Published online Jan 15, 2025. doi: 10.4251/wjgo.v17.i1.99376
Revised: October 5, 2024
Accepted: November 1, 2024
Published online: January 15, 2025
Processing time: 144 Days and 0.7 Hours
Esophageal squamous cell carcinoma (ESCC) is a malignant tumor with high morbidity and mortality, and easy to develop resistance to chemotherapeutic agents. Telomeres are DNA-protein complexes located at the termini of chro
To investigate the efficacy and underlying mechanisms of BIBR1532, a telomerase inhibitor, in ESCC.
KYSE150 and KYSE410 cells were cultured and exposed to various concentrations of BIBR1532. Cell viability was assessed at 48 hours and 72 hours to determine the IC50 values. The effects of BIBR1532 on ESCC cell proliferation, migration, and cellular senescence were evaluated using the cell counting kit-8 assay, plate colony formation assay, scratch assay, transwell assay, and β-galactosidase staining, respectively. Western blotting was performed to detect the expression of proteins in BIBR1532-treated ESCC cells, such as human telomerase reverse transcriptase (hTERT), key molecules involved in DNA damage response (DDR) or cellular senescence, as well as telomere-binding proteins. Additionally, a tumor-bearing nude mouse model was established to evaluate the anti-cancer effect of BIBR1532 in vivo.
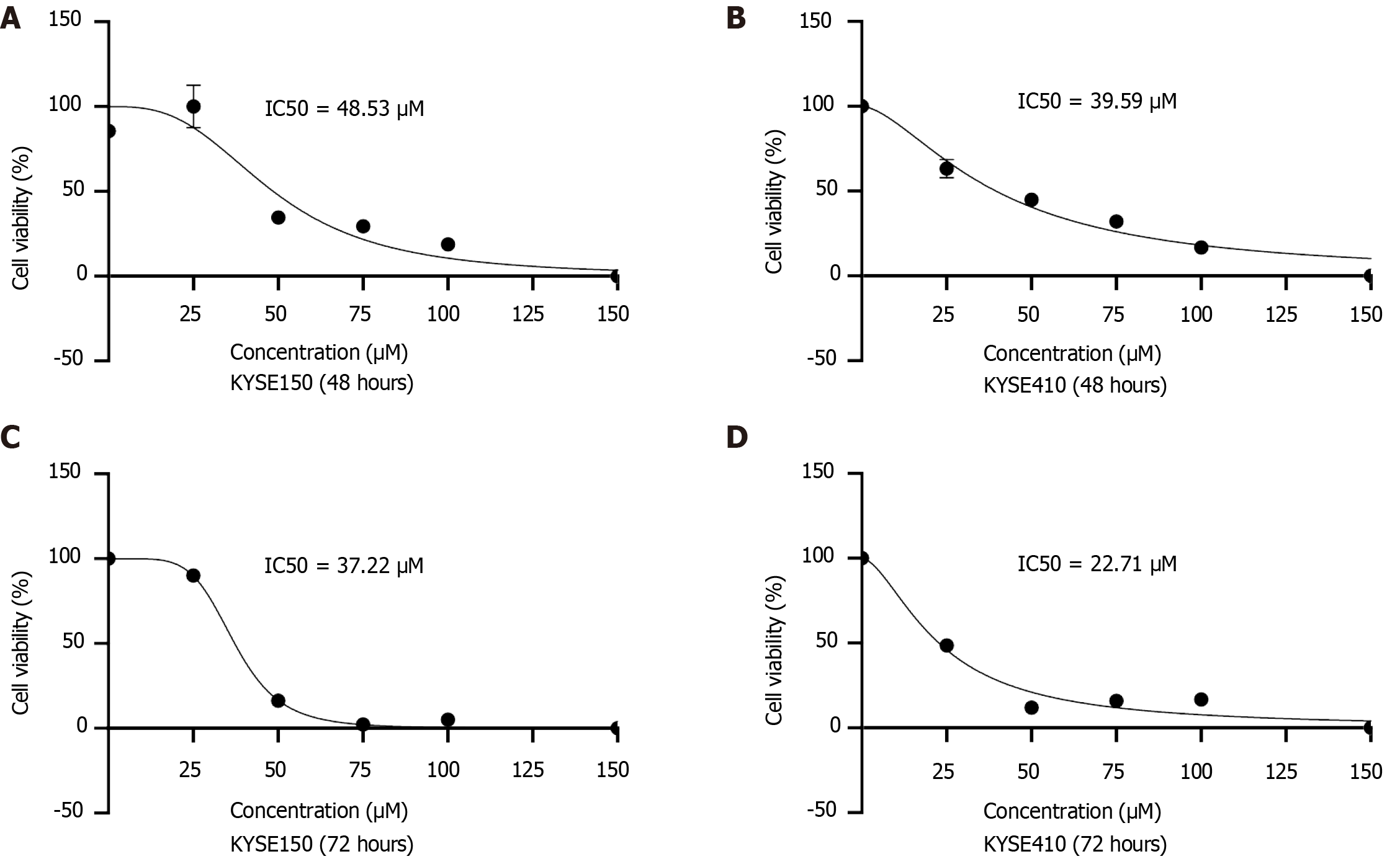
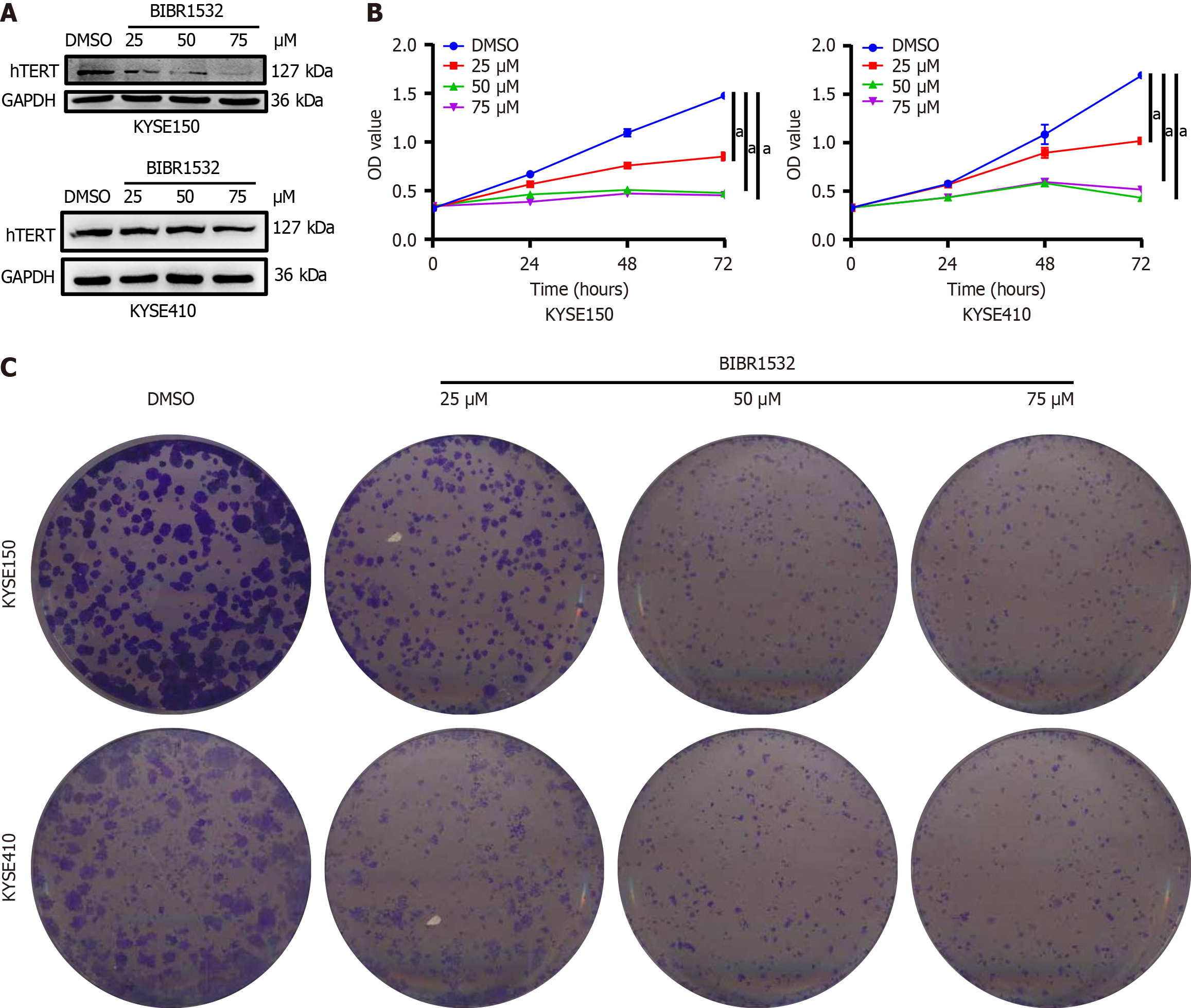
The IC50 values for KYSE150 and KYSE410 cells after 48 hours of BIBR1532 exposure were 48.53 μM and 39.59 μM, respectively. These values decreased to 37.22 μM and 22.71 μM, respectively, following a longer exposure of 72 hours. BIBR1532 exhibited dose-dependent effects on KYSE150 and KYSE410 cells, including decreased hTERT expression, inhibition of proliferation and metastasis, and induction of cellular senescence. Mechanistically, BIBR1532 upregulated the expression of the DDR protein, γ-H2AX, and activated the ataxia telangiectasia and Rad3-related protein (ATR)/ check point kinase 1 (CHK-1) and ataxia-telangiectasia mutated gene (ATM)/CHK2 pathways. BIBR1532 downregulated the expression of telomere-binding proteins, including telomeric-repeat binding factor 1 (TRF1), TRF2, protection of telomeres 1, and TIN2-interacting protein 1. In a nude mouse xenograft model, BIBR1532 significantly suppressed tumor growth, reduced hTERT expression, and increased γ-H2AX protein levels. Hematoxylin and eosin staining of various organs, including the heart, liver, spleen, lungs, and kidneys, revealed no apparent adverse effects.
BIBR1532 exerts anti-cancer effects on ESCC by inducing DDR through the ATR/CHK1 and ATM/CHK2 pathways and downregulating the expression of telomere-binding proteins.
Core Tip: BIBR1532 inhibited proliferation and metastasis of esophageal squamous cell carcinoma (ESCC) cells in a dose-dependent manner, which also effectively blocked the growth of ESCC in tumor-bearing nude mouse model. Mechanistically, BIBR1532 downregulated the expression of telomere-binding proteins, upregulated the expression of phosphorylated histone H2AX, ataxia telangiectasia and Rad3-related protein/check point kinase 1 (CHK-1) and ataxia-telangiectasia mutated gene/CHK2 pathways. The in vitro and in vivo studies showed that BIBR1532 is a potential chemotherapeutic drug for ESCC.
- Citation: Wang Q, Li QR, Xu L, Yuan ZC, Liu X, Tang MJ, Luo M, Zhong XW, Ma Q, Guo XL. BIBR1532 inhibits proliferation and metastasis of esophageal squamous cancer cells by inducing telomere dysregulation. World J Gastrointest Oncol 2025; 17(1): 99376
- URL: https://www.wjgnet.com/1948-5204/full/v17/i1/99376.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i1.99376
Esophageal cancer (EC) is one of the most frequently occurring malignant tumors worldwide, ranking seventh in terms of incidence and sixth in terms of mortality[1]. Approximately 600000 new cases of EC are reported globally each year, and over half of these occur in China. Esophageal squamous cell carcinoma (ESCC) is the predominant histological variety, constituting more than 90% of cases[1-3]. The incidence of ESCC has been correlated with various dietary and lifestyle factors, including the consumption of very hot food and drinks, alcohol intake, and cigarette smoking. Additional risk factors include the consumption of mold-contaminated food, food preparation methods involving charring or smoking, drinking water quality, soil composition, and environmental microbial communities[4]. According to the EC Diagnosis and Treatment Guidelines (version 2022), ESCC treatment modalities include surgery, radiotherapy, chemotherapy, targeted therapy, immunotherapy, and combination therapies[4]. However, the initial stages of EC are often asymp
Telomeres are DNA-protein complexes located at the termini of chromosomes in eukaryotic cells. They consist of short repetitive sequences of telomeric DNA (5’-TTAGGG-3’) and associated proteins [telomeric-repeat binding factor 1 (TRF1), TRF2, TIN2-interacting protein 1 (TPP1), protection of telomeres 1 (POT1), TRF1-interacting nuclear protein 2 (TIN2), and repressor activation protein 1 (RAP1)]. The primary role of telomeres is to shield chromosome ends from degradation or aberrant recombination[9,10]. Telomere length homeostasis depends on cellular division and telomerase activity. Telomerase is an RNA-dependent DNA polymerase comprising RNA molecules with telomeric template sequences and a protein catalytic subunit telomerase reverse transcriptase (TERT)[11]. Several studies have identified human TERT (hTERT) as a key regulator of telomerase activity[12]. Although telomerase remains transcriptionally inactive in most somatic cells, it is reactivated in over 85% of human cancers, primarily through upregulation of the typically silent hTERT gene[13]. Our previous study demonstrated that the expression of hTERT mRNA in ESCC tissues is significantly upregulated and that hTERT overexpression substantially enhances the proliferation and metastatic potential of cancer cells[14]. These findings suggest that hTERT contributes to ESCC progression and is a potential therapeutic target in afflicted patients.
For nearly two decades, targeting telomerase has been the focus of cancer research. Therapeutic strategies include immunotherapy, small-molecule telomerase inhibitors, oligonucleotide inhibitors, and plant compounds[15]. Among these, the small molecule inhibitor BIBR1532 is a non-nucleoside, non-competitive antagonist that selectively inhibits hTERT[16]. Lavanya et al[17] demonstrated that BIBR1532 dose-dependently curtails telomerase activity in human glioblastoma LN18 cells, inducing apoptosis through downregulation of telomerase at both the transcriptional and translational levels. Ding et al[18] revealed that lower concentrations of BIBR1532 effectively suppress telomerase activity and exacerbate radiation-induced telomeric dysfunction, compromising chromosome stability and inhibiting the ataxia-telangiectasia mutated gene (ATM)/check point kinase 1 (CHK-1) pathway, thereby impeding DNA damage response (DDR). Another study indicated that telomerase inhibition could regulate epithelial-mesenchymal transition (EMT) in breast cancer, suggesting that telomerase inhibitors primarily eradicate breast cancer stem cells, constrain cellular migration and invasiveness, and ultimately prevent breast cancer cell metastasis[19]. Collectively, these studies imply that BIBR1532 may function as a broad-spectrum anti-cancer agent. However, the effects of BIBR1532 on ESCC have yet to be reported. This study aimed to decipher the biological impact and molecular mechanisms of the telomerase inhibitor BIBR1532 in ESCC cells and provide data for its future clinical use as a potential therapeutic agent for ESCC.
RPMI 1640 medium was obtained from Gibco (Grand Island, NY, United States). Fetal bovine serum (FBS) was purchased from OPCEL (Inner Mongolia, China). Penicillin and Streptomycin were procured from SolarBio (Beijing, China). BIBR1532 was obtained from Pottery (Shanghai, China). Cell counting kit-8 (CCK-8) and β-galactosidase (β-gal) detection kits were obtained from Beyotime Biotechnology (Shanghai, China). The cell cycle detection kit was provided by KeyGen Biotech (Nanjing, Jiangsu Province, China). Immunohistochemistry (IHC) kit was purchased from BOSTER (Wuhan, Hubei Province, China). Antibodies against GAPDH, CHK1, CHK2, phosphorylated histone H2AX (γ-H2AX), phosphorylated ataxia telangiectasia and Rad3-related protein (p-ATR), phosphorylated ATM, hTERT, and goat anti-rabbit IgG were purchased from Cell Signaling Technology (Beverly, CA, United States). Goat anti-rabbit IgG antibody was purchased from BOSTER (Wuhan, Hubei Province, China).
The human ESCC cell lines KYSE150 and KYSE410 were obtained from the Translational Medicine Research Center of the North Sichuan Medical College. All the cells were cultured in DMEM supplemented with 10% FBS at 37 °C in a humidified atmosphere containing 5% CO2.
CCK-8 assay was performed to assess the viability of KYSE150 and KYSE410 cells. Briefly, the cells were seeded in 96-well plates at a density of 3 × 103 cells/well and incubated at 37 °C in 5% CO2. After 24 hours, cells were treated with DMSO (vehicle control) or a series of BIBR1532 concentrations (25 μM, 50 μM, 75 μM, and 100 μM) for 48 hours or 72 hours. At the indicated time points, 10 μL of the CCK-8 reagent was added to each well. The plates were incubated for 2 hours in the dark, and the absorbance (A) was measured at 450 nm using a microplate reader.
KYSE150 and KYSE410 cells were seeded in six-well plates at a density of 2 × 103 cells/well. After 24 hours of incubation, cells were treated with either DMSO or different concentrations of BIBR1532 (25 μM, 50 μM, and 75 μM). Cells were cultured for 1-2 weeks until visible clones emerged in control wells. Subsequently, cells were fixed with methanol for 10 minutes and stained with crystal violet for 10 minutes. Finally, plates were washed, air-dried, and imaged.
KYSE150 and KYSE410 cells were seeded in six-well plates at a density of 5 × 105 cells/well. When the cell monolayer reached approximately 90% confluence, two parallel scratches were created across the cell layer by using a sterile pipette tip. The plates were washed three times with PBS and fresh culture medium was added. Initial images (0 hour) were captured, and the cells were treated with either DMSO or a series of BIBR1532 concentrations (25 μM, 50 μM, and 75 μM). After 24 hours, images of the same locations were obtained using an optical microscope to assess the wound closure.
KYSE150 and KYSE410 cells were cultured, trypsinized (0.25% w/v), and resuspended in serum-free RPMI 1640. The cell density was adjusted to 5 × 105 cells/mL. Subsequently, 500 μL of RPMI 1640 medium containing 10% FBS was added to the lower chamber of a 12-well plate, and 200 μL of the cell suspension was added to the upper chamber of the Transwell insert. The cells were then treated with DMSO or different concentrations of BIBR1532 (25 μM, 50 μM, and 75 μM). After 24 hours, the non-migrated cells on the upper surface of the membrane were removed, and the migrated cells on the lower surface were fixed with methanol for 10 minutes and stained with crystal violet for 20 minutes. Images were captured using an optical microscope.
KYSE150 and KYSE410 cells were seeded into six-well plates at a density of 1 × 105 cells/well and treated with either DMSO or different concentrations of BIBR1532 (25 μM, 50 μM, and 75 μM). After treatment, cells were washed twice with PBS and fixed at room temperature for 15 minutes. Subsequently, the cells were washed three times with PBS and incubated overnight at 37 °C in β-gal staining solution. The plates were sealed with plastic wrap to prevent evaporation, and the senescent cells were observed under an optical microscope.
Male 6-week-old BALB/c nude mice (approximately 20 g) were purchased from Beijing Laboratory Animal Research Center (Beijing, China). Animal care and experiments were performed with the approval of the Animal Ethics Committee of North Sichuan Medical College. KYSE150 cells in the logarithmic phase of growth were harvested and adjusted to a density of 1 × 107 cells/mL. Subsequently, 100 μL of the cell suspension was subcutaneously implanted into the upper right flank of each mouse. When the tumor volume reached approximately 100 mm3, mice were randomly divided into two groups (n = 4 per group). The treatment group received intraperitoneal injections of BIBR1532 (50 mg/kg), whereas the control group received an equal volume of DMSO. Injections were administered every two days. Tumor dimensions were measured using a Vernier caliper every two days, and tumor volumes were calculated using the following formula: Volume (mm3) = length × width2/2. On day 20, all mice were euthanized and tumor tissues were collected and fixed in 4% paraformaldehyde solution for 24 hours. Fixed tissues were embedded in paraffin for further analysis.
Paraffin-embedded xenograft tissues were sectioned and subjected to immunohistochemical staining. Briefly, sections were deparaffinized, rehydrated, and subjected to antigen retrieval using sodium citrate buffer. The sections were incubated with primary antibodies against hTERT, Ki-67, and γ-H2AX at 4 °C overnight. Subsequently, the sections were stained using an immunostaining kit (BOSTER, Wuhan, Hubei Province, China), according to the manufacturer’s instructions.
Paraffin-embedded tissue sections were deparaffinized and rehydrated. The nuclei were stained with hematoxylin, and the cytoplasm was counterstained with eosin. Finally, the sections were dehydrated, cleared, mounted, and observed under a microscope.
Tissue samples were homogenized, and total proteins were extracted using RIPA buffer. Proteins were separated using SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were washed and blocked with 5% skimmed milk for 1 hour at room temperature. Subsequently, the membranes were incubated with the primary antibodies overnight at 4 °C. After washing, membranes were incubated with secondary antibodies for 1 hour at room temperature. Finally, the protein bands were detected using an enhanced chemiluminescence system (VILBER FUSION FX7, France).
The experimental data were analyzed using the GraphPad Prism 8 software. Data are presented as mean ± SD of three independent experiments. Comparisons between two groups were performed using the Student’s t-test, and comparisons between multiple groups were performed using analysis of variance (ANOVA). Differences were considered statistically significant at P < 0.05.
To assess the potential toxicity of BIBR1532 in ESCC cells, KYSE150 and KYSE410 cells were treated with various concentrations of BIBR1532 (25 μM, 50 μM, 75 μM, and 100 μM) for 48 hours or 72 hours. Cell viability was evaluated, and the half-maximal inhibitory concentration (IC50) values were calculated. Our results indicated a significant dose-dependent decrease in the viability of both KYSE150 and KYSE410 cells post-treatment at both time points, the IC50 of BIBR1532 was 48.53 μM and 39.59 μM in KYSE150 and KYSE410 cells treated for 48 hours, and 37.22 μM and 22.71 μM in abovementioned two cell lines treated for 72 hours (Figure 1). Based on these findings, BIBR1532 concentrations of 25 μM, 50 μM, and 75 μM and a 48-hour treatment duration were selected for subsequent experiments.
Since BIBR1532 is a telomerase inhibitor, we first evaluated whether it regulates the expression of hTERT, a core catalytic subunit of telomerase. The results showed that BIBR1532 significantly downregulated the expression of hTERT in KYSE150 and KYSE410 cells in a dose-dependent manner (Figure 2A). Subsequently, we evaluated the effect of BIBR1532 on the proliferation of KYSE150 and KYSE410 cells. As shown in Figure 2B, BIBR1532 significantly inhibited the viability of both the cell lines. The inhibitory effect on cell viability increased gradually with higher concentrations of BIBR1532 and longer incubation times, in a time- and dose-dependent manner. Plate colony formation assays further confirmed the ability of BIBR1532 to inhibit proliferation of KYSE150 and KYSE410 cells (Figure 2C).
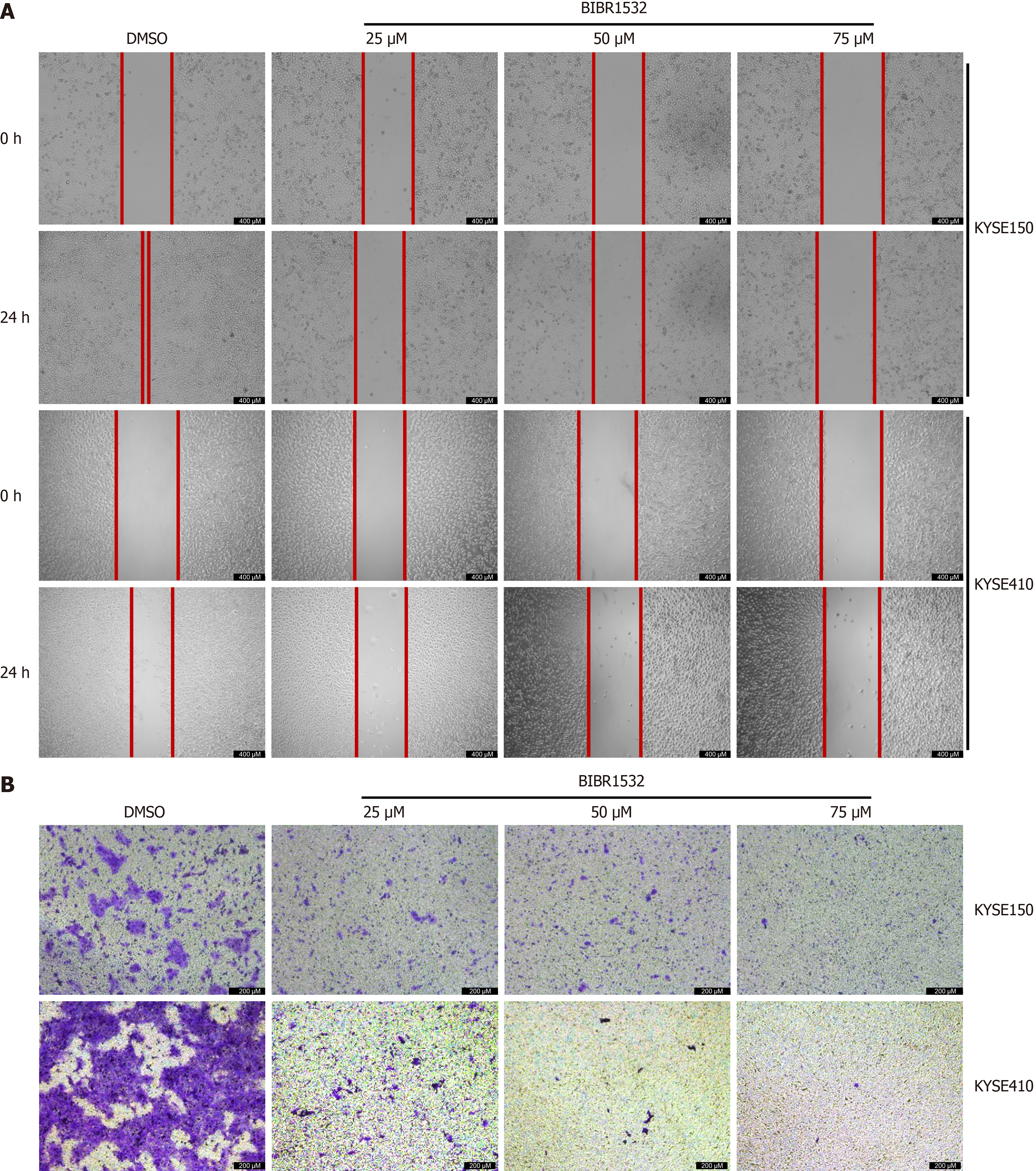
To explore whether BIBR1532 regulates the migration capacity of EC cells, scratch and transwell assays were used to evaluate the migration of KYSE150 and KYSE410 cells after exposure to BIBR1532. The results showed that the migration distance of KYSE150 and KYSE410 cells in the BIBR1532-treated group was significantly shorter than that in the DMSO control group, in a dose-dependent manner (Figure 3A). Similarly, transwell assays revealed that the number of migrated cells in the BIBR1532-treated group was significantly lower than that in the DMSO group (Figure 3B). These results suggested that BIBR1532 significantly inhibits the migration of EC cells.
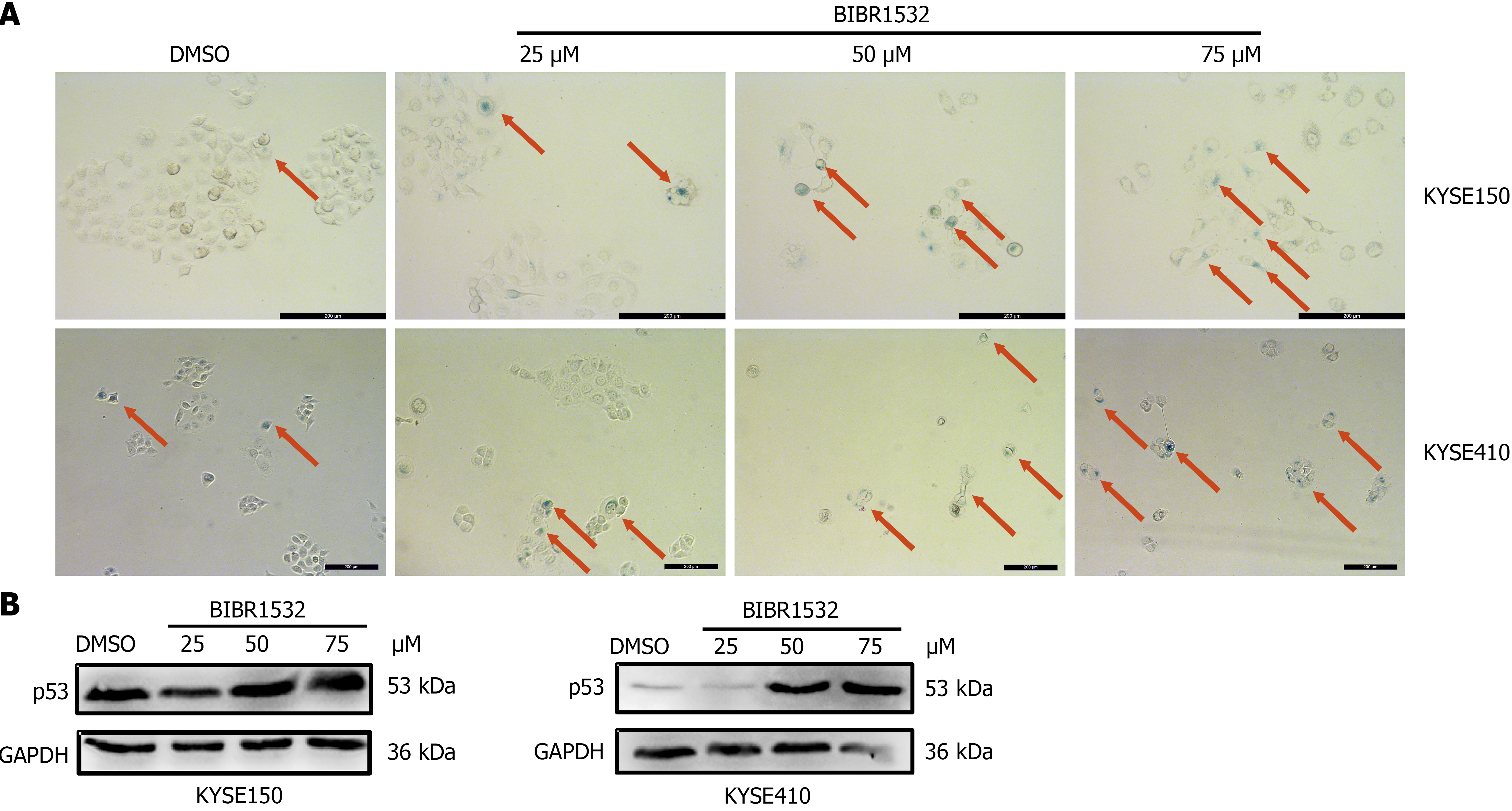
Because BIBR1532 is a telomerase inhibitor, we investigated whether it induced cellular senescence. β-gal staining assay was employed to detect the effect of BIBR1532 on the senescence of KYSE150 and KYSE410 cells. The number of senescent cells in the BIBR1532-treated group was significantly higher than that in the DMSO control group (Figure 4A). In addition, the senescent marker P53 was evidently upregulated after BIBR1532 exposure (Figure 4B). These results showed that BIBR1532 induces senescence in ESCC.
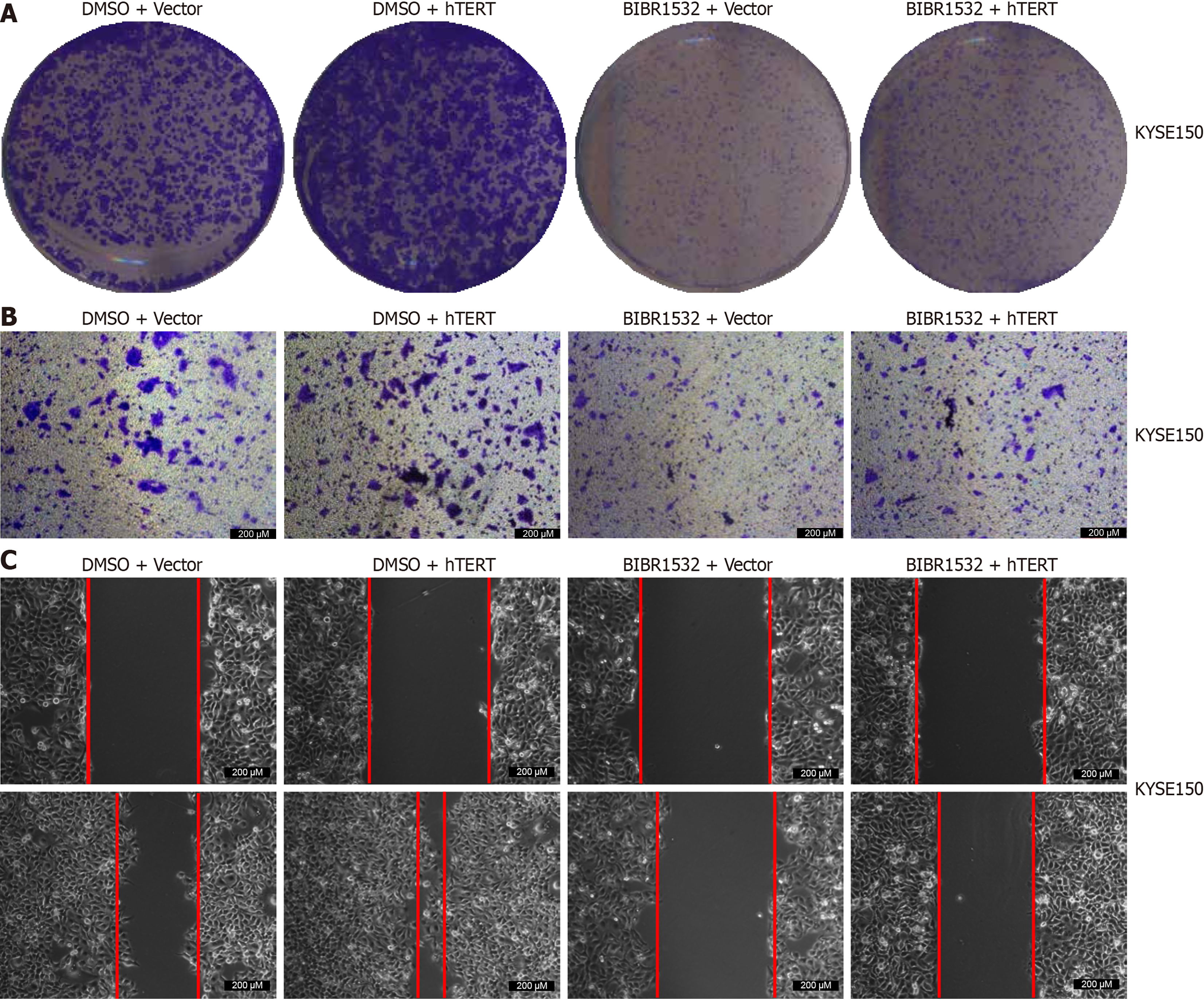
To determine whether BIBR1532 exerted its anti-cancer effect on ESCC cells by downregulating hTERT expression, we treated the cells with BIBR1532 (50 μM) combined with hTERT overexpression. The results showed that BIBR1532 inhibited the proliferation and migration of KYSE150 cells. Moreover, hTERT overexpression in KYSE150 cells promoted ESCC cell proliferation and migration. Importantly, hTERT overexpression alleviated the tumor-suppressive effects of BIBR1532 in KYSE150 cells (Figure 5). These results indicated that BIBR1532 exerts anti-cancer effects on ESCC by downregulating hTERT expression.
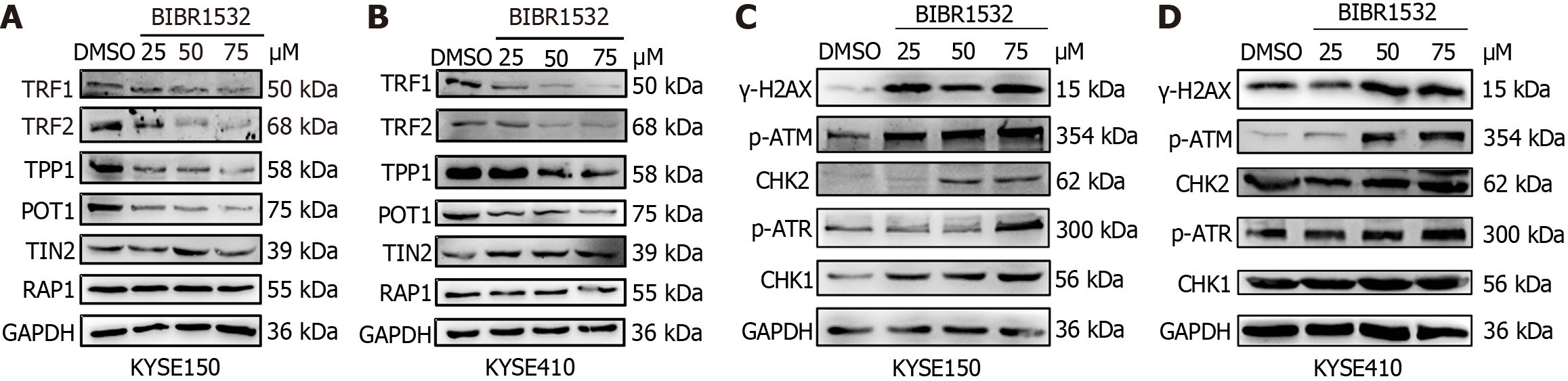
Studies have demonstrated that BIBR1532 is cytotoxic and can cause direct damage to telomere structure, leading to the loss of TRF2 binding, thereby inducing telomere dysfunction[20]. To investigate whether BIBR1532 regulated the expression of other telomere protection proteins, the expression of shelterin proteins (TRF1, TRF2, TPP1, POT1, TIN2, and RAP1) was evaluated in KYSE150 and KYSE410 cells treated with BIBR1532. The results showed that BIBR1532 significantly downregulated the expression of TRF1, TRF2, TPP1, and POT1 in both cell lines, but had no significant effect on the expression of TIN2 and RAP1 (Figure 6A and B). These results indicated that BIBR1532 may lead to the dysregulation of telomere structure and function.
The main function of telomere protection proteins is to protect DNA ends from being recognized as double-strand breaks that trigger DDR[15]. Excessive lengthening or shortening of telomeres results in an imbalance in genome homeostasis, ultimately inducing DDR. Given that BIBR1532 inhibits the expression of hTERT and the telomere protection proteins TRF1, TRF2, TPP1, and POT1, we hypothesized that BIBR1532 can induce DDR in ESCC cells. As shown in Figure 6C and D, the expression of γ-H2AX in KYSE150 and KYSE410 cells was significantly upregulated after treatment with the indicated concentrations of BIBR1532. ATM- and ATR-mediated protein expression levels of CHK2 and CHK1, which are important downstream regulators of the DNA damage repair pathway, were also upregulated. These results suggested that BIBR1532 may induce DDR in ESCC cells through the ATM-CHK2 and ATR-CHK1 pathways.
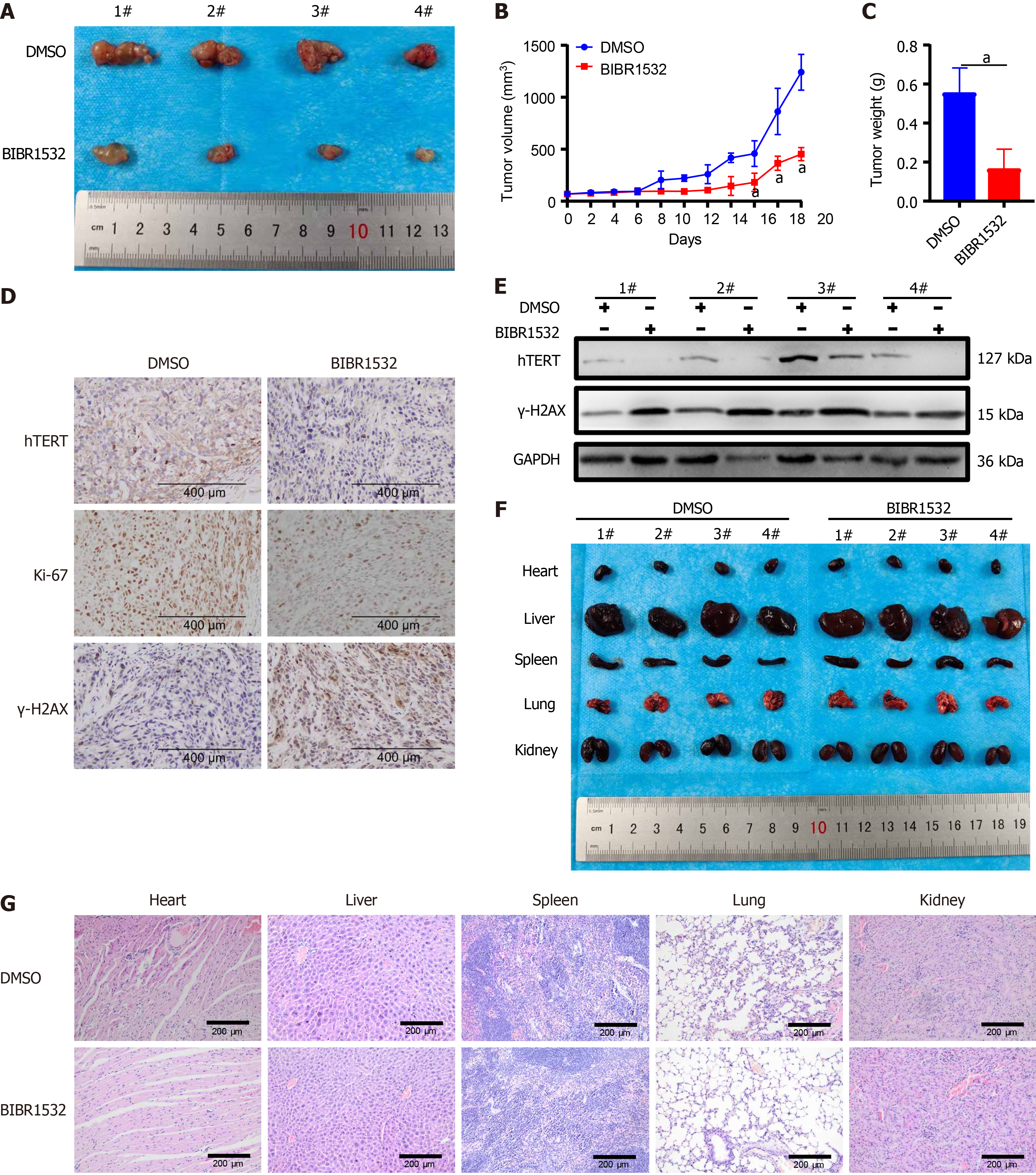
To further investigate the anti-cancer effect of BIBR1532 in vivo, nude mouse xenograft models were constructed by subscapular injection of KYSE150 cells. The results showed that tumor growth was inhibited in xenograft mice after treatment with BIBR1532 (Figure 7A and B). Moreover, the tumor weight was significantly lower in BIBR1532-treated mice than in DMSO-treated controls (Figure 7C). Furthermore, IHC results showed that the expression of hTERT and Ki-67 was reduced, whereas that of γ-H2AX was increased in tumor tissues treated with BIBR1532 (Figure 7D). Consistent with the IHC results, western blot analysis confirmed that the expression of hTERT was significantly downregulated, whereas that of γ-H2AX was upregulated in tumors treated with BIBR1532 (Figure 7E). Importantly, macroscopic images and hematoxylin and eosin (H&E) staining of the heart, liver, spleen, lungs, and kidneys of mice showed no obvious differences between the BIBR1532-treated group and the DMSO group (Figure 7F and G). Taken together, these data demonstrated that BIBR1532 inhibits ESCC growth in vivo without apparent toxicity.
Telomerase, an enzyme comprising catalytic proteins and RNA templates, is responsible for telomere elongation in cells. Normally, telomerase expression is suppressed in somatic cells, resulting in telomere shortening after cell division[21]. During the malignant transformation of cells, telomerase is reactivated, promoting uncontrolled cell growth. Studies have shown that telomerase maintains tumor cell proliferation capacity and promotes tumor development by regulating telomere length[22]. Previous research has demonstrated that hTERT is highly expressed in various tumors, including ESCC[23-25]. Our previous studies also confirmed that hTERT overexpression promotes the proliferation and metastasis of EC cells[14]. Therefore, targeting hTERT holds great potential for EC treatment.
Various telomerase inhibitors have been developed as potential therapeutic agents. Among them, BIBR1532 is a selective non-competitive inhibitor that primarily impairs DNA substrate extension after extending to the 5’ end of the template, reducing the number of TTAGGG repeats, and inhibiting long reactant formation[26]. BIBR1532 inhibits the proliferation, migration, and invasion of endometrial cells in patients with endometriosis by reducing the cascade reaction of telomerase activity and the MAPK signaling pathway[27]. In addition to benign diseases, BIBR1532 is primarily used in cancer therapy. For instance, it inhibits proliferation and migration, while inducing apoptosis in anaplastic thyroid cancer SW1736 cells[28]. Consistent with previous studies, we found that BIBR5132 downregulated hTERT expression and inhibited ESCC cell proliferation in a dose-dependent manner. Moreover, BIBR1532 significantly inhibited EC cell migration in a dose-dependent manner. Next, we explored the in vivo anti-EC effect of BIBR1532. These results demonstrated that BIBR1532 inhibited the growth of transplanted tumors in nude mice. H&E staining of the heart, liver, spleen, lung, and kidney tissues showed that BIBR1532 did not cause obvious microscopic changes in the key organs of nude mice, indicating its low toxicity in vivo. These findings further demonstrated that BIBR1532 is a potential therapeutic drug for ESCC.
Telomeres play a crucial role in the maintenance of chromosomal integrity. In eukaryotic cells, telomeres are protected by the telomere protection protein complex to prevent chromosome end DNA molecules from being recognized as broken double-stranded DNA, which induces ATM- and ATR-dependent DDR[29,30]. When DNA is damaged, upstream ATM and/or ATR protein kinases are activated, subsequently activating downstream signal transduction kinases, CHK1 and/or CHK2, as well as a series of receptors (BRCA1, MDC1, and 53BP1). These kinase cascades enhance DNA damage signals and initiate different response systems through the effectors CDC25, p53, and SMC1, ultimately leading to cell cycle arrest, activation of DNA damage repair pathways, and cell apoptosis and/or senescence[31]. In this study, we found that the expression of telomere protection proteins (TRF1, TRF2, TPP1, and POT1) was downregulated in BIBR1532 treated EC cells KYSE150 and KYSE410. Concurrently, the expression of the DNA damage-related response proteins γ-H2AX, p-ATM, CHK1, p-ATR, and CHK2 was significantly upregulated. These findings indicate that BIBR1532 inhibits telomerase activity and downregulates the expression of telomere protection proteins, inducing DDR and activating the ATM/ATR signaling pathway in EC cells. Our findings are consistent with those of a previous study showing that the knockdown of TPP1 induces DDR in EC cells[32]. Additionally, we observed that BIBR1532 induced cellular senescence in a concentration-dependent manner and upregulated the expression of the senescence marker P53, which is consistent with a series of reactions following telomere attrition, such as triggering the DDR pathway, cellular senescence, and apoptosis[33,34].
In summary, we found that BIBR1532 inhibited the proliferation and migration of ESCC cells, which is likely associated with the activation of DDR and the ATM/ATR signaling pathway, ultimately leading to telomere dysregulation and cellular senescence. Although this study presents novel findings, it has several limitations. First, we were unable to uncover the detailed mechanisms by which BIBR1532 downregulates hTERT expression, or whether it affects telomerase activity. Second, the mechanism by which BIBR1532 downregulates the expression of the four telomere protective proteins remains unclear. In the near future, we will further investigate the role of BIBR1532 in ESCC to provide more information regarding its potential therapeutic application. This may include exploring the direct effects of BIBR1532 on telomerase activity, elucidating the molecular pathways involved in the downregulation of telomere-protective proteins, and investigating potential combination therapies to enhance its anti-cancer effects.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68698] [Article Influence: 13739.6] [Reference Citation Analysis (201)] |
| 2. | Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1256] [Article Influence: 157.0] [Reference Citation Analysis (1)] |
| 3. | Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 499] [Article Influence: 83.2] [Reference Citation Analysis (1)] |
| 4. | National Health Commission of the People’s Republic of China Medical Administration and Medical Administration. [Standardization for diagnosis and treatment of esophageal cancer (2022 edition)]. Zhonghua Xiaohua Waike Zazhi. 2022;21:1247-1268. [DOI] [Full Text] |
| 5. | Ajani JA, Barthel JS, Bentrem DJ, D'Amico TA, Das P, Denlinger CS, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Mulcahy MF, Orringer MB, Osarogiagbon RU, Posey JA, Sasson AR, Scott WJ, Shibata S, Strong VE, Varghese TK Jr, Warren G, Washington MK, Willett C, Wright CD; National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 6. | Shoji Y, Koyanagi K, Kanamori K, Tajima K, Ogimi M, Ninomiya Y, Yamamoto M, Kazuno A, Nabeshima K, Nishi T, Mori M. Immunotherapy for esophageal cancer: Where are we now and where can we go. World J Gastroenterol. 2024;30:2496-2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (3)] |
| 7. | Jiang HF, Wang MS. [Current status of targeted therapy for esophageal cancer]. Shijie Linchuang Yaowu. 2015;36:378-383. [DOI] [Full Text] |
| 8. | Malhotra GK, Yanala U, Ravipati A, Follet M, Vijayakumar M, Are C. Global trends in esophageal cancer. J Surg Oncol. 2017;115:564-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 9. | Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2511] [Cited by in RCA: 2673] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 10. | Zakian VA. Structure and function of telomeres. Annu Rev Genet. 1989;23:579-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 447] [Article Influence: 12.1] [Reference Citation Analysis (1)] |
| 11. | Blackburn EH, Collins K. Telomerase: an RNP enzyme synthesizes DNA. Cold Spring Harb Perspect Biol. 2011;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 12. | Leão R, Apolónio JD, Lee D, Figueiredo A, Tabori U, Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. J Biomed Sci. 2018;25:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (1)] |
| 13. | Shay JW. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016;6:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 490] [Article Influence: 49.0] [Reference Citation Analysis (1)] |
| 14. | Li Q, Ma Q, Xu L, Gao C, Yao L, Wen J, Yang M, Cheng J, Zhou X, Zou J, Zhong X, Guo X. Human Telomerase Reverse Transcriptase as a Therapeutic Target of Dihydroartemisinin for Esophageal Squamous Cancer. Front Pharmacol. 2021;12:769787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 15. | Jäger K, Walter M. Therapeutic Targeting of Telomerase. Genes (Basel). 2016;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 16. | Chen X, Tang WJ, Shi JB, Liu MM, Liu XH. Therapeutic strategies for targeting telomerase in cancer. Med Res Rev. 2020;40:532-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 17. | Lavanya C, Venkataswamy MM, Sibin MK, Srinivas Bharath MM, Chetan GK. Down regulation of human telomerase reverse transcriptase (hTERT) expression by BIBR1532 in human glioblastoma LN18 cells. Cytotechnology. 2018;70:1143-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 18. | Ding X, Cheng J, Pang Q, Wei X, Zhang X, Wang P, Yuan Z, Qian D. BIBR1532, a Selective Telomerase Inhibitor, Enhances Radiosensitivity of Non-Small Cell Lung Cancer Through Increasing Telomere Dysfunction and ATM/CHK1 Inhibition. Int J Radiat Oncol Biol Phys. 2019;105:861-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 19. | Kusoglu A, Goker Bagca B, Ozates Ay NP, Gunduz C, Biray Avci C. Telomerase inhibition regulates EMT mechanism in breast cancer stem cells. Gene. 2020;759:145001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 20. | Gao J, Pickett HA. Targeting telomeres: advances in telomere maintenance mechanism-specific cancer therapies. Nat Rev Cancer. 2022;22:515-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (1)] |
| 21. | Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1223] [Article Influence: 58.2] [Reference Citation Analysis (1)] |
| 22. | Mizukoshi E, Kaneko S. Telomerase-Targeted Cancer Immunotherapy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 23. | Pal J, Gold JS, Munshi NC, Shammas MA. Biology of telomeres: importance in etiology of esophageal cancer and as therapeutic target. Transl Res. 2013;162:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 24. | Yang R, Han Y, Guan X, Hong Y, Meng J, Ding S, Long Q, Yi W. Regulation and clinical potential of telomerase reverse transcriptase (TERT/hTERT) in breast cancer. Cell Commun Signal. 2023;21:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 25. | Zalewska-Ziob M, Dobija-Kubica K, Biernacki K, Adamek B, Kasperczyk J, Bruliński K, Ostrowska Z. Clinical and prognostic value of hTERT mRNA expression in patients with non-small-cell lung cancer. Acta Biochim Pol. 2017;64:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 26. | Pascolo E, Wenz C, Lingner J, Hauel N, Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K, Schnapp A. Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J Biol Chem. 2002;277:15566-15572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 27. | Zhao X, Luo D, Liu T, Zhang H, Xie Y, Kong W. BIBR1532 Affects Endometrial Cell Proliferation, Migration, and Invasion in Endometriosis via Telomerase Inhibition and MAPK Signaling. Gynecol Obstet Invest. 2023;88:226-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 28. | Turkmen E, Sogutlu F, Erdogan M, Biray Avci C. Evaluation of the anticancer effect of telomerase inhibitor BIBR1532 in anaplastic thyroid cancer in terms of apoptosis, migration and cell cycle. Med Oncol. 2023;40:196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (3)] |
| 29. | Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2234] [Cited by in RCA: 2447] [Article Influence: 128.8] [Reference Citation Analysis (0)] |
| 30. | Li Z, Pearlman AH, Hsieh P. DNA mismatch repair and the DNA damage response. DNA Repair (Amst). 2016;38:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 31. | d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 2087] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 32. | Wen J, Zhong X, Gao C, Yang M, Tang M, Yuan Z, Wang Q, Xu L, Ma Q, Guo X, Fang L. TPP1 Inhibits DNA Damage Response and Chemosensitivity in Esophageal Cancer. Crit Rev Eukaryot Gene Expr. 2023;33:77-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 33. | Engin AB, Engin A. The Connection Between Cell Fate and Telomere. Adv Exp Med Biol. 2021;1275:71-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Frias C, Pampalona J, Genesca A, Tusell L. Telomere dysfunction and genome instability. Front Biosci (Landmark Ed). 2012;17:2181-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/