. Effects of thermal environment on sleep and circadian rhythm. J Physiol Anthropol. 2012;31:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 98. | Basner M, Babisch W, Davis A, Brink M, Clark C, Janssen S, Stansfeld S. Auditory and non-auditory effects of noise on health. Lancet. 2014;383:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1057] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 99. | Riemann D, Espie CA, Altena E, Arnardottir ES, Baglioni C, Bassetti CLA, Bastien C, Berzina N, Bjorvatn B, Dikeos D, Dolenc Groselj L, Ellis JG, Garcia-Borreguero D, Geoffroy PA, Gjerstad M, Gonçalves M, Hertenstein E, Hoedlmoser K, Hion T, Holzinger B, Janku K, Jansson-Fröjmark M, Järnefelt H, Jernelöv S, Jennum PJ, Khachatryan S, Krone L, Kyle SD, Lancee J, Leger D, Lupusor A, Marques DR, Nissen C, Palagini L, Paunio T, Perogamvros L, Pevernagie D, Schabus M, Shochat T, Szentkiralyi A, Van Someren E, van Straten A, Wichniak A, Verbraecken J, Spiegelhalder K. The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023. J Sleep Res. 2023;32:e14035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 377] [Article Influence: 125.7] [Reference Citation Analysis (0)] |
| 100. | Barker MJ, Greenwood KM, Jackson M, Crowe SF. Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: a meta-analysis. Arch Clin Neuropsychol. 2004;19:437-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 198] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 101. | Rapoport MJ, Lanctôt KL, Streiner DL, Bédard M, Vingilis E, Murray B, Schaffer A, Shulman KI, Herrmann N. Benzodiazepine use and driving: a meta-analysis. J Clin Psychiatry. 2009;70:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 102. | Yue JL, Chang XW, Zheng JW, Shi L, Xiang YJ, Que JY, Yuan K, Deng JH, Teng T, Li YY, Sun W, Sun HQ, Vitiello MV, Tang XD, Zhou XY, Bao YP, Shi J, Lu L. Efficacy and tolerability of pharmacological treatments for insomnia in adults: A systematic review and network meta-analysis. Sleep Med Rev. 2023;68:101746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 103. | Stranks EK, Crowe SF. The acute cognitive effects of zopiclone, zolpidem, zaleplon, and eszopiclone: a systematic review and meta-analysis. J Clin Exp Neuropsychol. 2014;36:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 104. | Schreiber S, Pick CG. Trazodone and mirtazapine: A possible opioid involvement in their use (at low dose) for sleep? Med Hypotheses. 2020;136:109501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Everitt H, Baldwin DS, Stuart B, Lipinska G, Mayers A, Malizia AL, Manson CC, Wilson S. Antidepressants for insomnia in adults. Cochrane Database Syst Rev. 2018;5:CD010753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 106. | Choi K, Lee YJ, Park S, Je NK, Suh HS. Efficacy of melatonin for chronic insomnia: Systematic reviews and meta-analyses. Sleep Med Rev. 2022;66:101692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 107. | Marupuru S, Arku D, Campbell AM, Slack MK, Lee JK. Use of Melatonin and/on Ramelteon for the Treatment of Insomnia in Older Adults: A Systematic Review and Meta-Analysis. J Clin Med. 2022;11:5138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 108. | Wu X, Xue T, Chen Z, Wang Z, Chen G. Orexin Receptor Antagonists and Insomnia. Curr Psychiatry Rep. 2022;24:509-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Han Y, Yuan K, Zheng Y, Lu L. Orexin Receptor Antagonists as Emerging Treatments for Psychiatric Disorders. Neurosci Bull. 2020;36:432-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 110. | Hertenstein E, Trinca E, Wunderlin M, Schneider CL, Züst MA, Fehér KD, Su T, Straten AV, Berger T, Baglioni C, Johann A, Spiegelhalder K, Riemann D, Feige B, Nissen C. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: A systematic review and meta-analysis. Sleep Med Rev. 2022;62:101597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (1)] |
| 111. | Furukawa Y, Sakata M, Yamamoto R, Nakajima S, Kikuchi S, Inoue M, Ito M, Noma H, Takashina HN, Funada S, Ostinelli EG, Furukawa TA, Efthimiou O, Perlis M. Components and Delivery Formats of Cognitive Behavioral Therapy for Chronic Insomnia in Adults: A Systematic Review and Component Network Meta-Analysis. JAMA Psychiatry. 2024;81:357-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 73] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 112. | Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med Rev. 2015;22:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 560] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 113. | Baillargeon L, Demers M, Ladouceur R. Stimulus-control: nonpharmacologic treatment for insomnia. Can Fam Physician. 1998;44:73-79. [PubMed] |
| 114. | Kyle SD, Siriwardena AN, Espie CA, Yang Y, Petrou S, Ogburn E, Begum N, Maurer LF, Robinson B, Gardner C, Lee V, Armstrong S, Pattinson J, Mort S, Temple E, Harris V, Yu LM, Bower P, Aveyard P. Clinical and cost-effectiveness of nurse-delivered sleep restriction therapy for insomnia in primary care (HABIT): a pragmatic, superiority, open-label, randomised controlled trial. Lancet. 2023;402:975-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 115. | Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163:191-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 686] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 116. | Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep. 2007;30:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 117. | Kim D, Ahmed A, Kushida C. Continuous Positive Airway Pressure Therapy on Nonalcoholic Fatty Liver Disease in Patients With Obstructive Sleep Apnea. J Clin Sleep Med. 2018;14:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 118. | Giannaki CD, Sakkas GK, Hadjigeorgiou GM, Manconi M, Bargiotas P. Unfolding the role of exercise in the management of sleep disorders. Eur J Appl Physiol. 2024;124:2547-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 119. | Zhang J, Zhang Z, Huang S, Qiu X, Lao L, Huang Y, Zhang ZJ. Acupuncture for cancer-related insomnia: A systematic review and meta-analysis. Phytomedicine. 2022;102:154160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 120. | Kim SA, Lee SH, Kim JH, van den Noort M, Bosch P, Won T, Yeo S, Lim S. Efficacy of Acupuncture for Insomnia: A Systematic Review and Meta-Analysis. Am J Chin Med. 2021;49:1135-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 121. | Gaballah S, El-Deen DS, Hebeshy MI. Effect of effleurage massage therapy on sleep disturbance, fatigue, pain, and anxiety in patients with multiple sclerosis: A quasi-experimental study. Appl Nurs Res. 2023;73:151719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 122. | Xu Z, Duan W, Yuan S, Zhang X, You C, Yu JT, Wang J, Li JD, Deng S, Shu Y. Deep brain stimulation alleviates Parkinsonian motor deficits through desynchronizing GABA release in mice. Nat Commun. 2025;16:3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 123. | Krauss JK, Lipsman N, Aziz T, Boutet A, Brown P, Chang JW, Davidson B, Grill WM, Hariz MI, Horn A, Schulder M, Mammis A, Tass PA, Volkmann J, Lozano AM. Technology of deep brain stimulation: current status and future directions. Nat Rev Neurol. 2021;17:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 492] [Article Influence: 82.0] [Reference Citation Analysis (0)] |